TGN1412, a superagonistic CD28-specific antibody, was shown to require Fc-cross-linking or immobilization as a prerequisite to mediate T-cell proliferation and cytokine release in vitro. We used primary human umbilical vein endothelial cells (HUVECs) to study their ability to induce activation of TGN1412-treated T cells. We confirmed that peripheral primary human T cells do not show activation upon stimulation with soluble TGN1412 alone. Nevertheless, cocultivation of TGN1412-treated T cells with HUVECs induced T-cell activation that was further enhanced using cytokine prestimulated HUVECs. Unexpectedly, Fc-FcγR interaction was dispensable for endothelial cell–mediated proliferation of TGN1412-treated T cells. Transwell-culture assays showed that TGN1412-treated T cells need direct cell-to-cell contact to HUVECs to induce proliferation. We found that costimulatory ICOS-LICOS interaction between T cells and endothelial cells is critically involved in TGN1412-mediated effects. Blocking LICOS reduced TGN1412-mediated T-cell proliferation significantly, whereas recombinant LICOS fully conferred TGN1412-mediated T-cell proliferation. Of note, cytokine stimulation enhanced LICOS expression on HUVECs and ICOS-LICOS interaction up-regulated ICOS expression on TGN1412-treated T cells. Hence, we provide a model of positive feedback conferred by ICOS-LICOS interaction between TGN1412-treated T cells and endothelial cells.
Introduction
Monoclonal antibodies (mAbs) are widely used for therapeutic applications. Although, therapeutic mAbs are potent and effective agents, they can induce severe adverse events including cytokine release syndrome (CRS), a cascade of systemic cytokine release.
The first mAb approved in 1986 as a drug for humans, muromonab (orthoclone OKT3), is a murine anti–human CD3 mAb, indicated for the treatment of acute renal, steroid-resistant cardiac, or steroid-resistant hepatic allograft rejection. In particular during the first infusion, OKT3-mediated T-cell activation can induce massive cytokine release, possibly culminating in a CRS.1,2 Currently, approximately 29 mAbs are available on the market in the European Union, including those directly influencing T-cell function.
For proper T-cell activation, at least 2 signals are required: the first signal is provided by the T-cell receptor (TCR) upon recognition of antigen:MHC (major histocompatibility complex) complexes on the surface of antigen presenting cells (APCs). However, antigen alone is not sufficient to drive activation of naive T cells. To fully activate resting naive T lymphocytes, a second signal, which emerges from triggering of so called costimulatory molecules, must be provided (reviewed by Sharpe3 ). The transmembrane CD28 homodimer is the most prominent costimulatory molecule. Its ligands B7-1 (CD80) and B7-2 (CD86) are up-regulated on APCs upon triggering of cells with danger signals derived from pathogens, such as bacteria or viruses, or upon cytokine stimulation.4 As for signal one, triggering of CD28 alone is not capable of inducing T-cell activation, whereas simultaneous engagement of the TCR and CD28 leads to activation of resting T lymphocytes.
Another important costimulatory molecule is ICOS (inducible costimulator; CD278) binding to its ligand LICOS (CD275), expressed on APCs, B cells, and endothelial cells.5,6 Homodimeric ICOS, a member of the CD28 immunoglobulin super family, is up-regulated after T-cell activation, initiating a cascade of events that can shape key aspects of immune responses including T-cell differentiation, proliferation, and secretion of Th1, Th2, and Th17 associated cytokines, such as TNF-α and IFN-γ, IL-4, IL-5, IL-10, and IL-17.7,8
TGN1412 belongs to a class of superagonistic anti-CD28 antibodies, able to activate T cells without any additional TCR/CD3 triggering. During the last 2 decades, those antibodies have been used to analyze CD28-mediated signaling pathways and to assess how CD28 facilitates activation and differentiation of murine, rat, and human T lymphocytes. In 2006, TGN1412, intended for the treatment of rheumatoid arthritis and B-cell chronic lymphocytic leukemia, was applied to healthy volunteers during a phase 1 clinical trial, inducing unexpected serious adverse events. These were associated with the induction of a CRS, that is the release of high amounts of proinflammatory cytokines, most notably TNF-α and IFN-γ.9
We and others showed that T-cell proliferation and cytokine release upon TGN1412 in vitro stimulation of either T cells, whole blood, or peripheral blood mononuclear cells (PBMCs) fails upon stimulation with soluble TGN1412. Immobilized or IgG cross-linked TGN1412 indeed was able to induce cell activation indicating contribution of the antibody's Fc-part to its function.10,,–13 So far, it is not clear which cell and which Fc receptor (FcR) mediates TGN1412 cross-linking, and to which extent FcR expressing cells contribute to cytokine release or T-cell stimulation.
Interestingly, Fc ligation by FcγRs was reported to contribute to OKT3-mediated CRS. Here, the interaction of T-cell bound antibody with FcRs on monocytes and other cells critically contributed to antibody-mediated CRS.14,–16 In studies with chimpanzees using OKT3 variants unable to bind FcRs, induction of less severe side effects was observed compared with OKT3.17,18 Likewise, OKT3 variants, which show a 100-fold reduced binding to FcγRI (CD64) and FcγRII (CD32), were much less effective in inducing human T-cell proliferation and cytokine release (eg, TNF-α and IFN-γ).19
In this study, we show that primary human umbilical vein endothelial cells (HUVECs) are able to confer TGN1412-mediated T-cell activation. Unexpectedly, Fc-FcR interaction is completely dispensable, whereas ICOS-LICOS interaction between TGN1412-treated T cells and HUVECs is both necessary and sufficient to mediate TGN1412-induced T-cell proliferation. Of note, cytokine stimulation enhances LICOS expression on HUVECs and ICOS-LICOS interaction up-regulates ICOS expression on TGN1412-treated T cells suggesting a model of positive feedback conferred by ICOS-LICOS interaction.
Methods
T-cell and B-cell purification
PBMCs were isolated from buffy coats from healthy donors (Blutspendedienst, Frankfurt am Main, Germany) by Ficoll (Biochrom) density gradient centrifugation. Human T cells or B cells were further purified by non–T-cell or non–B-cell depletion using the Pan T-cell isolation kit II or B-cell isolation kit II (Miltenyi Biotec). Purity of T cells usually exceeded 97%. All cells were cultured at 37°C with 5% CO2 in 96-well (2 × 105 cells in 200 μL medium) or 24-well (1 × 106 cells in 1 mL medium) flat-bottom tissue culture plates (Sarstedt) using X-VIVO 15 medium (Lonza).
Isolation and stimulation of HUVECs
HUVECs were isolated from human umbilical cords obtained from healthy donors (Uniklinikum, Mannheim, Germany) by incubation of the vein with 0.1% collagenase D (Roche) for 35 minutes. HUVECs were grown in endothelial cell growth medium (PromoCell) supplemented with 50 μg/mL Gentamycin (Gibco) and 5 ng/mL amphotericin B (Sigma-Aldrich) at 37°C with 5% CO2 in 75-cm2 or 175-cm2 tissue culture flasks (Greiner). Culture medium was changed twice per week. HUVECs were harvested at a confluence of approximately 80% by short exposure of monolayers to 0.25% trypsin plus 1% ethylenediaminetetraacetic acid (EDTA). HUVECs in passage 1 to 6 of 2 to 3 donors were pooled, irradiated, and used for experiments. HUVECs were characterized by morphology and positive staining for von Willebrand factor (clone 9F2-A9) and CD31 (clone WM59; both from BD Pharmingen; data not shown).
For in vitro stimulation, HUVECs were seeded at 8 × 104/24-well in 1 mL endothelial cell growth medium (PromoCell). Cytokines were added to the medium with final concentrations of 200 U/mL TNF-α and 100 U/mL IFN-γ (both from Peprotech) for 3 days.
Stimulating antibodies
Usage of the humanized superagonistic anti-CD28 antibody TGN1412 was kindly permitted by TheraMAB GmbH. Murine anti-CD3 antibody Orthoclone OKT3 was purchased from Janssen Cilag.
In vitro proliferation assays
Flat-bottom tissue culture plates, 96-well or 24-well, (Sarstedt) were coated with 5 μg/mL TGN1412, recombinant LICOS protein or recombinant CD40 protein (both from R&D Systems) in 100 μL or 200 μL BisTris (Sigma-Aldrich) per well at 4°C for 24 hours. Plates were washed twice with 200 μL BisTris to remove unbound antibody or protein before addition of T cells in 200 μL or 1 mL X-VIVO 15 medium (Lonza). OKT3 (Janssen-Cilag) was coated in phosphate-buffered saline (PBS; Biochrom). T cells cultured on BisTris or PBS-treated wells without antibody or protein were used as negative control. Alternatively, T cells were incubated with 1 μg/mL soluble TGN1412 with or without cross-linking anti-IgG mAb (2 μg/mL; BD Pharmingen). For cross-linking experiments, both antibodies were individually added to each well at the same time. As a control, T cells were stimulated with 500 ng/mL ionomycin plus 10 ng/mL 12-O-tetradecanoylphorbol-13-acetate (TPA; both from Sigma-Aldrich). For coculture experiments with HUVECs, 1 × 106 T cells per 24-well were added to adherent HUVECs (8 × 104), which were washed twice with 1 mL PBS (Biochrom) before addition of T cells. Subsequently, cocultures were treated with 1 μg/mL TGN1412. For cocultivation of HUVECs and T cells, 0.5 mL endothelial cell growth medium (PromoCell) and 0.5 mL X-VIVO 15 medium (Lonza) per 24-well was used. For coculture experiments with B cells, 2 × 105 T cells in 100 μL X-VIVO 15 medium (Lonza) per 96-well were added to irradiated B cells (2 × 105 in 100 μL X-VIVO 15 medium [Lonza]). Subsequently, cocultures were treated with 1 μg/mL TGN1412.
T-cell proliferative responses were measured by a flow cytometry-based assay using PKH26 red fluorescent cell linker kit (Sigma-Aldrich) according to the manufacturer's instructions.
Flow cytometric analysis
For flow cytometric analysis, T cells or HUVECs were stained for 20 minutes at 4°C using the following monoclonal antibodies diluted to the optimal concentration: anti–CD3-APC (clone UCHT1), anti–CD25-PE (clone M-A251), anti–CD31-FITC (clone WM59), anti–CD40-PE-Cy5 (clone 5C3), anti–CD69-FITC (clone FN50), anti–FcγRI-FITC (clone 10.1), anti–FcγRII-FITC (clone FLI8.26), and anti–FcγRIII-PE (clone 2G8; all from BD Pharmingen); anti–CD40L-PE-Cy5 (clone 24-31), anti–CD86-PE (clone IT2.2), anti–ICOS-PE (clone C398.4A), anti–LICOS-PE (clone 9F.8A4), and anti–MHC II-APC-Cy7 (clone L243; all from BioLegend); anti–CD80-APC (clone MEM-233; EuroBioScience); and anti–MHC I-PE-Cy5 (clone W6/32; eBioscience). For intracellular staining of Ki-67, surface markers were stained first, followed by permeabilization using Cytofix/Cytoperm (BD Pharmingen) and staining with anti–Ki-67–PE (clone Ki-67; BioLegend). Cells were analyzed using LSR II with BD FACS DIVA Version 6.1.3 (BD Biosciences) and FlowJo Version 7.6.4 software.
Blocking experiments
For blocking experiments, HUVECs were incubated for 1 hour in 200 μL medium/24-well at 37°C with 5% CO2 with the following antibodies: polyglobin (Bayer); anti–IL-2 (clone 5334), anti-CD40 (clone 82 102), anti-CD80 (clone 37 711), anti-CD86 (clone 37 301), and anti-LICOS (clone 136726; all from R&D Systems); anti–MHC I (clone W6/32), and anti–MHC II (clone L243; all from BioLegend); anti-FcγRI (clone 10.1), anti-FcγRII (clone AT10), and anti-FcγRIII (clone LNK16; all from AbD Serotec). Subsequently, T cells were added and TGN1412-stimulated where indicated. ICOS on T cells was blocked using an anti-ICOS antibody (clone ANC6C6; Ancell) for 1 hour before adding blocked T cells to coated recombinant LICOS protein.
Transwell assays
For transwell assays, transwell permeable supports (Corning) with 0.4-μm pore size polycarbonate membrane or polyester membrane were used. After an initial equilibrium period of the transwell permeable supports, irradiated HUVECs were cultured for 3 days in 24-well, or 12-mm diameter transwell inserts. On day 3, HUVECs were washed with PBS (Biochrom) and T cells were added to transwell inserts or 24-wells, respectively.
Analysis of cytokine secretion
After stimulation as described, cell-free supernatant was collected and analyzed by FlowCytomix Th1/Th2 11plex kit (Bender MedSystems) according to the manufacturer's recommendations.
Preparation of deglycosylated TGN1412
To yield deglycosylated TGN1412 with an otherwise intact core protein structure, the sample was enzymatically deglycosylated under nondenaturing conditions. Residual glycosylated forms were eliminated by affinity chromatography using immobilized wheat germ lectin (WGA-Agarose; Calbiochem). One-hundred microlitres of TGN1412, corresponding to 1 mg protein, were incubated with 20 μL N-glycosidase F (Roche, 1000 U/mL) for 16 hours at 37°C. One-hundred microlitres thereof was mixed with PBS (150mM NaCl; 10mM sodium phosphate pH 7.5) and loaded onto a WGA-agarose column equilibrated to the same buffer. Unbound protein was eluted with PBS and the protein-containing fraction was collected.
A negative control was prepared by incubating TGN1412 without N-glycosidase F followed by mock affinity chromatography on a nonlectin-containing agarose, which was blocked with ethanolamine (included in wheat germ agglutinin [WGA]-agarose kit from Calbiochem). This control preparation is termed TGN1412* in the following section.
Protein concentration of both glycosylated and deglycosylated antibody preparations was determined using high performance liquid chromatography (HPLC) size-exclusion chromatography. The success of deglycosylation was verified by capillary sodium dodecyl sulfate (SDS) electrophoresis using the Beckman IgG heterogeneity test kit.
Results
Endothelial cells confer TGN1412-mediated T-cell activation
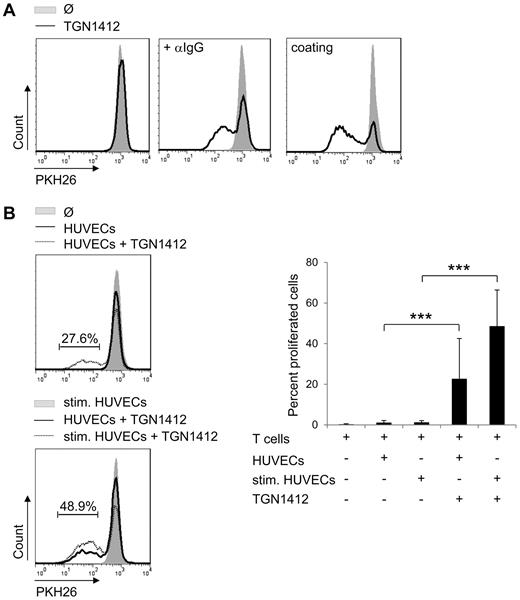
T-cell proliferation can be mediated by cross-linking TGN1412 via a secondary anti-IgG antibody or by immobilization via coating the antibody onto tissue culture plates (Figure 1A). Here, cross-linking of TGN1412 via an anti-IgG antibody was performed in aqueous solution. This experimental setup was shown to confer TGN1412-mediated T-cell activation in previous studies.12 TGN1412 was added at 0.1 μg/mL, 1 μg/mL or 10 μg/mL. In all experimental settings tested, 1 μg/mL induced the strongest T-cell proliferation (data not shown). Therefore, all following experiments were performed at 1 μg/mL. This also resembles the concentration of TGN1412 that theoretically was present in the blood of volunteers treated during the phase 1 clinical trial of the antibody.13
Endothelial cells confer TGN1412-mediated T-cell proliferation. (A) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were stimulated with 1 μg/mL TGN1412 (solid line left panel) followed by cross-linking with 2 μg/mL anti-IgG mAb (solid line middle panel). Five μg/mL TGN1412 was coated on 96-wells and PKH26-labeled T cells were added (solid line right panel). Controls were left untreated (no TGN1412; gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Data shown are representative for experiments using T cells of at least 20 independent donors. (B) HUVECs (8 × 104) were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated and PKH26-labeled human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. As controls, T cells were left untreated or were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations from data obtained with T cells of 30 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells. The statistical analysis was performed with SAS/STAT Version 9.3 software (SAS System for Windows; ***P < .001).
Endothelial cells confer TGN1412-mediated T-cell proliferation. (A) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were stimulated with 1 μg/mL TGN1412 (solid line left panel) followed by cross-linking with 2 μg/mL anti-IgG mAb (solid line middle panel). Five μg/mL TGN1412 was coated on 96-wells and PKH26-labeled T cells were added (solid line right panel). Controls were left untreated (no TGN1412; gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Data shown are representative for experiments using T cells of at least 20 independent donors. (B) HUVECs (8 × 104) were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated and PKH26-labeled human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. As controls, T cells were left untreated or were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations from data obtained with T cells of 30 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells. The statistical analysis was performed with SAS/STAT Version 9.3 software (SAS System for Windows; ***P < .001).
On intravenous application of any antibody, peripheral blood cells and endothelial cells are the first cell types getting into contact with the antibody. In our study, we used primary HUVECs and analyzed their ability to induce activation of TGN1412-treated T cells. We confirmed that primary human T cells did not show proliferation upon stimulation with soluble TGN1412 alone (Figure 1A). Nevertheless, cocultivation of TGN1412-treated T cells with HUVECs induced T-cell proliferation that was augmented using cytokine prestimulated HUVECs (Figure 1B). For prestimulation TNF-α and IFN-γ were used, 2 cytokines markedly induced upon TGN1412 treatment in vivo.9 In absence of TGN1412, HUVECs alone, either untreated or cytokine prestimulated, were not able to confer T-cell proliferation (Figure 1B) making any allo-reaction related effects or effects of potential residual cytokines unlikely. Thus, primary human endothelial cells confer TGN1412-mediated T-cell proliferation.
T-cell proliferation was measured on day 1, day 3, and day 5 of stimulation. Upon all experimental settings tested, the strongest proliferative responses were observed between day 3 and day 5. Stimulation for an additional 2 days did not increase T-cell proliferation (data not shown).
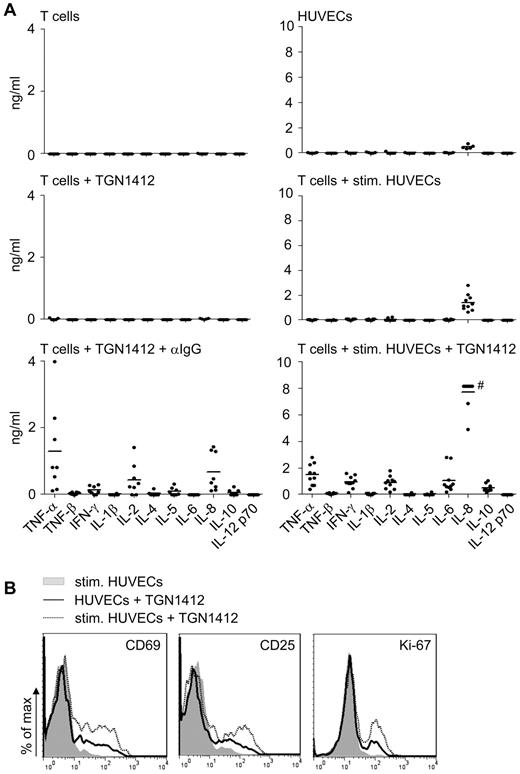
Already after 24 hours short-term cocultivation of TGN1412-treated T cells and TNF-α plus IFN-γ prestimulated endothelial cells, TGN1412-mediated cytokine release was detectable (Figure 2A). Cytokine patterns induced upon cross-linking TGN1412 using an anti-IgG antibody differed from that obtained when primary human endothelial cells were used to mediate activation of TGN1412-treated T cells (Figure 2A). In particular, we found higher levels of IFN-γ, IL-6, and IL-8 in cocultures at that early time point. At day 5 of stimulation, cross-linking TGN1412 via an anti-IgG antibody induced an enhanced and broader cytokine pattern, although some cytokines present in the coculture system were no longer detectable (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Adding an anti–IL-2 blocking antibody diminished endothelial cell–mediated proliferation of TGN1412-treated T cells by more than 80% (supplemental Figure 2) indicating that the contribution of this induced cytokine to T-cell proliferation.
Endothelial cells confer TGN1412-mediated cytokine release and early T-cell activation. (A) Freshly isolated human T cells (1 × 106) per 24-well were left unstimulated, stimulated with 1 μg/mL TGN1412, or 1 μg/mL TGN1412 followed by cross-linking with 2 μg/mL anti-IgG monoclonal antibody (left panel). HUVECs (8 × 104) per 24-well were irradiated and cultured unstimulated for 24 hours. Alternatively, HUVECs were stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated human T cells were added to HUVECs and left unstimulated or stimulated with 1 μg/mL TGN1412 (right panel). Twenty-four hours after the indicated stimulations, cell-free supernatant was collected and analyzed for TNF-α, TNF-β, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12 p70 by FlowCytomix Th1/Th2 11plex analysis. Data shown are from 5 to 10 independent T-cell donors. #, data points are in the saturation area of the assay. (B) HUVECs (8 × 104) were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves). Twenty-four or 48 hours after cocultivation, T cells were harvested and stained with an anti-CD69, anti-CD25 (both after 24 hours) or anti–Ki-67 antibody (after 48 hours). Data shown are representative for 4 or 2 different T-cell donors, respectively.
Endothelial cells confer TGN1412-mediated cytokine release and early T-cell activation. (A) Freshly isolated human T cells (1 × 106) per 24-well were left unstimulated, stimulated with 1 μg/mL TGN1412, or 1 μg/mL TGN1412 followed by cross-linking with 2 μg/mL anti-IgG monoclonal antibody (left panel). HUVECs (8 × 104) per 24-well were irradiated and cultured unstimulated for 24 hours. Alternatively, HUVECs were stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated human T cells were added to HUVECs and left unstimulated or stimulated with 1 μg/mL TGN1412 (right panel). Twenty-four hours after the indicated stimulations, cell-free supernatant was collected and analyzed for TNF-α, TNF-β, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12 p70 by FlowCytomix Th1/Th2 11plex analysis. Data shown are from 5 to 10 independent T-cell donors. #, data points are in the saturation area of the assay. (B) HUVECs (8 × 104) were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves). Twenty-four or 48 hours after cocultivation, T cells were harvested and stained with an anti-CD69, anti-CD25 (both after 24 hours) or anti–Ki-67 antibody (after 48 hours). Data shown are representative for 4 or 2 different T-cell donors, respectively.
In line with early cytokine secretion, early T-cell activation markers CD69 and CD25 were up-regulated on a proportion of TGN1412-treated T cells already 24 hours after cocultivation with endothelial cells and mitogenic activity of TGN1412 was detectable already upon 48 hours cocultivation (Figure 2B).
Fc-FcR interaction is dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation
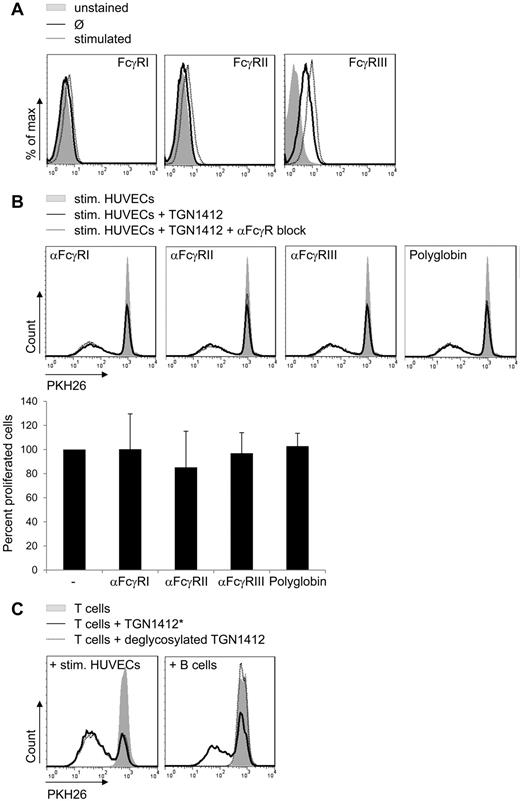
Analysis of HUVECs from 8 different donors revealed no FcγRI or II and only little FcγRIII surface expression, and FcγR expression levels were only slightly enhanced upon cytokine treatment of HUVECs (Figure 3A). Consequently, blocking Fc-receptors on HUVECs did not reduce proliferation of cocultured TGN1412-treated T cells (Figure 3B). For blocking we used either antibodies specific for each single FcγR, FcγRI, FcγRII, or FcγRIII, or we used polyglobin, human polyclonal immunoglobulins, for blocking all FcRs on the cell surface. Notably, polyglobin completely blocked B-cell conferred TGN1412-mediated T-cell proliferation (data not shown) that is Fc-FcR dependent as reported by L.Y.S. et al (L.Y.S., Z.W., P. Bartholomaeus, E. Corrales-Aguilar, E. Wiechec, B. Schraven, H. Hengel, U.K., unpublished data).
Fc-FcR interaction is dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation. (A) Untreated HUVECs (2 × 105; solid lines) or HUVECs treated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days (dotted lines) were stained with fluorochrome-conjugated anti-FcγRI, II or III antibody and analyzed by flow cytometry. Controls were left unstained (gray-shaded curves). Data shown are representative for experiments using HUVECs of 8 independent donors. (B) HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing with PBS, HUVECs' FcγRs were blocked specifically with anti-FcγRI, anti-FcγRII, anti-FcγRIII antibodies, or with Polyglobin for 1 hour. Freshly isolated and PKH26-labeled human T cells (1 × 106) were added to HUVECs and stimulated with 1 μg/mL TGN1412 (dotted lines). As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves) or with TGN1412 but without blocking antibodies (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations from at least 7 independent T-cell donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples without blocking antibody). (C) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were cocultivated with 2 × 105 freshly isolated and irradiated human B cells or 1 × 106 freshly isolated and PKH26-labeled human T cells per 24-well were cocultivated with prestimulated HUVECs as described in Figure 1B. Subsequently, T cells were stimulated with 1 μg/mL control TGN1412* (see “Methods”; solid line) or 1 μg/mL deglycosylated TGN1412 (dotted lines). Controls were left untreated (no TGN1412; gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometry. Data shown are representative for at least 6 independent T-cell donors.
Fc-FcR interaction is dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation. (A) Untreated HUVECs (2 × 105; solid lines) or HUVECs treated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days (dotted lines) were stained with fluorochrome-conjugated anti-FcγRI, II or III antibody and analyzed by flow cytometry. Controls were left unstained (gray-shaded curves). Data shown are representative for experiments using HUVECs of 8 independent donors. (B) HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing with PBS, HUVECs' FcγRs were blocked specifically with anti-FcγRI, anti-FcγRII, anti-FcγRIII antibodies, or with Polyglobin for 1 hour. Freshly isolated and PKH26-labeled human T cells (1 × 106) were added to HUVECs and stimulated with 1 μg/mL TGN1412 (dotted lines). As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves) or with TGN1412 but without blocking antibodies (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations from at least 7 independent T-cell donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples without blocking antibody). (C) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were cocultivated with 2 × 105 freshly isolated and irradiated human B cells or 1 × 106 freshly isolated and PKH26-labeled human T cells per 24-well were cocultivated with prestimulated HUVECs as described in Figure 1B. Subsequently, T cells were stimulated with 1 μg/mL control TGN1412* (see “Methods”; solid line) or 1 μg/mL deglycosylated TGN1412 (dotted lines). Controls were left untreated (no TGN1412; gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometry. Data shown are representative for at least 6 independent T-cell donors.
It is well established that FcγRs are not able to ligate deglycosylated IgG (reviewed by Arnold et al,20 Nimmerjahn and Ravetch,21 and Woof and Burton22 ). Cocultivation of stimulated HUVECs with T cells treated with deglycosylated TGN1412 did not reduce T-cell proliferation compared with unmodified (compare Figure 1B) or mock-modified TGN1412 (TGN1412*), respectively, confirming that Fc-FcγR interaction was dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation. In contrast, cocultivation of B cells with T cells treated with deglycosylated TGN1412 completely abolished T-cell proliferation (Figure 3C). These results indicate that Fc-FcR interactions are not involved in endothelial cell–mediated proliferation of TGN1412-treated T cells.
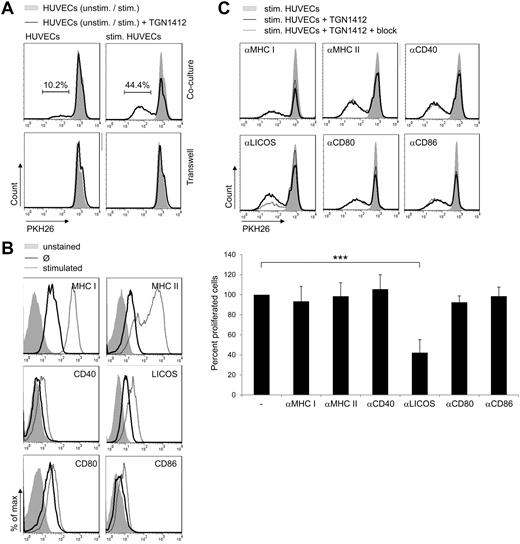
To get insight into the kind of interaction between T cells and endothelial cells needed to mediate antibody-induced T-cell proliferation, transwell assays were performed. We used both polycarbonate or polyester membrane transwell inserts and performed assays with either T cells in the insert and endothelial cells in the culture well or vice versa. All settings revealed similar results (data not shown). Membrane pore sizes were chosen to allow cytokines and antibodies to pass the membrane. T-cell proliferation was completely inhibited when TGN1412-treated T cells and endothelial cells were cultured in different compartments (Figure 4A). Of note, in cocultures of cells from the same donors, HUVECs were able to confer TGN1412-mediated T-cell proliferation as seen previously (Figure 4A; compare with Figure 1B). Hence, direct cell-to-cell contact between TGN1412-treated T cells and endothelial cells is needed to confer antibody-mediated T-cell proliferation.
ICOS-LICOS interaction facilitates TGN1412-induced T-cell proliferation. (A) HUVECs were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days in 24-wells or transwell inserts. After washing HUVECs with PBS, 1.9 × 106 freshly isolated and PKH26-labeled human T cells per 24-well or 1 × 106 per transwell insert were added and stimulated with 1 μg/mL TGN1412 (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. As controls, T cells were cocultivated with unstimulated or cytokine prestimulated HUVECs without the addition of TGN1412 (gray-shaded curves). The frequency of T-cell proliferation is shown as percentage of total T cells. Data shown are representative for at least 4 independent T-cell donors. (B) Unstimulated (solid lines) or with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days stimulated HUVECs (2 × 105; dotted lines) were stained with fluorochrome-conjugated anti–MHC I, anti–MHC II, anti-CD40, anti-LICOS, anti-CD80 or anti-CD86 antibodies and analyzed by flow cytometry. Controls were left unstained (gray-shaded curves). Data shown are representative for at least 9 independent HUVEC donors. (C) HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing with PBS, HUVECs MHC I, MHC II, or costimulatory molecules were blocked specifically with anti–MHC I, anti–MHC II, anti-CD40, anti-LICOS, anti-CD80, or anti-CD86 antibodies for 1 hour. Freshly isolated and PKH26-labeled human T cells (1 × 106) were added to HUVECs and stimulated with 1 μg/mL TGN1412 (dotted lines). As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves) or with TGN1412 but without blocking antibodies (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations of data retrieved from experiments performed with T cells derived of at least 8 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples without blocking antibody). The statistical analysis was performed with SAS/STAT Version 9.3 software (SAS System for Windows; ***P < .001).
ICOS-LICOS interaction facilitates TGN1412-induced T-cell proliferation. (A) HUVECs were irradiated and cultured either unstimulated or stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days in 24-wells or transwell inserts. After washing HUVECs with PBS, 1.9 × 106 freshly isolated and PKH26-labeled human T cells per 24-well or 1 × 106 per transwell insert were added and stimulated with 1 μg/mL TGN1412 (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. As controls, T cells were cocultivated with unstimulated or cytokine prestimulated HUVECs without the addition of TGN1412 (gray-shaded curves). The frequency of T-cell proliferation is shown as percentage of total T cells. Data shown are representative for at least 4 independent T-cell donors. (B) Unstimulated (solid lines) or with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days stimulated HUVECs (2 × 105; dotted lines) were stained with fluorochrome-conjugated anti–MHC I, anti–MHC II, anti-CD40, anti-LICOS, anti-CD80 or anti-CD86 antibodies and analyzed by flow cytometry. Controls were left unstained (gray-shaded curves). Data shown are representative for at least 9 independent HUVEC donors. (C) HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing with PBS, HUVECs MHC I, MHC II, or costimulatory molecules were blocked specifically with anti–MHC I, anti–MHC II, anti-CD40, anti-LICOS, anti-CD80, or anti-CD86 antibodies for 1 hour. Freshly isolated and PKH26-labeled human T cells (1 × 106) were added to HUVECs and stimulated with 1 μg/mL TGN1412 (dotted lines). As controls, T cells were cocultivated with cytokine prestimulated HUVECs without TGN1412 (gray-shaded curves) or with TGN1412 but without blocking antibodies (solid lines). At day 5 of cocultivation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations of data retrieved from experiments performed with T cells derived of at least 8 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples without blocking antibody). The statistical analysis was performed with SAS/STAT Version 9.3 software (SAS System for Windows; ***P < .001).
ICOS-LICOS interaction is necessary and sufficient to mediate TGN1412-induced T-cell proliferation
Both MHC:TCR-complex interaction and costimulation are crucial signals and prerequisite to initiate proper T-cell activation.23,24 As shown in Figure 4B, both MHC I and II are expressed on HUVECs and up-regulated upon cytokine stimulation of the cells. Moreover, costimulatory molecules LICOS, CD80, and CD86 are expressed on these cells and are up-regulated upon cytokine treatment to some minor extent. Costimulatory molecule CD40 is expressed on the surface of cytokine-treated HUVECs, whereas it is not expressed by untreated HUVECs (Figure 4B). To investigate the role of MHC:TCR-complex interaction and costimulation for endothelial cell–mediated proliferation of TGN1412-treated T cells, all of the aforementioned molecules were blocked using specific blocking antibodies. As shown in Figure 4C, blocking of MHC I and II, CD40, CD80, and CD86 did not affect proliferation of TGN1412-treated T cells mediated by endothelial cells either cytokine prestimulated or unstimulated (Figure 4C and data not shown). However, blocking LICOS on cytokine prestimulated endothelial cells significantly reduced TGN1412-mediated T-cell proliferation by more than 50% (Figure 4C). As expected, when using unstimulated endothelial cells, inducing less TGN1412-mediated T-cell proliferation compared with cytokine prestimulated HUVECs (Figure 1B), blocking LICOS was even more effective (data not shown). Importantly, this inhibition of T-cell proliferation was not related to any toxicity of the blocking antibody as ionomycin/TPA- or OKT3-mediated T-cell proliferation was not affected by addition of the anti-LICOS antibody (data not shown). As both anti-LICOS and anti-CD40 blocking antibody share the same subclass (IgG2b), anti-CD40 antibody can also serve as an isotype control for the anti-LICOS blocking antibody. Thus, interaction between LICOS on HUVECs and ICOS on T cells is necessary to mediate proliferation of TGN1412-treated T cells. Of note, upon TGN1412 treatment, some donor T cells already responded when cocultivated with HUVECs not pretreated with cytokines by T-cell proliferation of approximately 38%. Interestingly, these “high responders” were less responsive to anti-LICOS blocking (data not shown).
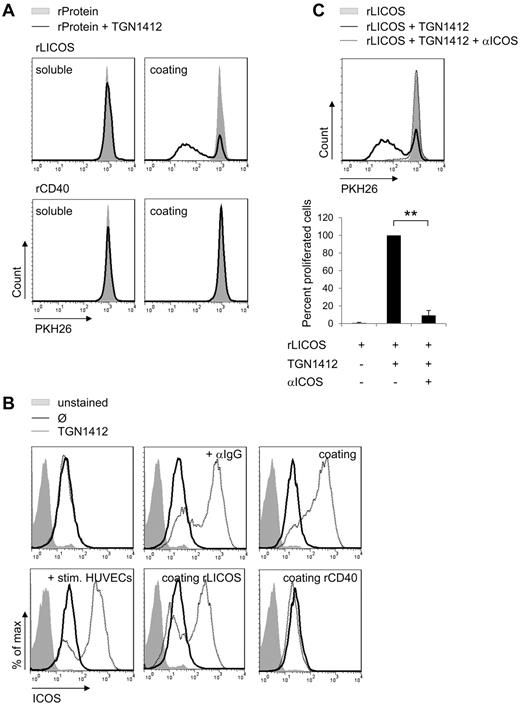
To test whether ICOS-LICOS interaction was not only necessary but sufficient to confer TGN1412-mediated T-cell proliferation, we used recombinant LICOS protein (rLICOS) to stimulate TGN1412-treated T cells. When rLICOS was coated onto cell culture plates, TGN1412-treated T cells were able to proliferate without the addition of any cross-linking reagent or any additional cell type (Figure 5A). This rLICOS-mediated proliferation of TGN1412-treated T cells was completely abolished by adding anti-LICOS blocking antibodies (data not shown). Interestingly, when rLICOS was added soluble into culture medium of TGN1412-treated T cells, no T-cell proliferation was observed (Figure 5A). In line with anti-CD40 blocking experiments, which did not inhibit HUVECs-mediated proliferation of TGN1412-treated T cells (compare Figure 4C), recombinant CD40 protein (rCD40) did not induce proliferation of TGN1412-treated T cells (Figure 5A). Thus, ICOS-LICOS interaction is both necessary and sufficient to mediate TGN1412-induced T-cell proliferation in our experimental setting.
ICOS-LICOS interaction initiates ICOS up-regulation of TGN1412-treated T cells. (A) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were stimulated with 3 μg/mL soluble recombinant LICOS protein (rLICOS) or 4 μg/mL soluble recombinant CD40 protein (rCD40; left panel). Alternatively, 5 μg/mL rLICOS or rCD40 were coated on 96-wells and 2 × 105 PKH-labeled T cells were added (right panel). Subsequently, PKH26-labeled T cells were stimulated with 1 μg/mL TGN1412 (solid lines). Controls were left unstimulated (gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Data shown are representative for at least 3 independent T-cell donors. (B) Freshly isolated human T cells (2 × 105) per 96-well were stimulated with 1 μg/mL TGN1412 followed by cross-linking with 2 μg/mL anti-IgG monoclonal antibody or 5 μg/mL TGN1412 were coated on 96-wells and T cells were added subsequently (top panel dotted lines). HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated and human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. 5 μg/mL rLICOS or rCD40 were coated on 96-wells. Subsequently, T cells were added and stimulated with 1 μg/mL TGN1412 (bottom panel dotted lines). Controls were left unstained (gray-shaded curves) or were stained but not TGN1412-stimulated (solid lines). At day 5 of stimulation, T cells were harvested, stained with an anti-CD3 and anti-ICOS antibody, and ICOS expression on CD3+ T cells was measured by flow cytometric analysis. Data shown are representative for at least 3 independent T-cell donors. (C) Freshly isolated and PKH26-labeled human T cells (1 × 106) per 24-well were treated with 10 μg/mL anti-ICOS blocking antibody for 1 hour. Subsequently, these T cells were added to 24-wells coated with 5 μg/mL rLICOS protein and stimulated with 1 μg/mL TGN1412. Controls were left unstimulated (gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations of data retrieved from experiments performed with T cells derived of 3 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples stimulated with TGN1412 but without blocking antibody). The statistical analysis was performed with SAS/STAT software (**P < .01).
ICOS-LICOS interaction initiates ICOS up-regulation of TGN1412-treated T cells. (A) Freshly isolated and PKH26-labeled human T cells (2 × 105) per 96-well were stimulated with 3 μg/mL soluble recombinant LICOS protein (rLICOS) or 4 μg/mL soluble recombinant CD40 protein (rCD40; left panel). Alternatively, 5 μg/mL rLICOS or rCD40 were coated on 96-wells and 2 × 105 PKH-labeled T cells were added (right panel). Subsequently, PKH26-labeled T cells were stimulated with 1 μg/mL TGN1412 (solid lines). Controls were left unstimulated (gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Data shown are representative for at least 3 independent T-cell donors. (B) Freshly isolated human T cells (2 × 105) per 96-well were stimulated with 1 μg/mL TGN1412 followed by cross-linking with 2 μg/mL anti-IgG monoclonal antibody or 5 μg/mL TGN1412 were coated on 96-wells and T cells were added subsequently (top panel dotted lines). HUVECs (8 × 104) were irradiated and stimulated with 200 U/mL TNF-α + 100 U/mL IFN-γ for 3 days. After washing HUVECs with PBS, 1 × 106 freshly isolated and human T cells were added to HUVECs and stimulated with 1 μg/mL TGN1412. 5 μg/mL rLICOS or rCD40 were coated on 96-wells. Subsequently, T cells were added and stimulated with 1 μg/mL TGN1412 (bottom panel dotted lines). Controls were left unstained (gray-shaded curves) or were stained but not TGN1412-stimulated (solid lines). At day 5 of stimulation, T cells were harvested, stained with an anti-CD3 and anti-ICOS antibody, and ICOS expression on CD3+ T cells was measured by flow cytometric analysis. Data shown are representative for at least 3 independent T-cell donors. (C) Freshly isolated and PKH26-labeled human T cells (1 × 106) per 24-well were treated with 10 μg/mL anti-ICOS blocking antibody for 1 hour. Subsequently, these T cells were added to 24-wells coated with 5 μg/mL rLICOS protein and stimulated with 1 μg/mL TGN1412. Controls were left unstimulated (gray-shaded curves). At day 5 of stimulation, PKH26-labeled T cells were harvested, stained with an anti-CD3 antibody, and T-cell proliferative responses were measured by flow cytometric analysis. Error bars indicate standard deviations of data retrieved from experiments performed with T cells derived of 3 independent donors. The frequency of T-cell proliferation is shown as percentage of total T cells, normalized to the control (samples stimulated with TGN1412 but without blocking antibody). The statistical analysis was performed with SAS/STAT software (**P < .01).
ICOS-LICOS interaction induces ICOS up-regulation on TGN1412-treated T cells
We showed that cytokine stimulation up-regulated LICOS expression on human endothelial cells and that blocking LICOS on these cells significantly reduced proliferation of TGN1412-treated T cells in coculture (Figure 4B-C, respectively). Next, we addressed the question whether this cell to cell interaction also affected expression of LICOS interaction partner ICOS on TGN1412-treated T cells. In contrast to stimulation of T cells with TGN1412 alone, ICOS expression on T cells was strongly up-regulated upon TGN1412 cross-linking using an anti-IgG antibody and upon addition of T cells to wells coated with TGN1412. Importantly, also cocultivation of cytokine prestimulated HUVECs with TGN1412-treated T cells up-regulated ICOS expression on T cells (Figure 5B). Moreover, experiments using rLICOS coated onto cell culture plates revealed that ICOS-LICOS interaction was sufficient to up-regulate ICOS on TGN1412-treated T cells (Figure 5B). This rLICOS-mediated up-regulation of ICOS on TGN1412-treated T cells was completely abolished by adding anti-LICOS blocking antibodies (data not shown). As a control, rCD40 was coated which did not induce proliferation of TGN1412-treated T cells (Figure 5A). Likewise, CD40 ligand (CD40L) expression on T cells was not altered under any experimental conditions tested (data not shown). Thus, interaction of LICOS with ICOS on TGN1412-treated T cells up-regulates ICOS expression on T cells possibly establishing a positive feedback mechanism of expression. Importantly, TGN1412-mediated T-cell proliferation as conferred by rLICOS protein could also be abolished when an anti-ICOS blocking antibody was used (Figure 5C).
Collectively, our data indicate that human primary endothelial cells confer TGN1412-mediated T-cell activation via ICOS-LICOS interaction. In our experimental setting, ICOS-LICOS interaction is both necessary and sufficient to mediate TGN1412-induced T-cell proliferation, whereas Fc-FcR interaction is dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation. Our data can be summarized in the following model: ICOS-LICOS interaction between T cells and endothelial cells facilitates TGN1412-mediated T-cell activation. This interaction leads to ICOS up-regulation on T cells. Moreover, cytokine stimulation induces LICOS up-regulation on the surface of endothelial cells, which collectively enhances T cell–endothelial cell interaction and TGN1412-mediated T-cell activation.
Discussion
TGN1412 is a superagonistic anti-CD28 antibody which in a phase 1 clinical trial induced severe CRS in all treated volunteers. Fc-cross-linking or immobilization of TGN1412, respectively, was shown to be a prerequisite to mediate T-cell proliferation and cytokine release in vitro.10,,–13
We used primary HUVECs to study their ability to induce activation of TGN1412-treated T cells. Using an endothelial-like cell line, Stebbings et al showed that endothelial cells indeed can confer TGN1412-mediated T-cell activation.11 Here, we confirmed that peripheral primary human T cells do not show proliferation upon stimulation with soluble TGN1412 alone (Figure 1A). Nevertheless, cocultivation of TGN1412-treated T cells with HUVECs induced T-cell proliferation (Figure 1B). Moreover, we detected a broad spectrum of cytokines after short-term stimulation of T cell cocultures with endothelial cells (prestimulated with TNF-α and IFN-γ) including higher levels of IFN-γ and IL-8, and with IL-6 secretion compared with cross-linking TGN1412 via an anti-IgG antibody (Figure 2A). After 5 day-incubation, we observed an enlarged spectrum of cytokine expression also when TGN1412 was cross-linked, whereas expression of some cytokines in cocultures was reduced, indicating that different mechanisms of cell activation might be involved in both experimental settings. In line with early cytokine secretion upon endothelial cell/T-cell cocultivation, we also found expression of early T-cell activation markers CD69 and CD25 and detection of Ki-67, 24 or 48 hours after stimulation, respectively (Figure 2B).
It will be a matter of future investigations, whether TGN1412-mediated T-cell activation conferred by endothelial cells affects the same subsets (effector memory T cells) as reported by Eastwood and Römer.25,26
Importantly, Fc-FcR interaction was dispensable for endothelial cell-conferred TGN1412-mediated T-cell proliferation as shown by FcR blocking and using deglycosylated TGN1412 (Figure 3B-C). This is in line with data by Findlay et al, who could not detect binding of TGN1412 to HUVECs.27 Hence, there might be distinct mechanisms conferring TGN1412-mediated T-cell activation: an FcR-independent one in the context of coincubation with endothelial cells and an FcR-dependent one in the context of other cells.
Endothelial cells play an important role in the recruitment of T cells and their activation at sites of inflammation.28,–30 We and others showed that human endothelial cells constitutively express and up-regulate MHC II molecules after cytokine exposure, and it was shown that endothelial cells can restimulate T cells in an antigen-specific manner.31,32 Compared with professional APCs such as dendritic cells, endothelial cells are rather low or negative for the expression of classic costimulatory molecules CD80 and CD86. Nevertheless, they express alternative costimulatory molecules, such as CD58 (LFA-3), CD134L (OX40L), and LICOS (CD275; Figure 4B and Khayyamian et al,6 Pober,29 Rose,30 Hughes et al,33 and Kunitomi et al,34 ). Interestingly, endothelial cells were shown to augment T-cell proliferation of, and cytokine release by, T cells incubated with anti-CD3 antibody OKT3. Under such conditions, interaction between CD58 on endothelial cells and CD2 on T cells was involved.33
Römer et al recently showed that in a high-density PBMC culture assay system peripheral T cells show TGN1412-mediated activation, which was dependent on HLA class I and II molecules expressed by monocytes mediating “subthreshold priming of the TCR signaling.”26 However, endothelial cell–mediated activation of TGN1412-treated T cells does not seem to rely on MHC-mediated TCR activation because blocking MHC molecules on endothelial cells did not reduce T-cell proliferation (Figure 4C). In addition, coblocking both MHC II and LICOS on endothelial cells cocultured with TGN1412-treated T cells showed no synergistic effects over those mediated by blocking LICOS alone (data not shown).
We found that interaction of LICOS, expressed on HUVECs, with ICOS on T cells is both necessary and sufficient to mediate TGN1412-induced T-cell proliferation (Figures 4C, 5A). Cytokine stimulation enhanced LICOS expression on HUVECs and furthermore, ICOS-LICOS interaction up-regulated ICOS expression on TGN1412-treated T cells (Figures 4B-5B). For cytokine stimulation of HUVECs, we used TNF-α and IFN-γ, which are also secreted in our endothelial cell/T-cell cocultivation assay after short-term incubation (Figure 2A). Hence, we provide a model of positive feedback conferred by ICOS-LICOS interaction between TGN1412-treated T cells and endothelial cells. In our experimental model, TNF-α and IFN-γ were added exogenously. Whether cytokine prestimulation of endothelial cells is needed to initiate this positive feedback loop or needed to enhance it will be a matter of future investigations.
However, blocking ICOS-LICOS interaction did not completely abolish TGN1412-induced T-cell proliferation mediated by HUVECs suggesting that additional molecular interactions between both cell types could be involved in TGN1412-mediated T-cell activation. On the other hand, it cannot be ruled out that the anti-LICOS antibody is not blocking with a 100% effectiveness, as is has been reported for other blocking antibodies.35 This latter suggestion is supported by our experiments using an anti-ICOS blocking antibody which reduced rLICOS-mediated proliferation of TGN1412-treated T cells by more than 90% (Figure 5C).
In this study, we used HUVECs as one of the best characterized and established models of primary human endothelial cells. However, HUVECs resemble a model of macrovascular endothelial cells. Even though LICOS up-regulation on microvascular endothelial cells upon cytokine treatment has been reported,6 it will be a matter of future investigations to analyze the role of ICOS-LICOS interaction in TGN1412-mediated T-cell activation using a microvascular endothelial cell model.
ICOS is a member of the B7 family of costimulatory ligands. In contrast to constitutively expressed CD28, ICOS is up-regulated on T-cell activation. Several sets of data indicate that ICOS regulates cytokine production in activated T cells, whereas it is less effective on naive T cells. However, Mesturini et al demonstrated that ICOS cooperation with CD28 modulates activation of human naive CD4 T cells.36 In line with this, we observed ICOS up-regulation when TGN1412-treated T cells were activated by ICOS-LICOS interaction with HUVECs (Figure 5B). The strong impact of ICOS-LICOS interaction on T cell–mediated immune responses in vivo became evident by ICOS gene knockout in mice. ICOS-deficient mice show defects in isotype class switch in T-cell dependent B-cell responses, and are defective in IL-4 and IL-13 production.37,–39 Likewise, ICOS null CVID patients have reduced secretion of IFN-γ, IL-4, IL-10, and IL-17.40 Moreover, it was shown that human ICOShi CD4 T cells produce more IFN-γ compared with ICOSlo CD4 T cells suggesting that ICOShi T cells have relevant effector functions.41,42 In line with this, we found that T cells proliferating upon TGN1412/LICOS stimulation showed higher ICOS expression than those showing less responsiveness to the same treatment (data not shown).
Yao et al showed that in addition to ICOS-LICOS interaction, human LICOS is a costimulatory ligand for CD28 facilitating anti-CD3–mediated T-cell activation.35 Of note, in our experimental setting using coated recombinant LICOS protein, the interaction between rLICOS and CD28 on T cells does not seem to play an important role because blocking ICOS on T cells was efficient in inhibiting TGN1412-mediated T-cell proliferation as well (Figure 5C).
Collectively, we provide data indicating molecular mechanisms underlying TGN1412-mediated T-cell activation. We show that ICOS-LICOS interaction between TGN1412-treated T cells and HUVECs is both necessary and sufficient to induce HUVEC-mediated T-cell activation. The role of this molecular interaction in TGN1412-mediated CRS in the phase 1 clinical trial of the antibody can only be speculated. Furthermore, ICOS-LICOS interaction between monkey cells (animals, which did not show CRS on preclinical testing of TGN1412) is not investigated by now. Moreover, our data along with those presented by others (L.Y.S., Z.W., P. Bartholomaeus, E. Corrales-Aguilar, E. Wiechec, B. Schraven, H. Hengel, U.K., unpublished data), suggest that T-cell activation upon TGN1412 treatment is a multifactorial event involving FcγR-dependent as well as FcR-independent mechanisms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Regine Schaffelder for provision of human umbilical cords; Kay-Martin Hanschmann for statistical analyses; Max Bastian for technical advice; Jörg Kirberg and Heinfried H. Radeke for helpful discussion; and Stefanie Bauer, Janine Beetz, and Julius Kühn for expert technical assistance. Furthermore, they thank Sergey Chuvpilo for kindly permitting the usage of TGN1412.
L.Y.S. was partially supported by the German Research Council (DFG, SFB854, B15).
Authorship
Contribution: S.W., L.Y.S., and S.C. performed experiments; U.K. and J.M.-B. analyzed results; and Z.W. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zoe Waibler, Junior Research Group, Novel Vaccination Strategies and Early Immune Responses, Paul-Ehrlich-Institut, D-63225 Langen, Germany; e-mail: zoe.waibler@pei.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal