Graft-versus-host disease is the major complication that occurs after allogeneic bone marrow transplantation (BMT) and is classically thought to be the response of donor lymphocytes to foreign antigens of the recipient or host.2 The onset of this posttransplantation complication, termed acute GVHD, is characterized by cutaneous eruptions, rash, and purpuric lesions in the skin along with infiltrates of T cells into the epidermis.3 Pathologic changes consistent with an acute inflammatory response can also be detected in the gastrointestinal tract and the liver during acute GVHD.
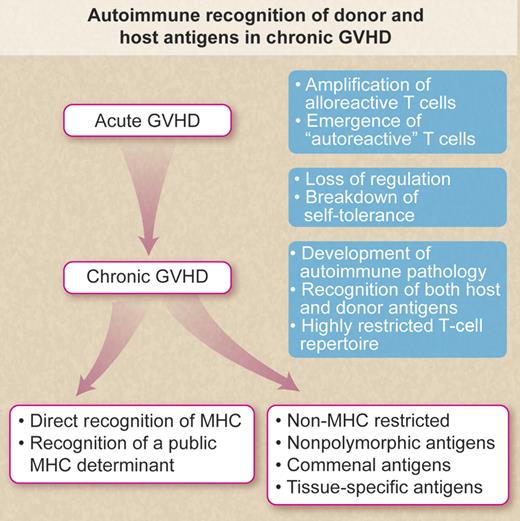
Autoimmune recognition of donor and host antigens in chronic GVHD. Schematic illustration indicating potential mechanism accounting for “autoimmune” recognition of donor and host antigens in chronic GVHD after allogeneic bone marrow transplantation. Professional illustration by Debra T. Dartez.
Autoimmune recognition of donor and host antigens in chronic GVHD. Schematic illustration indicating potential mechanism accounting for “autoimmune” recognition of donor and host antigens in chronic GVHD after allogeneic bone marrow transplantation. Professional illustration by Debra T. Dartez.
After this initial inflammatory response, it is common for patients to develop a late form of GVHD called chronic GVHD. Less commonly, chronic GVHD can arise de novo after BMT in the absence of any evidence of acute GVHD.2 Interestingly, chronic GVHD is characterized by fibrosis and sclerosis along with T cell–mediated destruction of the dermis.2,3 The pathologic changes in chronic GVHD, in fact, are similar to autoimmune diseases such as Sjogren syndrome, lichen planus, and scleroderma.2,3 There has been an ongoing debate about whether chronic GVHD is truly an autoimmune disease and whether this post-BMT complication is mediated by alloreactive or autoreactive T cells.
Clonotypic analysis of the effector T cells in an autoimmune model of chronic GVHD after allogeneic BMT by Rangarajan et al reveals that the repertoire was highly skewed with the reactive T cells capable of recognizing cells and tissues from both donor and host.1 A highly skewed repertoire suggests that the T cells are responding to a limited number of antigens. This is in contrast to a diverse repertoire that would be detected if the T cells are responding to many different antigens. Furthermore, the studies by Rangarajan et al reveal that many of the T cells analyzed in the autoimmune model of chronic GVHD expressed identical CDR3 domains.1 This domain governs the peptide specificity of the effector cells, and use of a common CDR3 domain would again suggest that the response is to a limited antigen set. Of critical importance is the finding that the clonotypically defined effector T cells recognized target tissues of both donor and host (self and nonself). Thus, the effector T cells were capable of recognizing self or “autoreactive.”
The emergence of the autoreactive T cells in the BMT recipient can occur via 2 distinct pathways. Quiescent autoreactive T cells can be carried over with the bone marrow graft. In the absence of effective immune regulation (schematically illustrated in the figure), the cells are activated and clonally amplified during GVHD leading to tissue destruction. Alternatively, the thymic epithelium is destroyed by acute GVHD effector T cells resulting in a failure to delete autoreactive T cells.4 Thus, self-tolerance is broken followed by the development of autoimmune or chronic GVHD. Interestingly, recent studies clearly suggest that the rapid reconstitution of CD4+CD25+ regulatory T cells prevents the development of chronic GVHD after clinical allogeneic BMT.5
The specificity of the effector T cells in chronic GHVD remains unclear (as illustrated in the figure). Rangarajan et al suggest that the effector T cells in their studies recognized nonpolymorphic antigens, perhaps tissue-specific antigens expressed on both donor and host tissues. Recognition of a commensal organism shared between donor and host is also a possible explanation. However, it is unclear whether the effector T cells in the studies by Rangarajan et al were restricted by class I or class II MHC determinants or whether the cells directly recognized the nonpolymorphic antigens. Of importance in this regard are the results from a number of recent studies in models of both alloimmune and autoimmune chronic GVHD.4,6,–8 The autoreactive T cells in these studies promiscuously recognize MHC class II determinants and effectively target these antigens on donor, host, and third-party cells. Like the studies of Rangarajan et al, clonotypic analysis revealed that the cells were highly restricted with respect to Vβ T cell–receptor gene use. In this setting, it is possible that a public MHC class II determinant (nonpolymorphic) was recognized. On the other hand, the effector T cells may have been positively selected and matured without first undergoing negative selection mechanisms in the thymus. Recognition of MHC class II would most likely be dependent only on the “goodness” of fit between the T-cell receptor and the MHC class II determinant(s). Recent studies suggest that recognition of a highly conserved peptide from the MHC class II–associated invariant chain may govern the promiscuous recognition of this determinant and may also account for the skewing of the repertoire.9
The analysis of the cytokine profile of the effector T cells in the model developed by Rangarajan et al may provide additional insight into immunologic complexity of acute and chronic GVHD. Recent studies suggest that both acute and chronic GVHD have distinct cytokine responses.3 In this regard, recent studies also suggest that the effector T cells shift from a type 1 profile to a type 2 profile during the transition from acute to chronic GVHD.8 This transition appears to be the result of a breakdown in self-tolerance. Analysis of the T-cell repertoire provides a unique insight into the complexity of chronic GVHD and the development of “autoimmunity” after allogeneic BMT.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal