Serial plasma aliquots (50 mL) obtained from 10 commercial donors who converted from hepatitis C virus (HCV) RNA negative to positive were transfused into 2 chimpanzees to assess infectivity during early HCV infection. Plasma, obtained 4 days before HCV RNA detectability by licensed assays, transmitted HCV infection to chimpanzee X355. The infectious PCR-negative plasma was subsequently shown to be positive in 2 of 23 replicates using a sensitive transcription-mediated amplification (TMA) assay, and estimated to contain 1.2 HCV RNA copies/mL (60 copies/50 mL transfused). Plasma units obtained up to 8 weeks earlier were not infectious in a second susceptible chimp, even when from donors with low-level, intermittent HCV RNA detection. Chimp x355 developed acute viremia with subsequent seroconversion, but cleared both virus and Ab in 17 weeks. When rechallenged 38 months later with 6000 RNA copies/mL from the same donor, X355 was transiently reinfected and again rapidly lost all HCV markers. We conclude that: (1) transfusions can transmit HCV infection before RNA detection, but the interval of test-negative infectivity is very brief; (2) early “blips” of HCV RNA appear noninfectious and can be ignored when calculating residual transfusion risk; and (3) markers of HCV infection can be lost rapidly after exposure to low-dose inocula.
Introduction
The phase between the onset of hepatitis C virus (HCV) infection and sustained systemic viremia is known as the eclipse or previremic window phase of infection.1,–3 At its conclusion, plasma HCV RNA concentrations rise exponentially in what has been termed the ramp-up phase of infection.1,2 The eclipse phase, by definition, is characterized by lack of detectable plasma viremia by commercially available HCV RNA assays, which are primarily based on PCR technologies. However, by performing quadruplicate analyses of plasma donations from acutely infected source plasma donors using more sensitive qualitative transcription-mediated amplification (TMA) assays for HCV RNA, we found previously that 108 of 225 eclipse-phase donations from 50 donors unexpectedly demonstrated the presence of intermittent, low-level HCV RNA.1 A second study of source plasma donors found a similar phenomenon,4 as did studies in injection drug users (IDU)5,6 and transfusion recipients.7 Thus, HCV dynamics in the eclipse phase may follow one of 2 patterns: no viremia until a sustained rapid increase in serum RNA levels occurs (ramp-up) or intermittent low-level HCV RNA detection (previously referred to in HIV and SIV infections as “blip” viremia)8,9 that precedes ramp-up by varying intervals up to 2 months.
Because HCV RNA levels are very low in this very early phase of infection, it has not been possible to determine whether the RNA is contained in infectious virions or to characterize the sequence integrity of HCV RNA genomes during these “blips” relative to the sequences observed during the ramp-up period from the same subjects. With respect to blood safety, if the “blips” are found to be infectious, then current residual risk modeling might be underestimating HCV transfusion-transmission risk because such modeling assumes that donations given before the extrapolated beginning of the ramp-up period are noninfectious.10,–12
The chimpanzee model of HCV infection has played a key role in our understanding of determinants of viral transmission and replication, the development of antiviral immune responses, assessment of potential viral and host factors that lead to resolved versus chronic infection, and evaluation of therapeutics and vaccines. Of particular note, the chimpanzee model is considered an extremely sensitive model for assessment of parenteral infectivity relevant to transfusion safety.13,,,,,,,,,,–24 For this reason, we conducted multistep inoculation experiments in 2 chimpanzees, designed to study the infectivity of serially collected plasma donations from source plasma donors recently infected with HCV. First, we evaluated infectivity from donations initially assessed as occurring immediately before the onset of ramp-up–phase viremia. Next, we evaluated infectivity of nonviremic and low-level HCV RNA-positive (blip) donations from donors who demonstrated intermittent HCV RNA detection in the eclipse phase of HCV infection. We also studied the early immunology of HCV infection after a low-dose exposure and the influence of this exposure on subsequent reinfection with the same HCV isolate. In addition to contributing to our understanding of transfusion-transmission, these experiments provide data relevant to broader issues of HCV transmissibility, the ability to document previous HCV infection using RNA and Ab diagnostics, and the early immunologic events in HCV infection.
Methods
Preparation of HCV-RNA–positive plasma donor panels
Plasma for chimp inoculation studies was selected from a collection of 50 source plasma panels used in a previous study of early HCV viral dynamics (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).1 Each panel consisted of aliquots of a series of donations (serial donations) from a given donor. These donations occurred at approximately twice-weekly intervals in both the eclipse and ramp-up phases of acute HCV infection. We based the classification of ramp-up phase infection on the specific rate of increase in HCV viral load for each individual donor.1 We then back-extrapolated from the slope of ramp-up viremia for each donor to establish a time when the estimated viral load would have been < 0.05 copies/mL; all RNA-positive donations collected before this time point were classified as occurring in the eclipse phase (ie, before the extrapolated beginning of the ramp-up phase). Donations given before quantifiable ramp-up viremia were then further evaluated for HCV RNA by performing TMA testing (Procleix discriminatory HCV [dHCV] assay; Gen-Probe) on 4 replicates of 0.5 mL of plasma for each donation.1 Multiple additional replicates (n = 20 or 23) of selected eclipse-phase samples were also further tested for HCV RNA either by the dHCV assay or by the Procleix duplex (HIV-HCV) assay (Gen-Probe) as part of a previously published study.25 Based on this testing, we characterized 37 of the 50 panels as having intermittent HCV RNA-positive donations (at least 1 dHCV TMA-positive replicate result) during the eclipse phase interspersed with nonviremic (all replicates negative by TMA) donations.
Selection of plasma donor panels for infusion into chimps
We selected 10 ABO-compatible serial donation panels for chimpanzee infectivity studies. We used 5 of the 13 panels that did not have evidence of intermittent viremia as well as 5 of the 37 that showed intermittent HCV RNA detection (blips). The 5 cases without intermittent viremia were selected to have the most frequent serial donations over the 3 weeks preceding RNA detection by quantitative HCV PCR and qualitative HCV TMA assays. The 5 cases with intermittent HCV RNA during the eclipse phase were selected to have at least 3 reactive results among the 4 replicate HCV TMA results on 2 or more specimens collected > 2 weeks before ramp-up viremia; they were also selected to include intervening donations that tested negative on a total of up to 27 replicate TMA assays.
General format of chimp inoculation experiments
Two healthy adult female chimpanzees (X331 and X355) were housed in a containment facility maintained by the Texas Biomedical Research Institute and Southwest National Primate Research Center, an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility. Housing conditions and animal manipulations were approved by the institutional animal care and use committee and all animal procedures were performed with prior administration of appropriate sedatives and anesthesia. Fifty milliliters of donor plasma units were intravenously administered; these could be either individual unit infusions, multiple units infused one after another, or a premixed pool of 5 U (250-mL total volume). Further details of the timing of the infusions in the various experiments are provided in Figure 1 and supplemental Figure 1, and in “Results.”
Experimental design and sequence of plasma infusions and follow-up of chimpanzees. Experiment I assessed the infectivity of plasma that tested HCV RNA negative by licensed diagnostic assays and was obtained in the days just before ramp-up viremia. (A) Fifty milliliters of pre–ramp-up phase plasma from each of 5 commercial apheresis donors was infused sequentially during a single experimental procedure into chimp X355. (B) When transmission was linked to 1 donor by phylogenetic sequencing, 50-mL plasma samples from each of 4 earlier donations from that implicated donor were transfused to a second animal (X331) at 9-week intervals. Both animals were followed for virologic, serologic, and cell-mediated immune responses to assess evidence of HCV infection. Experiment II examined the infectivity of samples from 5 donors who had intermittent low-level HCV RNA (“blips”) detected during the eclipse phase of HCV infection by infusions into chimp X331. (C) Phase 1: Infusion of a pool of 250 mL (50 mL of plasma/donor from donations collected subsequent to blips) of HCV RNA-negative plasma from the eclipse phase. (D) Phase 2: 50-mL plasma samples from blip viremic units from the eclipse phase from the same 5 donors were sequentially infused at 6-week intervals. Phase 1 and phase 2 infusions did not transmit HCV infection to the recipient animal (chimp X331). (E) Phase 3: To confirm this chimp's susceptibility to HCV infection, 50-mL plasma samples from each of 3 progressively higher titer HCV RNA-positive donations collected during the early ramp-up phase of infection from one of these 5 donors were infused at 8-week intervals. In experiment III, chimp X355 who had spontaneously recovered from HCV infection and lost anti-HCV as well as virus, was rechallenged 3 years later to determine whether prior infection conferred protection against reinfection. (F1) Infusion of 50 mL of plasma containing an estimated 80 HCV RNA copies (1.6 copies/mL) from the previous infecting donation. (F2) Rechallenge with 50 mL of plasma containing an estimated 300 000 HCV RNA copies (6000 copies/mL) from a subsequent early ramp-up–phase donation from the same donor.
Experimental design and sequence of plasma infusions and follow-up of chimpanzees. Experiment I assessed the infectivity of plasma that tested HCV RNA negative by licensed diagnostic assays and was obtained in the days just before ramp-up viremia. (A) Fifty milliliters of pre–ramp-up phase plasma from each of 5 commercial apheresis donors was infused sequentially during a single experimental procedure into chimp X355. (B) When transmission was linked to 1 donor by phylogenetic sequencing, 50-mL plasma samples from each of 4 earlier donations from that implicated donor were transfused to a second animal (X331) at 9-week intervals. Both animals were followed for virologic, serologic, and cell-mediated immune responses to assess evidence of HCV infection. Experiment II examined the infectivity of samples from 5 donors who had intermittent low-level HCV RNA (“blips”) detected during the eclipse phase of HCV infection by infusions into chimp X331. (C) Phase 1: Infusion of a pool of 250 mL (50 mL of plasma/donor from donations collected subsequent to blips) of HCV RNA-negative plasma from the eclipse phase. (D) Phase 2: 50-mL plasma samples from blip viremic units from the eclipse phase from the same 5 donors were sequentially infused at 6-week intervals. Phase 1 and phase 2 infusions did not transmit HCV infection to the recipient animal (chimp X331). (E) Phase 3: To confirm this chimp's susceptibility to HCV infection, 50-mL plasma samples from each of 3 progressively higher titer HCV RNA-positive donations collected during the early ramp-up phase of infection from one of these 5 donors were infused at 8-week intervals. In experiment III, chimp X355 who had spontaneously recovered from HCV infection and lost anti-HCV as well as virus, was rechallenged 3 years later to determine whether prior infection conferred protection against reinfection. (F1) Infusion of 50 mL of plasma containing an estimated 80 HCV RNA copies (1.6 copies/mL) from the previous infecting donation. (F2) Rechallenge with 50 mL of plasma containing an estimated 300 000 HCV RNA copies (6000 copies/mL) from a subsequent early ramp-up–phase donation from the same donor.
The animals were monitored by testing blood samples for HCV RNA by PCR, anti-HCV by enzyme-linked immunoassay (EIA), liver enzyme levels, and T-cell assays. If infected, weekly or biweekly monitoring continued; however, if there was no detectable infection after 6 weeks, the animal was rested for an interval of up to 3 weeks and then became eligible for infusion with another donor plasma sample. This sequence was repeated with several different donor plasma samples until evidence of infection could be ascertained. After confirmation of infection, animals were monitored for a period of 1 year to determine the outcome of infection. No additional infusions with donor plasma were performed until completion of this 1-year period.
Laboratory assays
HCV RNA quantification in plasma donor panels.
Two different assays were performed as previously described.1 The COBAS Amplicor HCV Monitor (Version 2.0 assay; Roche Molecular Systems) HCV PCR assay was able to quantify HCV RNA down to a lower limit of 600 IU/mL. Samples that were negative on this assay were tested using the dHCV TMA assay using multiple 0.5-mL replicates of plasma. The limit of detection (LOD) of each replicate assay is 12.1 copies/mL (50% LOD; 95% confidence interval [CI] 11.1-13.2).26 HCV RNA concentration was determined based on the percentage of replicates that gave positive results using probit analyses.
HCV RNA detection and quantification in the recipient chimps.
HCV RNA detection was performed using the COBAS Amplicor Hepatitis C Virus Test (Version 2.0; Roche Molecular Systems) and quantification of HCV RNA was performed using the COBAS Amplicor HCV Monitor (Version 2.0 assay).
HCV Ab detection.
Initial screening of plasma donor panels was done using a third-generation HCV Ab EIA (Ortho Diagnostics). Repeat reactive samples were further evaluated by the Recombinant Immunoblot Assay (RIBA; Version 3; Novartis Diagnostics). Initial screening of chimp sera was done using an anti-HCV 2.0 EIA (Abbott Laboratories) with confirmation by RIBA.
HCV RNA extraction, amplification, cloning, sequencing, and phylogenetic analysis.
HCV RNA was extracted from 140 μL of plasma from one viremic time point from each of the 5 plasma donors implicated in HCV transmission to chimp X331 and from 2 viremic time points from the infected chimp using the QIAamp Viral RNA Mini Kit following the manufacturer's instructions (QIAGEN). Extraction and amplification of donor and chimp samples were performed on different days to eliminate the possibility of cross-contamination. Details of the amplification, cloning, sequencing, and phylogenetic analysis procedures are in supplemental Methods.
ALT and AST measurements.
Serum samples collected from the study animals were analyzed for alanine aminotransferase (AST) and asparate aminotransferase (ALT) levels with a Unicel DXC600 serum chemistry analyzer (Beckman Coulter). Enzyme levels were considered normal if they were within the normal range (AST 11-25 U/L; ALT 21-55 U/L) established at the primate center.
CD4 proliferative responses to HCV Ags.
PBMCs were isolated from ACD-anticoagulated chimpanzee blood via gradient centrifugation as described.27 Triplicate cultures of 200 000 PBMCs were stimulated with 1 μg/mL HCVcore, NS3, helicase, NS4, NS5A, NS5B proteins (Mikrogen) or buffer control as previously described.28 Cultures were labeled with 1 μCi [3H]thymidine (Amersham) on day 5 and harvested 16 hours later. Separate cultures stimulated with or without PHA (1 μg/mL; Murex Biotech Limited) were labeled with [3H]thymidine on day 2. The stimulation index (SI) was calculated as a ratio of the average number of counts per minute of 4 replicate cultures in the presence of Ag compared with control buffer or medium.
Results
Three chimp inoculation experiments were performed in multiple phases as described below and in Figure 1.
Experiment I: testing the infectivity of plasma immediately before the ramp-up phase
Experiment I, phase 1 involved infusions of 50 mL of plasma (during a single infusion episode) from each of 5 different source plasma donors into an HCV naive chimp (X355). The infused donations were from donors in whom there was no HCV RNA detected during the eclipse phase and were selected so as to be the donations that immediately preceded the first ramp-up viremia donation (Figure 2A, and supplemental Figure 1A-E). These donations, which tested negative for HCV RNA by quadruplicate dHCV TMA and viral load assays, were estimated by back-extrapolation from measured viral loads during the subsequent ramp-up phase of each donor to have been collected from 4 to 16 days before the onset of the ramp-up phase (defined as one HCV RNA copy present in 20 mL of plasma).
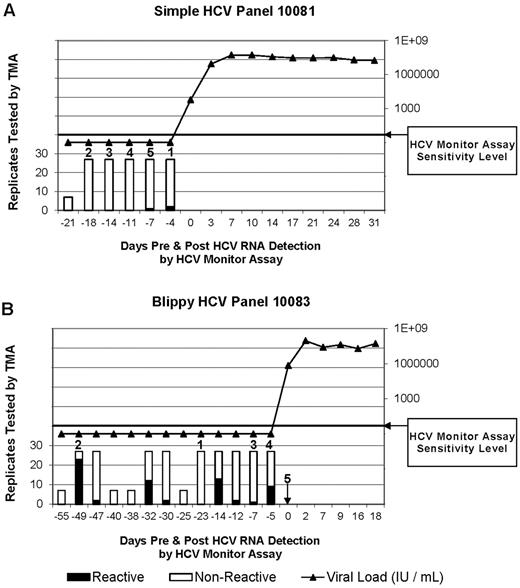
Representative viremia results from 2 of the 10 plasma donors used in chimpanzee infection experiments (data from all 10 panels is presented in supplemental Figure 1). (A) TMA and viral load results over time from plasma donor panel 10081, who did not demonstrate intermittent blips of HCV RNA before detection of ramp-up–phase viremia. Numbers above bars represent the order in which aliquots were infused into chimps. Infusion 1 was part of a transmitting pool that was infused into chimp X355. Infusions 2-5 were each infused into chimp X331 at intervals of 9 weeks between infusions. ▴ indicates viral load in the ramp-up and post–ramp-up phase of HCV infection in donor 10081. (B) TMA and viral load results over time from plasma donor panel 10083 who manifested intermittent blips of HCV RNA reactivity during the eclipse phase of acute HCV infection. Both HCV RNA-negative “valley” and HCV RNA-positive “blip” samples were infused into chimp X331 (see Figure 1, experiment II). Numbers above bars identify aliquots and the order infused into chimps. Note that the units were infused in a different order than they were collected and that infusion 1 was part of a nontransmitting pool. ▴ indicates viral load in donor 10083.
Representative viremia results from 2 of the 10 plasma donors used in chimpanzee infection experiments (data from all 10 panels is presented in supplemental Figure 1). (A) TMA and viral load results over time from plasma donor panel 10081, who did not demonstrate intermittent blips of HCV RNA before detection of ramp-up–phase viremia. Numbers above bars represent the order in which aliquots were infused into chimps. Infusion 1 was part of a transmitting pool that was infused into chimp X355. Infusions 2-5 were each infused into chimp X331 at intervals of 9 weeks between infusions. ▴ indicates viral load in the ramp-up and post–ramp-up phase of HCV infection in donor 10081. (B) TMA and viral load results over time from plasma donor panel 10083 who manifested intermittent blips of HCV RNA reactivity during the eclipse phase of acute HCV infection. Both HCV RNA-negative “valley” and HCV RNA-positive “blip” samples were infused into chimp X331 (see Figure 1, experiment II). Numbers above bars identify aliquots and the order infused into chimps. Note that the units were infused in a different order than they were collected and that infusion 1 was part of a nontransmitting pool. ▴ indicates viral load in donor 10083.
After infusion, chimp X355 was evaluated weekly for the first 11 weeks, then biweekly until week 29 after infusion, with a final assessment at 1 year after infusion. Tests performed were HCV RNA (qualitative and, when indicated, quantitative), HCV Ab, ALT, and AST. Assays for PBMC proliferative responses to HCV proteins were performed before infusion and biweekly until week 10 after infusion. As shown in Figure 3, chimp X355 developed mild hepatitis (peak ALT 68 IU/L) and transient HCV infection with viremia developing at week 2 and persisting until week 9. Peak viral load was 5.2 × 104 copies/mL at week 4. HCV Ab was first detected at week 8 and persisted until week 17, after which it became undetectable throughout follow-up (seroreversion). Samples collected from weeks 9 through 13 were RIBA positive but never showed Ab reactivity to the core (c22) Ag. Samples at weeks 15 and 17 showed very low Ab reactivity by RIBA (c33 at 1+, 511 and NS5 at +/−, and c22 nondetectable). ALT was elevated from weeks 5 through 8 with a peak value of 68 U/L. Proliferation of PBMC to HCV Ags was not detectable preinfusion or at week 2, but a clear peak was detectable at week 4. Responses were exclusively targeted against nonstructural HCV Ags as evidenced by SIs of 33 and 27 against NS5B and NS5A, respectively, 29 against NS4 and 31 against NS3. Responses were maintained at week 6 and 8 after infusion, and declined to slightly above baseline at week 10. By 17 weeks after infusion, HCV RNA and HCV Ab were undetectable and ALT levels were normal, thus leaving no residual evidence of prior HCV infection except for the cell-mediated immune response which remained detectable as late as week 21 after infusion.
Results of transmission experiment I in chimp X355. Fifty milliliters of plasma from each of 5 donors in the pre–ramp-up phase of HCV infection were infused in a single experimental procedure into chimp X355 (see Figure 1, experiment I) and infectivity monitored by weekly or biweekly measurement of ALT level (solid line), HCV RNA (dashed line and box), anti-HCV (box), and cell-mediated immune response (bottom panel). Transient HCV infection is demonstrated. Note that HCV RNA was no longer detectable by week 10 and that Ab to HCV disappeared by week 18 after infusion. Cell-mediated immune responses, measured in a proliferation assay, peaked at week 4 and considerably diminished by week 10.
Results of transmission experiment I in chimp X355. Fifty milliliters of plasma from each of 5 donors in the pre–ramp-up phase of HCV infection were infused in a single experimental procedure into chimp X355 (see Figure 1, experiment I) and infectivity monitored by weekly or biweekly measurement of ALT level (solid line), HCV RNA (dashed line and box), anti-HCV (box), and cell-mediated immune response (bottom panel). Transient HCV infection is demonstrated. Note that HCV RNA was no longer detectable by week 10 and that Ab to HCV disappeared by week 18 after infusion. Cell-mediated immune responses, measured in a proliferation assay, peaked at week 4 and considerably diminished by week 10.
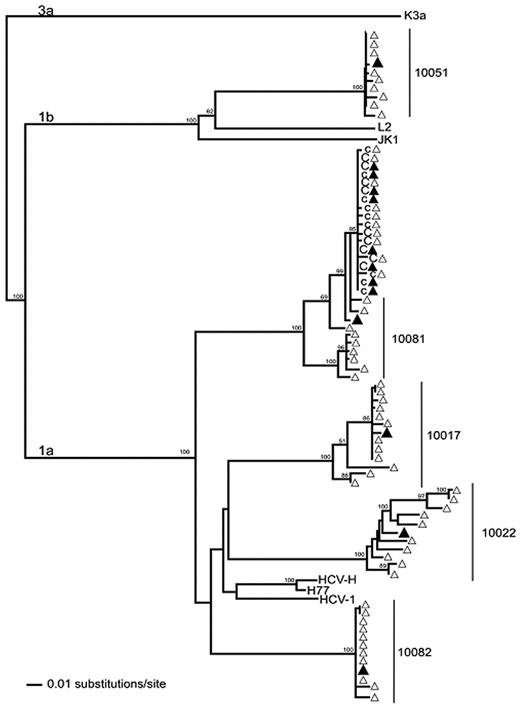
Sequencing of HCV RNA and phylogenetic analysis revealed that the chimp HCV isolate was very closely related to an HCV isolate obtained from a subsequent highly viremic donation from one of the 5 source plasma donors used in the infecting inocula (Figure 4). The transmitting donation had been collected 4 days before the first quantifiable HCV RNA-positive donation (viral load of 6 × 103 HCV RNA copies/mL) from this donor (donor 10081). To increase the sensitivity for detection of HCV RNA in the transmitting and nontransmitting source plasma donations, 23 additional 0.5-mL plasma replicates from the infused donations were tested under code for HCV RNA by the TMA assay. In total, 2 of the 27 replicate TMA assays were positive with plasma from the donation implicated as infectious by phylogenetic analysis, whereas similar multiple replicate TMA testing performed on identically processed frozen-thawed plasma from the other 4 donations in the transmitting pool gave negative results. A probit analysis indicated that the dHCV TMA-positive donation had an estimated HCV RNA concentration of 1.2 copies/mL (95% CI 0.5-1.9 copies/mL). In retrospect, this initially HCV RNA-negative donation was probably in the very early ramp-up phase of infection such that multiple replicate testing was required to detect the very low level of HCV RNA that was present. Because 50 mL of plasma from this donation was infused, we estimate that chimp X355 acquired HCV infection after being transfused with a total of ∼ 60 HCV RNA copies (95% CI: 25-95 copies).
Phylogenetic reconstruction of HCV E1/E2 region sequences from 5 plasma donors (10081, 10051, 10082, 10017, and 10022) and a chimpanzee (X355) infused with a pool of 50 mL of plasma from each of the donors. ▴ indicates population sequences corresponding to the first hypervariable region (HVR-1) of E1/E2 (404nt) for donors and the chimp; and ▵, cloned sequences. Samples from 2 time points 19 days apart were obtained from the chimpanzee. The first time point sequences are indicated with c and the second time point sequences by C. Viral sequences in the chimp are shown to be closely related to donor 10081. Reference sequences from genotypes 1a (HCV-H, H77, and HCV-1), 1b (L2, JK1), and 3a (K3a) were included. The maximum likelihood tree was rooted with the 3a reference sequence. Bootstrap values > 70% are indicated.
Phylogenetic reconstruction of HCV E1/E2 region sequences from 5 plasma donors (10081, 10051, 10082, 10017, and 10022) and a chimpanzee (X355) infused with a pool of 50 mL of plasma from each of the donors. ▴ indicates population sequences corresponding to the first hypervariable region (HVR-1) of E1/E2 (404nt) for donors and the chimp; and ▵, cloned sequences. Samples from 2 time points 19 days apart were obtained from the chimpanzee. The first time point sequences are indicated with c and the second time point sequences by C. Viral sequences in the chimp are shown to be closely related to donor 10081. Reference sequences from genotypes 1a (HCV-H, H77, and HCV-1), 1b (L2, JK1), and 3a (K3a) were included. The maximum likelihood tree was rooted with the 3a reference sequence. Bootstrap values > 70% are indicated.
To confirm that the donation that initially infected chimp X355 was the earliest infectious donation in the panel of serial donation samples from the implicated donor, we conducted phase 2 of this experiment. A second HCV naive chimp (chimp X331) was infused on separate occasions with 50 mL of plasma from HCV RNA-negative units donated by the transmitting donor that had been collected 14, 10, 7, and 3 days before the transmitting donation (Figure 2A). Chimp X331 was monitored weekly for 6 weeks after each 50-mL infusion (total of 24 weeks) to document evidence of HCV infection by HCV RNA, HCV Ab, and ALT/AST testing. There was no evidence for HCV infection in this second recipient chimp.
Experiment II: testing the infectivity of plasma during the eclipse phase
In experiment II, eclipse-phase RNA-positive and RNA-negative donations were selected from 5 of the 37 source plasma donors who had intermittent HCV RNA detected in the eclipse phase of infection (Figure 1, supplemental Figure 1F-J). Chimp X331, who had not been infected in phase 2 of experiment I, was used for these studies, which were begun 22.5 months after the completion of experiment I. In phase 1 of experiment II, a 250-mL plasma pool from nonviremic donations obtained during the eclipse phase of infection from 5 different source plasma donors was infused into chimp X331 (see supplemental Figure 1F-J for time points used in these infusions); these HCV RNA-negative donations were collected during the RNA-negative “valleys” between intermittent HCV RNA-positive eclipse-phase donations (blips). This chimp was monitored weekly for 6 weeks for HCV RNA, HCV Ab, ALT, and AST. There was no evidence for viremia, Ab seroconversion, or transaminase elevation, indicating that these HCV RNA-negative eclipse-phase donations were not infectious.
One month later, phase 2 of this second experiment involved infusions of 50 mL of low-level HCV RNA-positive eclipse-phase plasma from each of the same 5 source plasma donors into the same chimp (Table 1, supplemental Figure 1F-J). The infusions of the 5 units were separated by 6-week intervals during which the recipient chimp was monitored for evidence of HCV infection. There was no virologic evidence for transmission of HCV infection, and no evidence for humoral or cellular immune responses.
Estimated HCV RNA concentrations of eclipse-phase donations with blip viremia infused into chimp X331 (experiment II, phase 2)
| Donor/donation no. . | Days preceding ramp-up* . | No. of TMA-positive replicates (%) . | Imputed HCV RNA copies/mL (95% fiducial limits)† . | Infused HCV RNA copies . |
|---|---|---|---|---|
| 10050-04 | 41 | 6/27 (22) | 2.22 (1.23-3.17) | 111 |
| 10083-02 | 49 | 23/27 (85) | 11.22 (9.35-13.67) | 561 |
| 10011-02 | 49 | 2/27 (7) | 1.18 (0.53-1.91) | 60 |
| 10029-01 | 48 | 10/27 (38) | 3.37 (2.15-4.47) | 185 |
| 10085-02 | 49 | 10/27 (37) | 3.29 (2.08-4.38) | 164 |
| Donor/donation no. . | Days preceding ramp-up* . | No. of TMA-positive replicates (%) . | Imputed HCV RNA copies/mL (95% fiducial limits)† . | Infused HCV RNA copies . |
|---|---|---|---|---|
| 10050-04 | 41 | 6/27 (22) | 2.22 (1.23-3.17) | 111 |
| 10083-02 | 49 | 23/27 (85) | 11.22 (9.35-13.67) | 561 |
| 10011-02 | 49 | 2/27 (7) | 1.18 (0.53-1.91) | 60 |
| 10029-01 | 48 | 10/27 (38) | 3.37 (2.15-4.47) | 185 |
| 10085-02 | 49 | 10/27 (37) | 3.29 (2.08-4.38) | 164 |
TMA indicates transcription-mediated amplification.
Plasma from HCV RNA-negative donations from each of these five donors were previously infused into chimp X331 and resulted in no evidence of HCV infection. These donations were collected prior to ramp-up viremia at days −31, −23, −19, −21, and −18, respectively.
RNA copy levels were estimated from probit analysis of replicate testing of the HCV WHO International Standard (NIBSC Codes: 97/690); copies per milliliter were based on a conversion factor of 3.4 RNA copies/IU.
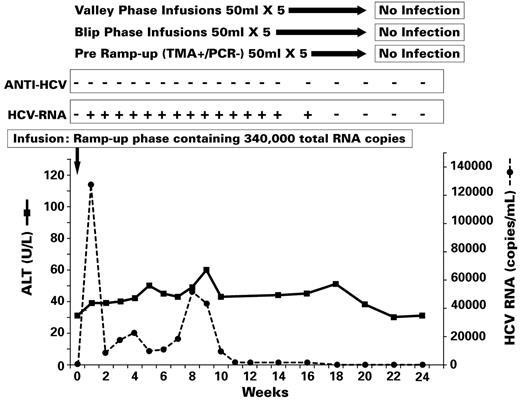
Finally, after an additional 6-month interval, phase 3 of this experiment was conducted. In light of the multiple exposures to HCV RNA from eclipse-phase infusions, the aim of this phase was to establish that chimp X331 was susceptible to HCV infection when infused with a sufficient HCV RNA dose. Three 50-mL plasma infusions from HCV RNA-positive units obtained either immediately before ramp-up or during the ramp-up phase of infection from one of the 5 donors (plasma donor 10083) used in phases 1 and 2 were infused at 8-week intervals (Figure 2B, Table 2). These 3 units had increasing HCV viral loads (1.5 copies/mL, 3.0 copies/mL, and 6.8 × 105 copies/mL). The chimp was monitored weekly for 8 weeks for HCV RNA, HCV Ab, and ALT/AST after the first 2 infusions and then weekly for 12 weeks and biweekly until week 24 after the third infusion. Liver biopsies were performed immediately before the infusion containing 6.8 × 105 copies/mL and at weeks 2, 3, 5, and 7 after that infusion. No HCV infection was detected over an 8-week follow-up period after each of the first 2 infusions (infusion of 74 HCV RNA copies [1.5 copies/mL × 50 mL] and 149 HCV RNA copies [3.0 copies/mL × 50 mL]). In contrast, after infusion of the donation with 6.8 × 105 HCV copies/mL (total infusion of 3.4 × 107 copies [6.8 × 105 copies/mL × 50 mL]), the recipient chimp developed viremia at 1-week postinfusion (peak titer 1.3 × 105 copies/mL) which persisted until week 16 (Figure 5). All liver biopsies were normal. Unexpectedly, HCV Ab never developed over the 24 weeks of follow-up.
Estimated HCV RNA concentrations of donations infused into chimp X331 to prove susceptibility to HCV infection (experiment II, phase 3)
| Donor/donation no. . | Phase of infection . | Days preceding ramp-up . | No. of TMA-positive replicates (%) . | HCV RNA copies/mL (imputed from replicate TMA testing* or measured by VL assay) . | Infused HCV RNA copies . |
|---|---|---|---|---|---|
| 10083-12 | Prior to ramp-up | 7 | 3/27 (11) | 1.48 (0.71-2.28) | 74 |
| 10083-13 | Prior to ramp-up | 5 | 9/27 (33) | 2.99 (1.83-4.05) | 149 |
| 10083-14 | Ramp-up | 0 | NA† | 6.8 × 105 | 3.4 × 107 |
| Donor/donation no. . | Phase of infection . | Days preceding ramp-up . | No. of TMA-positive replicates (%) . | HCV RNA copies/mL (imputed from replicate TMA testing* or measured by VL assay) . | Infused HCV RNA copies . |
|---|---|---|---|---|---|
| 10083-12 | Prior to ramp-up | 7 | 3/27 (11) | 1.48 (0.71-2.28) | 74 |
| 10083-13 | Prior to ramp-up | 5 | 9/27 (33) | 2.99 (1.83-4.05) | 149 |
| 10083-14 | Ramp-up | 0 | NA† | 6.8 × 105 | 3.4 × 107 |
TMA indicates transcription-mediated amplification; VL, viral load; and NA, not applicable.
RNA copy levels were estimated from probit analysis of replicate testing of the HCV WHO International Standard (NIBSC Codes: 97/690); copies per milliliter were based on a conversion factor of 3.4 RNA copies/IU.
This donation was positive by quantitative HCV RNA testing at a concentration of 6.8 × 105 copies/mL and was not subjected to replicate TMA testing.
Results of transmission experiments in chimp 331 (see Figure 1, experiment II). After demonstrating that valley phase, blip phase, and pre–ramp-up phase infusions (50 mL) from each of 5 plasma donors were not infectious in chimp X331, this animal was challenged with a ramp-up phase inoculum containing 340 000 total RNA copies in a volume of 50 mL. Chimp X331 was infected as indicated by the detection of HCV RNA from weeks 1 to 16, but then HCV RNA cleared; HCV Abs and cell-mediated immune responses to HCV were not detected and the ALT level was not elevated. By week 18, there was no residual evidence that this infection had occurred.
Results of transmission experiments in chimp 331 (see Figure 1, experiment II). After demonstrating that valley phase, blip phase, and pre–ramp-up phase infusions (50 mL) from each of 5 plasma donors were not infectious in chimp X331, this animal was challenged with a ramp-up phase inoculum containing 340 000 total RNA copies in a volume of 50 mL. Chimp X331 was infected as indicated by the detection of HCV RNA from weeks 1 to 16, but then HCV RNA cleared; HCV Abs and cell-mediated immune responses to HCV were not detected and the ALT level was not elevated. By week 18, there was no residual evidence that this infection had occurred.
Experiment III: testing whether transient HCV infection protected against rechallenge
Experiment III (Figure 1) was conducted to determine whether the acquisition of HCV infection from a low-dose inoculum, followed by viral clearance, would protect against rechallenge by the same HCV strain. In this experiment, performed 34 months after recovery from the initial experimental transmission of HCV to chimp X355, this same chimp was challenged with a 50-mL plasma infusion from the same low-viremic (1.6 copies/mL), early window–phase donation that had transmitted the initial HCV infection. The chimp was monitored weekly for 12 weeks during which time there was no evidence of a second HCV infection, implying some level of immunity even though anti-HCV and HCV-specific cell-mediated immunity were not detectable before or after the HCV challenge.
To further assess protective immunity, 4 weeks later, the chimp was rechallenged with a 50-mL plasma infusion from the subsequent ramp-up phase donation (donated 4 days later) given by this same donor, which contained 6000 HCV RNA copies/mL (for a total infusion of 300 000 HCV RNA copies). The chimp was monitored weekly for 10 weeks, then biweekly until week 24, and then monthly until week 52. Chimp X355 was reinfected with HCV as evidenced by development of low-level HCV viremia that was only present at weeks 1, 2, and 3 and only quantifiable at week 1 (1620 copies/mL). HCV Ab developed at week 5 and persisted through week 20, was intermittently detected at weeks 28 and 32 and then reverted to negative on 3 subsequent measurements. ALT remained normal throughout the 1-year follow-up period and this chimp did not mount any PBMC proliferative responses to the challenge inoculum. At week 52, the chimp expired from an unrelated pyelonephritis and Escherichia coli sepsis precluding any further follow-up.
Discussion
We studied the infectivity of human plasma collected in various stages of acute HCV infection to define the relationship between HCV RNA as detected by the most sensitive RNA assays and infectivity in the well-established chimp infection model. We demonstrated that infusions of 50 mL of plasma from each of 5 human source plasma donors thought to be in the late eclipse phase of infection (just before or at the beginning of the ramp-up phase) at the time of donation into an HCV-naive chimpanzee resulted in a mild and transient HCV infection characterized by low-level viremia, HCV Ab production, and HCV-specific T-cell immune responses. This was followed by viral clearance, rapid loss of HCV Ab, and marked diminution of the cellular immune response. Sequencing of isolates established that the infection was from a single plasma donor in the group of 5 used in the experimental infusion. Based on performing HCV TMA on 27 replicate samples from the transmitting donation, we established that this plasma had an estimated HCV RNA concentration of 1.2 copies/mL and hence the donor was probably in the early phase of HCV ramp-up viremia (rather than the eclipse phase) at the time of this donation. The definition of the transition between the eclipse and ramp-up phases is not precise and the mechanisms leading to this transition are not known, as discussed in Glynn et al.1 Based on the 50-mL plasma infusion from this donation, we estimated that 60 HCV RNA copies were infused into the recipient chimp. Samples collected from 3 to 14 days previously from the same donor were infused in 50-mL aliquots into another HCV naive chimp and did not cause infection, indicating that the transmitting donation was obtained at or near the time when the donor first became infectious. This also indicated that the duration of infectious viremia before detectable RNA by donor screening nucleic acid assay technology (NAT) assays was very brief.
This experimental transmission has some parallels with the first reported human case of transfusion-transmitted HCV infection from a unit retrospectively shown to be HCV RNA negative by a sensitive individual donation NAT (ID NAT) assay.29 In this human transmission, HCV RNA could be demonstrated inconsistently in the transmitting donation when evaluated in multiple replicates by dHCV TMA testing, leading to the conclusion that HCV RNA was present, but at a very low level (estimated at < 10 copies/mL). The donor of this unit may have been in the very early ramp-up phase with HCV RNA levels below the level of consistent detection by even the most sensitive ID NAT screening method, as occurred in our chimp transmission experiment. Data from this study in chimpanzees, other chimp experimental studies using serially diluted ramp-up phase plasma infusions,14,16 and the clinical case report of a breakthrough transmission29 demonstrate that ID NAT cannot completely eliminate the risk of transfusion transmission of HCV, given the high level infectivity of ramp-up phase virus. Of relevance to transfusion safety, it appears that blood transfusions from donors in the early stage of acute HCV infection can be infectious before the time of RNA detection by routinely performed NAT screening, including the most sensitive ID NAT test currently available which has a 50% limit of detection of 12.1 copies/mL (95% CI 11.1-13.2).26 Hence, conversion to individual donation NAT from currently used mini-pool NAT (MP NAT) screening will not close the infectious window period completely and may not be warranted given its projected very low incremental yield and poor cost-effectiveness.10,25,30
A further set of experiments was performed to evaluate the infectivity of 2 types of donations from the surprisingly prolonged eclipse phase of infection observed in 74% of the plasma donor panels we studied. These included donations that were HCV RNA positive and estimated to contain from 1 to 20 HCV RNA copies/mL (60 to 561 HCV RNA copies per infusion) and other intervening donations from the same donors that were HCV RNA negative by sensitive multireplicate testing. Infusions of 50 mL of plasma from either of these 2 donation types from each of 5 donors with such intermittent HCV RNA “blips” did not cause infection when transfused into a single chimp, who was later proven to be susceptible to HCV infection when infused with a ramp-up phase viremic sample from one of these same donors.
Thus, although HCV RNA was intermittently detected during the eclipse phase of infection for up to 8 weeks before ramp-up viremia, our results show that transfusion of such units was not infectious in chimpanzees. In contrast, we demonstrated infectivity with an early ramp-up phase sample at a total infusion dose of 60 HCV RNA copies. Japanese investigators have shown that even lower doses of HCV RNA (total inocula of 20 HCV RNA copies) from an acutely infected chimp transmitted HCV infection to recipient chimps.14 Hence, during the ramp-up phase of early HCV infection, infusions with very low copy numbers are highly infectious whereas infusions having equal or higher copy numbers of HCV RNA obtained during the eclipse phase are not infectious. This suggests critical differences in the presentation or packaging of HCV RNA during the eclipse and ramp-up phases of HCV infection as discussed below.
There are multiple possible explanations for the finding of intermittent HCV RNA detection in some donors during the eclipse phase. This may represent persistent low-level viremia that occurs at a concentration that is at or near the limit of assay detection and thus, based on assay limitations, is detectable in some samples and nondetectable in other serially collected specimens. Another explanation is that periods of HCV viremia alternate with nonviremic periods as a result of intermittent release of viral particles into the plasma from focal replication sites such as the liver and possibly even peripheral blood cells. Third, these episodes could represent repeated HCV exposures or reinfections which abort, until a particularly fit variant is finally transmitted and disseminates. Perhaps the most likely explanation is that early in HCV infection, HCV RNA can be released into plasma as nonvirion-associated nucleic acid or as defective, nonreplication competent virions. The data appear to be most consistent with the hypothesis that complete infectious particles likely do not circulate until immediately before the ramp-up phase of HCV infection, leaving a very narrow window of infectivity before detectability by sensitive blood donor screening and diagnostic assays.
Whatever the explanation, this series of experiments, and those of others,14,16 establish that HCV RNA intermittently detected during the eclipse phase of HCV infection can be ignored when performing mathematical modeling of residual transfusion-transmitted HCV risk. This lack of infectivity (or perhaps very rare infectivity, which we were not able to demonstrate in our small study) is consistent with reports of only a very few transfusion-transmissions from HCV NAT-screened units. Thus, risk modeling can use the time period from the extrapolated beginning of ramp-up phase viremia to detectable virus by the screening method used (ID NAT, MP NAT, fourth generation Ag/Ab assays), as reviewed in detail elsewhere.2
We have shown that a chimp infected with HCV from infusion of a low-dose early ramp-up inoculation developed a transient HCV infection as evidenced by low-level and short-lived viremia, and both humoral and cellular immune responses; by week 17, virologic, serologic, and biochemical evidence of HCV infection had disappeared and very low-level T-cell responses were the only residua of past HCV infection. A second chimp also inoculated with plasma from the early ramp-up phase of HCV infection developed transient viremia but failed to seroconvert. Similar examples of transient infections without seroconversion or with seroconversion followed by seroreversion have been reported in experimental chimp infectivity studies,15 and rarely in human HCV infections after transfusion and injection drug-use exposures.6,7,31,,,,–36 Our experimental findings corroborate these previously reported human observations in establishing that more HCV infections occur in persons than are clinically, virologically, or serologically identified by cross-sectional serologic or NAT screening.
Lastly, these experiments in a single chimp indicate that HCV infection from a low-dose inoculum did not confer protective immunity to rechallenge, as we demonstrated only limited protection when the chimp who developed but then lost detectable adaptive humoral and cellular immunity was rechallenged with a higher dose of the identical HCV strain.
A noteworthy limitation of our study is that we had access to only 2 HCV naive chimps to evaluate the infectivity of a large number of donations from 10 donors with different patterns of eclipse and ramp-up phase viremia. Consequently, we had to judiciously design experiments with reuse of the same chimps for multiple infusion experiments. This could have resulted in either immunization or tolerance to HCV, perturbing our ability to ascertain infectivity of donor plasma. However, we do not believe this seriously compromised interpretation of our experiments because all the chimps were carefully evaluated using sensitive assays for detection of HCV RNA and both humoral and cell-mediated immunity and each animal was negative by all these parameters before viral challenge. Furthermore, in the chimp (X331) that was uninfected after challenge with blip and valley eclipse-phase infusions, we confirmed HCV susceptibility by transfusing a high titer inoculum from the ramp-up phase of infection.
In summary, these studies demonstrate that: (1) Large volume plasma transfusions can transmit HCV infection before RNA detection by current donor screening assays, but the interval of infectivity before RNA detection appears to be very brief (4 days in this experiment). (2) The noninfectivity of eclipse-phase “blips” indicates that these may represent incomplete virions and can be ignored when calculating HCV residual transfusion risk. (3) Markers of HCV infection can be rapidly lost after exposure to low-dose inocula, suggesting that more HCV infections occur than are currently documented. (4) HCV infection from a low-dose inoculum does not necessarily confer protective immunity to rechallenge from a higher dose of the homologous strain.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stuart Armstrong and Abigail Schrock for expert assistance with figure development and preparation.
This work was supported in part by National Heart, Lung, and Blood Institute grant R01 HL-076902 and contract N01-HB-27091.
Authorship
Contribution: M.P.B., S.H.K., B.R., and H.J.A. designed the study; K.K.M. and H.J.A. conducted and monitored the animal research; D.F.H., B.L.H., E.L.D., V.R., J.C.Y., and B.R. conducted research on samples, and compiled and analyzed data; M.P.B., K.K.M., S.H.K., E.L.D., B.R., and H.J.A. analyzed data, and contributed to the final design and the development of the manuscript; and M.P.B., S.H.K., and H.J.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.L.H. is University of Sydney, Sydney, NSW, Australia. The current affiliation for V.R. is Department of Internal Medicine and Clinical Oncology, University of Bari Medical School, Bari, Italy. The current affiliation for J.C.Y. is Ewha Womans University School of Medicine, Seoul, Republic of Korea.
Correspondence: Michael P. Busch, MD, PhD, Blood Systems Research Institute, 270 Masonic Ave, San Francisco, CA 94118; e-mail: mbusch@bloodsystems.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal