In this issue of Blood, Jansen and colleagues use murine models to give new insights into the possible mechanisms of clearance of transfused refrigerated platelets.1
Today, platelet transfusions are used routinely in preventing or treating hemorrhage and the demand for platelet concentrates continues to rise, mainly because of the increasing number of patients undergoing marrow suppressive therapy. The main task for blood transfusion services is to maintain an adequate supply of safe and effective platelet products. Unfortunately, because of the potential risk of bacterial contamination, platelets have a very short shelf life of 5 days stored at 20-24°C with continuous gentle agitation.2 Consequently, platelet products are often in short supply. The processes used to collect and store platelets have evolved during past years to allow storage under optimal conditions to limit physiologic alterations in the cells known as the platelet storage lesion (PSL). The PSL is associated with decreased posttransfusion survival of transfused platelets and their rapid clearance from the bloodstream. Several approaches are presently being explored with the goal of better preserving cell viability and function during platelet storage, including adjusting the storage medium3 and/or modifying the platelets themselves. The latter approach has arisen from the publications of Hoffmeister and colleagues.4
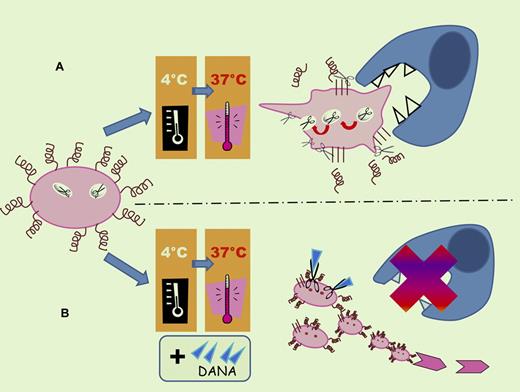
Refrigeration and platelet activation increases platelet surface expression of the sialidase Neu1 providing desialylation of the platelet VWF receptors. Cold-stored platelets undergo clustering of these receptors. GPIbα and GPV are cleaved from the surface of refrigerated platelets by metalloproteinase ADAM17. These phenomena result in increased clearance of the platelets because of the recognition of β-N-acetylglucosamine (β-GlcNac) residues on clustered GPIbα by the αMβ2 integrin receptor present on hepatic macrophages and of desialylated GPIbα by the Ashwell-Morell receptor present on hepatocytes (A). Addition of the sialidase inhibitor, DANA, enhances the recovery and circulation of refrigerated platelets, but did not completely prevent galactose exposure (B).
Refrigeration and platelet activation increases platelet surface expression of the sialidase Neu1 providing desialylation of the platelet VWF receptors. Cold-stored platelets undergo clustering of these receptors. GPIbα and GPV are cleaved from the surface of refrigerated platelets by metalloproteinase ADAM17. These phenomena result in increased clearance of the platelets because of the recognition of β-N-acetylglucosamine (β-GlcNac) residues on clustered GPIbα by the αMβ2 integrin receptor present on hepatic macrophages and of desialylated GPIbα by the Ashwell-Morell receptor present on hepatocytes (A). Addition of the sialidase inhibitor, DANA, enhances the recovery and circulation of refrigerated platelets, but did not completely prevent galactose exposure (B).
Four decades ago, Murphy et al showed that cold temperatures, which reduce the risk of bacterial growth, had deleterious effects on platelets.5 Considerable effort was subsequently expended on better understanding the phenomena underlying the metabolic and structural modifications related to cold storage to develop mitigation strategies and forestall on-going platelet shortages.
Hoffmeister et al demonstrated in 2003 that the reduced survival of short-term cold-stored platelets was because of the clustering of the von Willebrand factor (VWF) receptors at the platelet surface.6 The recognition of β-N-acetylglucosamine (β-GlcNac) residues on clustered GPIbα by the αMβ2 integrin receptor present on hepatic macrophages (Kupffer cells) produced in vivo platelet phagocytosis in mice. Subsequently, the same group showed the role of the hepatic asialoglycoprotein, the Ashwell-Morell receptor, in recognizing desialylated GPIbα and its importance in increased clearance of long-term refrigerated platelets.7
The article by Jansen et al here makes a novel and interesting contribution to the field.1 In their present study, the authors investigated the behavior of key enzymatic pathways in platelets after refrigeration, including the role of the platelet sialidases and the GPIbα metalloproteinase (MP) ADAM17-dependent cleavage in mice.
During a cold-rewarming process, Jansen at al have shown that refrigeration and platelet activation up-regulate platelet sialidase activity, causing desialylation of the platelet VWF receptors, specifically the GPIbα. The loss of sialic acid may therefore be a key point leading to accelerated platelet clearance. In light of these results, it was hypothesized that diminishing intrinsic sialidase activity by addition of the sialidase inhibitor, DANA, might improve transfused platelet survival in vivo. In mice, platelets stored with such an inhibitor had a higher initial recovery and enhanced survival compared with platelets stored in the cold without DANA. However, this treatment did not totally inhibit sialidase activity (see figure).
To more accurately characterize changes on the platelet surface after refrigeration that influenced in vivo platelet clearance, alterations in expression of the VWF receptors were analyzed. A decrease in the surface expression of the GPIbα and GPV after refrigerated storage and rewarming was noticeable. To address the role of the metalloproteinases, mouse platelets were refrigerated in plasma in the presence or absence of a metalloproteinase inhibitor. It appeared that ADAM17 mediates the GPIbα shedding from human and mouse platelets and inhibition of MP prevented shedding. Surprisingly, inhibition of the MP-dependent shedding did not improve refrigerated platelet survival transfused into mice. This was confirmed with platelets from mice expressing an inactive form of ADAM17. Using recombinant ADAM17 (rADAM17), Jansen et al further showed that sialic acid removal plays a role in GPIbα and GPV shedding, which is prevented by the addition of DANA. Moreover, addition of ADAM17 inhibitor to sialidase-treated platelets in-hibited receptor shedding by rADAM17. Therefore, desialylation of GPIbα and GPV was a prerequisite for ADAM17-mediated receptor shedding under these conditions.
Adjustment of the different pieces of the puzzle in such models for a better understanding of platelet senescence markers requires still more research. Although significant results have been obtained by investigating the hypothesis that inhibition of sialidase activity during platelet storage (even at room temperature) may improve platelet quality, translating these results from ex vivo experimentation or in vivo murine models to humans must be done with some caution. Questions remain and further research in this area is warranted.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■