Abstract
Deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), a VWF-cleaving protease, is the key factor in the pathogenesis of thrombotic thrombocytopenic purpura (TTP), a life-threatening thrombotic microangiopathy. It is well established that ADAMTS13 deficiency results in elevated plasma levels of ultra-large VWF multimers (ULVWF), which are prone to induce platelet aggregation; however, the actual trigger of TTP development remains uncertain. Here we describe a new animal model in which some TTP-like symptoms can be triggered in ADAMTS13 knockout mice by challenge with 2000 units/kg body weight of recombinant human VWF containing ULVWF multimers. Animals rapidly showed clinical symptoms and developed severe thrombocytopenia. Schistocytosis, a decrease in hematocrit, and elevated serum lactate dehydrogenase levels were observed. The heart was identified as the most sensitive target organ with rapid onset of extensive platelet aggregation in the ventricles and myocardial necrosis. Prophylactic administration of 200 units/kg recombinant human ADAMTS13 protected ADAMTS13 knockout mice from developing TTP. Therapeutic administration of 320 units/kg rhADAMTS13 reduced the incidence and severity of TTP findings in a treatment interval-dependent manner. We therefore consider this newly established mouse model of thrombotic microangiopathy highly predictive for investigating the efficacy of new treatments for TTP.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a blood coagulation disorder characterized by the presence of VWF- and platelet-rich microthrombi in the microvasculature of multiple organs. TTP was traditionally diagnosed by a pentad of symptoms: thrombocytopenia, microangiopathic hemolytic anemia with erythrocyte fragmentation, fever, renal dysfunction, and changes in mental status.1 In current practice, however, the presence of a nonimmune microangiopathic hemolytic anemia and thrombocytopenia without any alternative cause are often sufficient criteria to diagnose TTP.2 The key factor for the pathogenesis of the 2 clinically recognized forms of TTP is a deficiency in a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) because of either a genetic deficiency as in congenital TTP3 or an autoantibody-mediated deficiency as observed in acquired TTP.4,5 ADAMTS13 is a plasma protease that in a shear stress–dependent manner specifically cleaves the ultra-large (UL) multimer portion of newly released VWF (ULVWF) to smaller and less thrombogenic forms, thereby preventing spontaneous platelet aggregation and occlusive microvascular thrombosis in healthy persons.4,6,7 By contrast, plasma samples from patients with chronic relapsing TTP usually show elevated levels of ULVWF multimers8 and a high VWF to ADAMTS13 ratio (ie, elevated levels of VWF together with decreased ADAMTS13 activity levels).9,10
The identification of ADAMTS13 mutations11 in patients with hereditary TTP made ADAMTS13 knockout (KO) mice appear as an attractive animal model for human congenital TTP.11,12 However, somewhat unexpectedly, ADAMTS13 KO mice did not spontaneously develop TTP, even when mice of a genetic background with high plasma levels of VWF were studied.1,12,13 In these mice (CASA/Rk or CASA/Ei), exogenous triggers, such as Shiga toxin, were required to develop TTP in at least a subset of animals.14-16 Mice with low intrinsic VWF levels, such as the C57BL/6 background, remained nonsusceptible to TTP induction, even after Shiga toxin challenge. Genetic factors combined with an ill-defined VWF threshold level were therefore postulated to explain the unreliable responses in ADAMTS13 KO mice.14,15
On the other hand, treatment of ADAMTS13 KO mice with high doses of a recombinant human VWF (rhVWF) containing a portion of ULVWF multimers17 consistently led to exaggerated pharmacologic effects.18 Based on this observation, we hypothesized that a suitable animal model of TTP could be set up by challenging ADAMTS13 KO mice with a defined concentration of rhVWF, provided that the TTP-like symptoms which rhVWF triggers develop simultaneously and consistently in a majority of animals. Such a model would not only allow study of the early stages of TTP but also render preclinical screening of TTP therapy drug candidates feasible.
Here we analyzed rhVWF-challenged ADAMTS13 KO mice for signs of TTP development and used this setup to test the effect of rhADAMTS13,19 administered either before or after TTP symptoms had been triggered by rhVWF. The results obtained are discussed in terms of a pathophysiology of TTP that focuses on plasma levels of ULVWF multimers with rhADAMTS13 as a potential remedy.
Methods
rhVWF and recombinant human ADAMTS13
The preparation of rhVWF and recombinant human ADAMTS13 (rhADAMTS13) has been described.20,21 VWF ristocetin cofactor activity (VWF:RCo) was determined using a turbidimetric analyzer (BCS system; Siemens). ADAMTS13 activity (ADAMTS13:Ac) was analyzed using the synthetic fluorogenic fluorescence resonance energy transfer substrate units (FRETS-U)/VWF73 minimal peptide substrate (Peptanova), essentially as described.22 A pharmacokinetic analysis of rhVWF in VWF-deficient mice23 showed a terminal half-life of 4.5 hours and a mean residence time of 4.9 hours. Pharmacokinetics of rADAMTS13 in ADAMTS13 KO mice (B6.129-ADAMTS13tm1Dgi)24 showed a terminal half-life of 24.3 hours and a mean residence time of 26.7 hours.
Experimental design of the TTP model
All animal experiments were performed according to Austrian laws governing animal experimentation and were additionally approved by Baxter's Institutional Animal Care and Use Committee. Fifty-two ADAMTS13 KO mice (26 males and 26 females; B6.129-ADAMTS13tm1Dgi),24 12 to 18 weeks old, received a single slow bolus injection of 2000 VWF:RCoU/kg body weight (BW) of rhVWF into a lateral tail vein on day 0. Forty-two untreated ADAMTS13 KO mice (21 males and 21 females) of the same age range were used as negative controls. All animals were examined for clinical symptoms by a veterinarian at least twice daily for 1, 3, or 14 days. Clinical symptoms were recorded descriptively and scored as mild, moderate, or severe.
Seven groups of 4 ADAMTS13 KO mice (2 males and 2 females) were injected with 2000 VWF:RCoU/kg BW (2000 U/kg) of rhVWF. Specimens (blood, tissues) were collected from each group at 5 minutes, 15 minutes, 30 minutes, 1 hour, 3 hours, 6 hours, and 9 hours after injection. The specimens were analyzed for platelet counts and organ damage to monitor the acute onset of thrombocytopenia.
Efficacy of rhADAMTS13 in the TTP model
To assess prophylactic treatment, 6 ADAMTS13 KO mice (3 males and 3 females) received a bolus injection of 200 FRETS-U/kg BW (200 U/kg) rhADAMTS13 into a lateral tail vein, immediately followed by an intravenous administration of 2000 U/kg rhVWF. To test for the therapeutic effect of rhADAMTS13, 6 ADAMTS13 KO mice (3 males and 3 females) received a bolus injection of 320 U/kg rhADAMTS13 into a lateral tail vein 15, 30, or 180 minutes after intravenous administration of 2000 U/kg rhVWF. As a negative control for both settings, 6 animals (3 males and 3 females) received rhVWF only (2000 U/kg). All animals were followed for 1 day.
Body weight, hematology, and serum chemistry
The BW of all animals was assessed before application of rhVWF (day 0) and on days 1, 3, and 14. Relative BW changes are presented as means.
Blood was sampled by puncture of the caudal Vena cava. Hematocrit and platelet counts (ADVIA 120; Siemens) were assessed in EDTA-treated whole blood (Vacuette K3E K3EDTA; Greiner Bio-One), and lactate dehydrogenase (LDH) concentration (Dimension Xpand Plus; Siemens) was determined in serum (Vacuette Z Serum Clot Activator; Greiner Bio-One). A blood smear was done for a subset of 30 rhVWF-treated and 20 untreated animals and stained with H&E (Merck) to manually assess schistocytosis. A total of 1000 RBCs were evaluated for each sample. The results are presented as means.
Tissue preparation and analysis
At the end of the experimental phase, all animals were exsanguinated under deep anesthesia with subsequent necropsy. The heart, kidneys, lungs, brain, and liver of each animal were preserved in 10% neutral-buffered formalin, paraffin-embedded and cut into 2- to 3-μm-thick sections. The paraffin slides were stained with H&E (Merck) for routine tissue preparation, with phosphotungstic acid hematoxylin (Diapath SpA) to visualize fibrin and collagen, and Prussian Blue (VWR International) to visualize ferric iron and ferritin. Fraser Lendrum staining (Merck) was applied to visualize fibrin. Staining for VWF was achieved using polyclonal rabbit-anti–human VWF (Dako Austria) detecting both rhVWF and mouse VWF as a primary antibody and biotinylated polyclonal donkey-anti–rabbit VWF (Southern Biotechnology) as a detection antibody in combination with HRP-labeled streptavidin (Biocare Medical). Nonspecific rabbit IgG (Dako Austria) was used instead of the primary antibody as an isotype control. Finally, the slides were counterstained with hemalaun to visualize nuclei. Pathologic findings were scored (1 indicates mild; to 5, severe). Micrographs were taken at room temperature using a BX45TF-5 Ergonomic microscope (Olympus), a ColorView Illu digital camera, and UPlanApo N, UPlanSApo, or UPlanApo lens and cellD 3.2 (Olympus Soft Imaging Solutions).
Results
Infusion of a high dose of rhVWF into ADAMTS13 KO mice induces TTP-like symptoms
We have previously observed that administration of different doses of rhVWF ranging from 250 to 4000 U/kg caused exaggerated pharmacologic effects of increasing severity in ADAMTS13 KO mice on a C57BL/6 background.18 Based on these findings, we questioned whether a high dose of rhVWF would be sufficient to trigger TTP in these mice despite their reported nonsusceptibility to the development of TTP-like symptoms.16 A dose of 2000 U/kg was chosen for the entire study as most (17 of 20) of the animals treated at this concentration had shown clinical symptoms but none had died.18
Challenge of ADAMTS13 KO mice with 2000 U/kg rhVWF resulted in an increase in VWF antigen (VWF:Ag) from 0.15 U/mL, reflecting the endogenous mouse VWF level, to 55 U/mL after 5 minutes. Substantial amounts of rhVWF were still present in the circulation after 30 minutes (23 U/mL) and 3 hours (17 U/mL), and even after 24 hours, VWF:Ag (7 U/mL) was clearly above the endogenous mouse level (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Multimer analysis showed that 5 minutes after rhVWF treatment the multimer pattern was similar to that of the rhVWF material administered. Only a slow, successive removal of the highest molecular weight multimers became visible after 30 minutes and 3 hours (supplemental Figure 2 group B).
Clinical symptoms presented within minutes of the intravenous administration of 2000 U/kg rhVWF into ADAMTS13 KO mice. Animals showed mild to moderate behavioral depression (21 of 52), piloerection (9 of 52), dyspnea (2 of 52), cramps (3 of 52), a hunched posture (2 of 52), and a lying position (2 of 52). Most animals recovered by day 3, but behavioral depression and piloerection persisted until day 9 in 2 animals. No deaths occurred or necessity to kill animals for welfare reasons arose.
All animals lost weight during the first day after treatment with rhVWF (Table 1). Consistent with a slow recovery from clinical symptoms, BW loss decreased between day 0 and day 3, and by day 14 all animals had regained their pre-rhVWF administration BW. Untreated control animals did not show any relevant changes in BW throughout the study (Table 1).
Summary of body weight development, hematology, and serum chemistry of rhVWF-challenged ADAMTS13 KO mice
| Variable . | Time point, d . | TTP model . | ||||
|---|---|---|---|---|---|---|
| 2000 U/kg rhVWF* . | Untreated . | |||||
| Mean . | % . | n† . | Mean‡ . | n† . | ||
| Platelets, × 103/μL | 1 | 30 | 2.7 | 28 | 1128 (overall) | 41 |
| 3 | 416 | 36.9 | 13 | |||
| 14 | 1276 | 113.1 | 10 | |||
| Body weight, Δ% | 0-1 | −8.8 | NA | 40 | −0.9 | 33 |
| 0-3 | −4.8 | 13 | −0.9 | 10 | ||
| 0-14 | 0.1 | 10 | 0.8 | 10 | ||
| Hematocrit, % | 1 | 34.6 | 85.2 | 28 | 40.6 (overall) | 41 |
| 3 | 28.5 | 70.2 | 13 | |||
| 14 | 41.7 | 102.7 | 10 | |||
| LDH, U/L | 1 | 1598 | 482.8 | 12 | 331 | 12 |
| Schistocytes, 1000 RBCs | 1 | 27.5 | 9166.7 | 29 | 0.3 | 20 |
| Variable . | Time point, d . | TTP model . | ||||
|---|---|---|---|---|---|---|
| 2000 U/kg rhVWF* . | Untreated . | |||||
| Mean . | % . | n† . | Mean‡ . | n† . | ||
| Platelets, × 103/μL | 1 | 30 | 2.7 | 28 | 1128 (overall) | 41 |
| 3 | 416 | 36.9 | 13 | |||
| 14 | 1276 | 113.1 | 10 | |||
| Body weight, Δ% | 0-1 | −8.8 | NA | 40 | −0.9 | 33 |
| 0-3 | −4.8 | 13 | −0.9 | 10 | ||
| 0-14 | 0.1 | 10 | 0.8 | 10 | ||
| Hematocrit, % | 1 | 34.6 | 85.2 | 28 | 40.6 (overall) | 41 |
| 3 | 28.5 | 70.2 | 13 | |||
| 14 | 41.7 | 102.7 | 10 | |||
| LDH, U/L | 1 | 1598 | 482.8 | 12 | 331 | 12 |
| Schistocytes, 1000 RBCs | 1 | 27.5 | 9166.7 | 29 | 0.3 | 20 |
NA indicates not applicable.
U rhVWF = VWF:RCoU rhVWF.
Number of reported values can be smaller than group size because of missing values (eg, technical errors).
Means of untreated animals are set to 100% to compare means of rhVWF-challenged animals.
All animals showed severe thrombocytopenia on day 1 after administration of rhVWF (2.7% of normal; Figure 1; Table 1). Animals were still thrombocytopenic on day 3, but on day 14 platelet counts returned to normal or were even slightly elevated compared with untreated animals (Figure 1). In addition and parallel to the platelet count, the hematocrit dropped in rhVWF-treated animals to 85.2% and 70.2% of that of control animals by day 1 and 3, respectively, and completely recovered by day 14 (Table 1). Serum LDH concentration was assessed on day 1 of the study. Animals receiving rhVWF showed an almost 5-fold increase in the mean LDH concentration compared with the untreated controls (Table 1). Evaluation of schistocytosis on day 1 of the study demonstrated the presence of schistocytes in all blood smears of the rhVWF-challenged animals assessed (Table 1).
Reversible thrombocytopenia in rhVWF-challenged ADAMTS13 KO mice. Animals were administered 2000 VWF:RCoU/kg BW of rhVWF (●), and platelet counts were determined after 1, 3, and 14 days. A severe thrombocytopenia was noted on day 1 after the challenge. Thrombocytopenia persisted on day 3 but subsided by day 14 compared with untreated ADAMTS13 KO mice (○).
Reversible thrombocytopenia in rhVWF-challenged ADAMTS13 KO mice. Animals were administered 2000 VWF:RCoU/kg BW of rhVWF (●), and platelet counts were determined after 1, 3, and 14 days. A severe thrombocytopenia was noted on day 1 after the challenge. Thrombocytopenia persisted on day 3 but subsided by day 14 compared with untreated ADAMTS13 KO mice (○).
Necropsy revealed that macroscopic lesions were restricted to the heart and consisted of multifocal, well-delineated, dark-red discolorations of the myocardium 1 to 2 mm in diameter (acute myocardial hemorrhage and necrosis). On day 1, moderate to severe myocardial necrosis was accompanied by infiltration of large numbers of neutrophilic granulocytes, hemorrhage, and fibrin precipitation. These lesions were more pronounced in the proximal half of the heart (Figure 2C). On day 3, hemorrhage and myocardial lesions were observed with a similar distribution pattern as on day 1. In addition to the neutrophilic granulocytes, macrophages and lymphocytes were present and proliferation of fibroblasts was evident. On day 14, interstitial fibrosis was the predominant pathologic feature. VWF-specific immunohistochemical staining showed its presence in areas of hemorrhage and necrosis, with the overall staining intensity being weaker on day 3 than on day 1 (data not shown).
TTP-like pathohistologic changes in rhVWF-challenged animals. (A) The presence of large aggregates of platelets (arrowheads) within the left ventricle 30 minutes after TTP induction (H&E, original magnification ×100). Bar represents 200 μm. (B) Myocardial necrosis and hemorrhage (arrowheads) as well as rolling and extravasation of neutrophilic granulocytes (arrows) within the first 3 hours of TTP induction (H&E, original magnification ×400). Bar represents 50 μm. (C) Vacuolation of myocytes (arrow) and perivascular infiltration of neutrophilic granulocytes (arrowheads) within 24 hours after TTP induction (H&E, original magnification ×200). Bar represents 100 μm. (D) Renal tubular necrosis (arrowheads) characterized by loss of cellular detail, increased eosinophilia of cytoplasm, and karyopyknosis within 24 hours after TTP induction (H&E, original magnification ×400). Bar represents 50 μm. (E) Myocardial artery (arrows) immunohistochemically strongly positive for VWF 60 minutes after TTP induction (VWF immunohistochemistry, original magnification ×600). Bar represents 33 μm. (F) Myocardial artery (arrows) of an untreated control animal with minor background staining for VWF resulting from cross-reactivity of the antibody with endogenous murine VWF (VWF immunohistochemistry, original magnification ×200). Bar represents 100 μm.
TTP-like pathohistologic changes in rhVWF-challenged animals. (A) The presence of large aggregates of platelets (arrowheads) within the left ventricle 30 minutes after TTP induction (H&E, original magnification ×100). Bar represents 200 μm. (B) Myocardial necrosis and hemorrhage (arrowheads) as well as rolling and extravasation of neutrophilic granulocytes (arrows) within the first 3 hours of TTP induction (H&E, original magnification ×400). Bar represents 50 μm. (C) Vacuolation of myocytes (arrow) and perivascular infiltration of neutrophilic granulocytes (arrowheads) within 24 hours after TTP induction (H&E, original magnification ×200). Bar represents 100 μm. (D) Renal tubular necrosis (arrowheads) characterized by loss of cellular detail, increased eosinophilia of cytoplasm, and karyopyknosis within 24 hours after TTP induction (H&E, original magnification ×400). Bar represents 50 μm. (E) Myocardial artery (arrows) immunohistochemically strongly positive for VWF 60 minutes after TTP induction (VWF immunohistochemistry, original magnification ×600). Bar represents 33 μm. (F) Myocardial artery (arrows) of an untreated control animal with minor background staining for VWF resulting from cross-reactivity of the antibody with endogenous murine VWF (VWF immunohistochemistry, original magnification ×200). Bar represents 100 μm.
Minimal, acute tubular necrosis in the kidneys was seen in the majority of rhVWF-treated animals on day 1 (Figure 2D) and in a few animals on day 3. On day 14, no differences were noted between control and rhVWF-treated animals. The incidence and severity grade of regenerative tubuli were higher in the rhVWF-treated group than in untreated ADAMTS13 KO mice. The indices were also higher on day 3 than on day 1 (data not shown). Notably, no lesions were identified in the brain of rhVWF-treated animals.
Kinetics of the development of thrombocytopenia
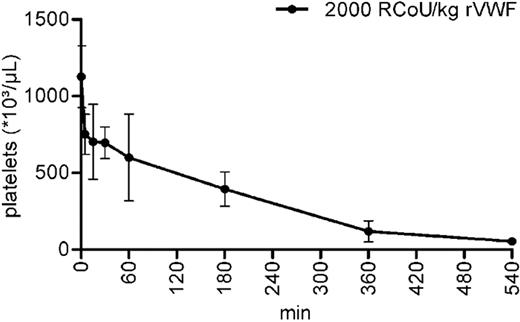
The striking observation of severe thrombocytopenia in all mice analyzed 1 day after administration of rhVWF followed by a slow improvement during the next 14 days prompted us to monitor the early stages of thrombocytopenia development more closely. To this end, platelets were counted at 5, 15, 30, 60, 180, 360, and 540 minutes after rhVWF injection. Platelet counts decreased by approximately one-third within 5 minutes. Thrombocytopenia worsened rapidly over time with as few as 5% of platelets remaining after 540 minutes (Figure 3), indicating that platelet depletion was already maximum 9 hours after rhVWF administration.
Kinetics of thrombocytopenia development in ADAMTS13 KO mice at early time points after rhVWF challenge. Animals were administered 2000 VWF:RCoU/kg BW of rhVWF (●), and platelet counts were determined at the time points (4 animals each) indicated up to 9 hours. Compared with untreated control animals (time point 0), platelet counts were already reduced by approximately 33% after 5 minutes and by 95% after 9 hours.
Kinetics of thrombocytopenia development in ADAMTS13 KO mice at early time points after rhVWF challenge. Animals were administered 2000 VWF:RCoU/kg BW of rhVWF (●), and platelet counts were determined at the time points (4 animals each) indicated up to 9 hours. Compared with untreated control animals (time point 0), platelet counts were already reduced by approximately 33% after 5 minutes and by 95% after 9 hours.
These hematologic findings were consistent with pathologic findings. Macroscopic lesions were seen as early as 6 hours after rhVWF treatment and were again restricted to acute myocardial hemorrhage and necrosis. Histopathologic changes included ventricular and vascular platelet aggregations (hyaline [micro-] thrombi) in animals killed between 15 minutes and 3 hours after rhVWF administration (Figure 2A). Furthermore, myocardial lesions were recorded as early as 15 minutes after administration of rhVWF and consisted of necrosis of individual myocytes and hemorrhage. After 3 hours, infiltration of neutrophilic granulocytes along with hemorrhage of increased severity became evident (Figure 2B). Minimal, peracute tubular necrosis in the kidneys was seen 1 hour after treatment in a few animals. However, after 9 hours, lesions were observed in the majority of animals in the rhVWF-treatment group (data not shown). Immunohistochemistry revealed intense VWF staining of hemorrhagic and necrotic areas in the myocardium as well as more intense intravascular staining at all time points within the first 9 hours (Figure 2E), whereas nontreated animals showed only minimal staining (Figure 2F).
Prophylactic efficacy of rhADAMTS13 in the TTP mouse model
The successful reproduction of many clinical aspects of human TTP in our new mouse model prompted us to test the prophylactic effect of rhADAMTS13 on the development of TTP-like symptoms. A group of 6 ADAMTS13 KO animals (3 males and 3 females) was observed for 1 day after treatment with a bolus injection of 200 U/kg rhADAMTS13 into a lateral tail vein, followed immediately by intravenous administration of 2000 U/kg rhVWF.
This treatment regimen caused the VWF:Ag levels to increase to 54.6 U/mL after 5 minutes and then to decline to 25.4 U/mL after 30 minutes and 17 U/mL after 3 hours. After 24 hours, VWF:Ag levels (0.54 U/mL) were already within the range of the endogenous mouse VWF level (supplemental Figure 1). Compared with the rhVWF material administered, a pronounced decrease in ULVWF multimers was already discernible 30 minutes after treatment, and the multimer size was further reduced after 3 hours (supplemental Figure 2).
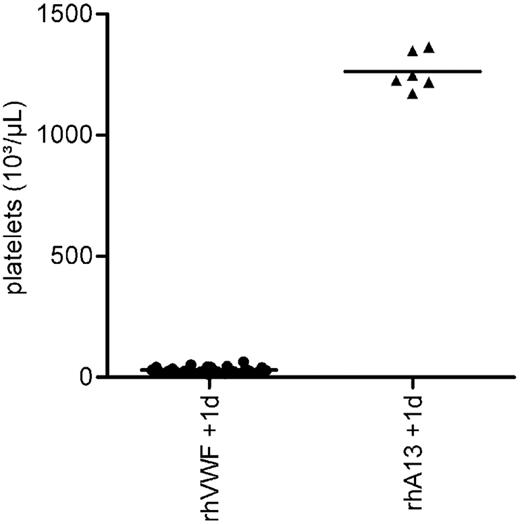
Animals receiving prophylactic treatment with rhADAMTS13 did not show any clinical symptoms or relevant changes in BW. Furthermore, they did not develop thrombocytopenia (Figure 4), had an almost normal hematocrit, and did not show an increase in serum LDH (Table 2). Moreover, animals treated with rhADAMTS13 before setting the TTP trigger had no macroscopic or histologic lesions characteristic of TTP on day 1.
Prophylactic efficacy of rhADAMTS13. Before the challenge with 2000 VWF:RCoU/kg BW of rhVWF, one group of animals received 200 FRETS-U/kg BW rhADAMTS13 (rhA13 + rhVWF), whereas the control group received rhVWF only (rhVWF). Platelet counts were determined after 1 day. Whereas all animals of the control group developed a severe thrombocytopenia (●), animals prophylactically treated with rhADAMTS13 (▴) were protected.
Prophylactic efficacy of rhADAMTS13. Before the challenge with 2000 VWF:RCoU/kg BW of rhVWF, one group of animals received 200 FRETS-U/kg BW rhADAMTS13 (rhA13 + rhVWF), whereas the control group received rhVWF only (rhVWF). Platelet counts were determined after 1 day. Whereas all animals of the control group developed a severe thrombocytopenia (●), animals prophylactically treated with rhADAMTS13 (▴) were protected.
Summary of body weight development, hematology, and serum chemistry of ADAMTS13 KO mice receiving treatment with rhADAMTS13 before or after challenge with rhVWF
| Variable . | Time point, d . | Prophylactic treatment: 200 U/kg rhADAMTS13* + 2000 U/kg rhVWF† . | Therapeutic treatment: 2000 U/kg rhVWF† + 320 U/kg rhADAMTS13* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| After 15 min . | After 30 min . | After 180 min . | |||||||
| Mean . | n . | Mean . | n . | Mean . | n . | mean . | n . | ||
| Body weight, Δ% | 0-1 | −0.7 | 6 | −2.5 | 6 | −3.8 | 6 | −3.0 | 6 |
| Platelets, × 103/μL | 1 | 1263 | 6 | 970 | 6 | 805 | 6 | 370 | 6 |
| Hematocrit, % | 1 | 36.9 | 6 | 39.3 | 6 | 38.3 | 6 | 34.1 | 6 |
| LDH, U/L | 1 | 296 | 6 | 256 | 6 | 222 | 6 | 206 | 6 |
| Variable . | Time point, d . | Prophylactic treatment: 200 U/kg rhADAMTS13* + 2000 U/kg rhVWF† . | Therapeutic treatment: 2000 U/kg rhVWF† + 320 U/kg rhADAMTS13* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| After 15 min . | After 30 min . | After 180 min . | |||||||
| Mean . | n . | Mean . | n . | Mean . | n . | mean . | n . | ||
| Body weight, Δ% | 0-1 | −0.7 | 6 | −2.5 | 6 | −3.8 | 6 | −3.0 | 6 |
| Platelets, × 103/μL | 1 | 1263 | 6 | 970 | 6 | 805 | 6 | 370 | 6 |
| Hematocrit, % | 1 | 36.9 | 6 | 39.3 | 6 | 38.3 | 6 | 34.1 | 6 |
| LDH, U/L | 1 | 296 | 6 | 256 | 6 | 222 | 6 | 206 | 6 |
U rADAMTS13 = FRETS-U rhADAMTS13.
U rhVWF = VWF:RCoU rhVWF.
Therapeutic efficacy of rhADAMTS13 in the TTP model
TTP-like symptoms were induced in 3 groups of 6 ADAMTS13 KO animals (3 males and 3 females) by a bolus injection of 2000 U/kg rhVWF into a lateral tail vein. The therapeutic efficacy of rhADAMTS13 was tested by subsequent intravenous administration of 320 U/kg of rhADAMTS13, 15, 30, or 180 minutes after the mice were challenged with rhVWF. The animals were observed for 1 day.
Animals treated 15 and 30 minutes after the VWF challenge did not show clinical symptoms, but 2 of 6 animals treated with rhADAMTS13 180 minutes after the challenge showed symptoms, including behavioral depression (2 of 6), cramps (1 of 6), and a lying position (1 of 6). All 3 treatment groups exhibited a mean BW loss after 1 day that was however less than that of the group treated with rhVWF only (Table 2).
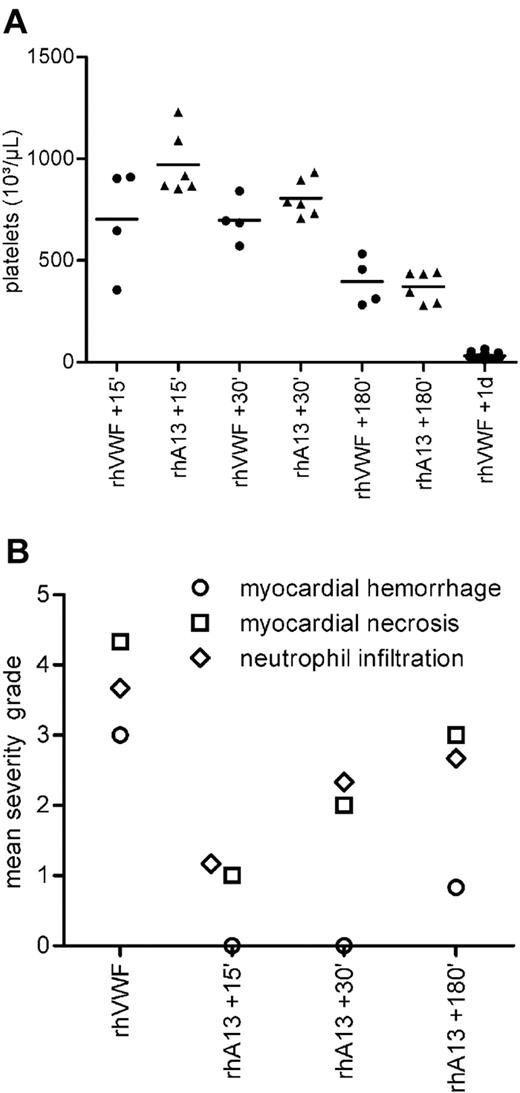
All animals treated therapeutically with rhADAMTS13 had normal mean serum LDH values (Table 2). The severity of thrombocytopenia was dependent on the time window between the challenge with rhVWF and therapeutic treatment with rhADAMTS13 (Table 2). The 3 groups of rhADAMTS13-treated mice had mean platelet counts after 1 day similar to those of mice 15, 30, or 180 minutes after rhVWF challenge without rhADAMTS13 treatment (Figure 5A). At day 1, all 3 groups had a markedly higher platelet count than rhVWF-challenged animals that did not receive rhADAMTS13 (Figure 5A).
Therapeutic efficacy of rhADAMTS13. (A) Platelet counts. All mice were challenged with 2000 VWF:RCoU/kg BW rhVWF at time point zero. Four groups of animals (●; rhVWF) did not receive any further treatment, and platelet count was assessed 15, 30, and 180 minutes and 1 day after challenge. These mice developed a thrombocytopenia (also shown in Figure 3). Three groups of mice received therapeutic treatment with 320 FRETS-U/kg BW of rhADAMTS13 (▴; rhA13), 15, 30, or 180 minutes after challenge with rhVWF. Platelet count of these groups was assessed on day 1. Data derived from the rhVWF-challenged controls (●; rhVWF) indicated that the animals allocated to treatment had already developed a thrombocytopenia at the time point of treatment. At day 1, platelet counts were higher for all rhADAMTS13-treated groups (▴) compared with the untreated group (●; rhVWF 1 day). Deductively, therapeutic administration of rhADAMTS13 caused a stabilization of the platelet counts at the respective time point of treatment (eg, rhVWF +15 minutes vs rhADAMTS13 + 15 minutes). (B) Pathologic changes. The same animals as in panel A were scrutinized on day 1 for pathologic changes in the heart. Incidences and severity of myocardial hemorrhage (○), myocardial necrosis (□), and neutrophil infiltration of the myocardium (♢) were highest in the nontreated control group (n = 6). Therapeutic treatment with 320 FRETS-U/kg BW of rhADAMTS13 (n = 6) had a beneficial treatment interval-dependent effect on the incidence and severity of pathologic changes.
Therapeutic efficacy of rhADAMTS13. (A) Platelet counts. All mice were challenged with 2000 VWF:RCoU/kg BW rhVWF at time point zero. Four groups of animals (●; rhVWF) did not receive any further treatment, and platelet count was assessed 15, 30, and 180 minutes and 1 day after challenge. These mice developed a thrombocytopenia (also shown in Figure 3). Three groups of mice received therapeutic treatment with 320 FRETS-U/kg BW of rhADAMTS13 (▴; rhA13), 15, 30, or 180 minutes after challenge with rhVWF. Platelet count of these groups was assessed on day 1. Data derived from the rhVWF-challenged controls (●; rhVWF) indicated that the animals allocated to treatment had already developed a thrombocytopenia at the time point of treatment. At day 1, platelet counts were higher for all rhADAMTS13-treated groups (▴) compared with the untreated group (●; rhVWF 1 day). Deductively, therapeutic administration of rhADAMTS13 caused a stabilization of the platelet counts at the respective time point of treatment (eg, rhVWF +15 minutes vs rhADAMTS13 + 15 minutes). (B) Pathologic changes. The same animals as in panel A were scrutinized on day 1 for pathologic changes in the heart. Incidences and severity of myocardial hemorrhage (○), myocardial necrosis (□), and neutrophil infiltration of the myocardium (♢) were highest in the nontreated control group (n = 6). Therapeutic treatment with 320 FRETS-U/kg BW of rhADAMTS13 (n = 6) had a beneficial treatment interval-dependent effect on the incidence and severity of pathologic changes.
Therapeutic treatment with rhADAMTS13 within 180 minutes after rhVWF challenge prevented the macroscopic lesions in the heart that had been observed in the rhVWF-challenged mice as early as 6 hours after the challenge. Microscopic lesions were not present or had a low severity grade when rhADAMTS13 was administered within 180 minutes after the induction of TTP-like symptoms by rhVWF (Figure 5B). After 24 hours, myocardial hemorrhage was seen in 1 of 6 animals treated after 15 or 30 minutes. In the group treated after 180 minutes, 4 of 6 animals were affected, as were all animals (6 of 6) in the untreated group. Myocardial necrosis was seen in all animals; however, the mean severity grade was lower in groups treated after 15 or 30 minutes compared with groups that were treated after 180 minutes or remained untreated (Figure 5B). Finally, parenchymal infiltration of the myocardium by neutrophilic granulocytes was observed in all animals, but the mean severity grade was lower in animals treated after 15 and 30 minutes than in animals treated after 180 minutes and untreated control animals (Figure 5B).
Acute tubular necrosis was not observed in animals treated after 15 minutes and only in 1 animal in the group treated after 30 minutes. Three of 6 animals in the group treated after 180 minutes and in the rhVWF-challenged control group developed acute tubular necrosis of low severity.
Discussion
In this study, we established and characterized a new animal model mimicking TTP in which the administration of a high dose of human rVWF to ADAMTS13 KO mice reliably provoked a TTP-like response and showed that human rADAMTS13 can act as a prophylactic and therapeutic agent in this model.
Pathophysiologic relevance of rVWF as a trigger of TTP
The recombinant human VWF used to trigger TTP has not been exposed to ADAMTS13 and consequently contains a portion of ULMW VWF multimers.20 It thereby resembles VWF that is newly released into plasma from Weibel-Palade bodies of endothelial cells and platelet α-granules.25 Although in healthy persons the UL portion of the newly released VWF is rapidly processed to smaller multimers by ADAMTS13, it persists for a much longer time in TTP patients who have a severe ADAMTS13 deficiency.8 Recombinant VWF thus allows the design of a rodent model that mimics certain aspects of human TTP in ADAMTS13-deficient mice. Challenge of ADAMTS13 KO mice on a C57BL/6 background with a high dose of human rhVWF effectively led to a rapid development of severe thrombocytopenia and other TTP-like symptoms.
The importance of high rhVWF levels for provoking TTP-like symptoms is underscored by a report showing that the KO mouse strain used is refractory to the development of TTP because of its low levels of VWF.16 Indeed, ADAMTS13 KO mice usually remain healthy, and only strains with higher endogenous levels of VWF, such as CASA/Rk and CAST/Ei, are considered susceptible to TTP development when challenged with a trigger, such as Shiga toxin.12,15,16 Inspection of littermates generated by crossing strains with low and high VWF levels enabled an estimation of the required VWF threshold level for TTP symptoms of being, at a minimum, twice as high as in the C57BL/6 background. On the other hand, various VWF levels above this threshold did not seem to impact the likelihood of an individual mouse's predisposition to TTP. As a consequence, genetic factors other than VWF were postulated to explain the onset of TTP-like symptoms.14 However, at that time, VWF levels were measured only before and not after challenge, although VWF levels and the ULMW fraction thereof in particular do rise significantly on exposure to Shiga toxin.
This becomes particularly evident when the isolated B subunit of Shiga toxin (ie, without its cytotoxic A subunit) is used as a trigger. This Shiga toxin variant seems to specifically cause an endothelial release of VWF without provoking any renal symptoms typical of hemolytic uremic syndrome. The VWF release sufficed for an onset of mild TTP-like symptoms in some animals (5 of 13).15 It is conceivable that, depending on the individual animal's response to the toxin trigger, different amounts of ULVWF were released that were not always sufficient to cause TTP. By contrast, administration of rhVWF as in our model results in a well-defined increase in the plasma concentration of ULVWF without the necessity of a preceding endothelial activation step. Indeed, it has become clear that rhVWF constitutes a more direct and more reliable trigger of TTP than Shiga toxin and the truncated variant thereof, as every animal treated developed thrombocytopenia.
The requirement for the very high concentration of 2000 U/kg of rhVWF, resulting in a maximum plasma concentration of 55 U/mL, to cause the variety of TTP-like symptoms was determined in a previous toxicology study. Administration of lower concentrations of rhVWF (1000 and 500 U/kg) to ADAMTS13 KO mice of the same breeding colony were sufficient to cause milder forms of thrombocytopenia and microthrombosis but did not cause an increase in LDH or any clinical signs.18 For future work, it will be interesting to see whether lower rhVWF concentrations can cause a profound onset of TTP in the more susceptible mouse strains CASA/Rk and CAST/Ei.
In contrast to mice, where a trigger appears requisite to achieve VWF levels sufficient to provoke signs of TTP in ADAMTS13-deficient animals, the normal ULMW VWF levels in baboons are apparently high enough to trigger TTP on inhibition of ADAMTS13.26 Other proteases, such as elastase and cathepsin G, may modulate the steady state of ULVWF levels to various extents.27 Different rates of endothelial VWF release may also account for this difference.
With regard to TTP patients, clinical data suggest that a high VWF to ADAMTS13 ratio could be the crucial criterion for predicting the risk of TTP occurrence.10 Thus, patients with residual ADAMTS13 activity might experience a TTP episode at much higher VWF levels than patients who entirely lack ADAMTS13, as in the Upshaw Schulman syndrome, which is reflected in a much earlier onset of the disease.28,29
TTP-like symptoms in the rhVWF-induced animal model of TTP
Challenge of ADAMTS13 KO mice with human rVWF rapidly led to a number of symptoms characteristic for patients with TTP. The most striking observation was the rapid development of severe thrombocytopenia. Both the consistency and the severity of the thrombocytopenia (< 5% of untreated animals) were much more pronounced than in mouse models of TTP formerly described.15,16 A similar homogeneous development of thrombocytopenia (< 5% of untreated animals) was observed in a baboon model of acquired TTP.26 The very low platelet numbers seen in our model resemble those of patients with an acute severe episode of TTP, where platelet counts less than 10% of normal are not unusual.2
Microangiopathic hemolytic anemia is another frequent hematologic disorder in patients with TTP. Animals challenged with human rhVWF had a decreased hematocrit (70%-80% of untreated animals), and schistocytes were found in blood smears, indicating hemolytic anemia. This finding accords with those in other animal models of TTP.15,16,26 Likewise, the serum LDH concentration, which is indicative of organ damage and hemolysis, was markedly increased in several of these models, including ours (5-fold increase over the untreated control).
Our study identified the heart as the most sensitive target organ. We noted a rapid onset of extensive aggregation of platelets in the ventricles as well as necrosis of the myocardium, which is also in line with findings in the baboon model of acquired TTP.26 Although dysfunction of the heart is not part of the classic pentad of TTP symptoms, patients very often have cardiac changes, including heart failure and arrhythmias.30-32 The histologic finding of time-dependent formation of hyaline thrombi in the heart correlated well with the decrease over time in platelet numbers in peripheral blood. Immunohistologic data further demonstrated a rapid accumulation of the exogenous human rVWF in the myocardium within minutes after application. The multifocal distribution pattern of myocardial necrosis and hemorrhage correlated well with microvascular thrombosis.13 The hemorrhages seen in our model are reminiscent of those seen in humans after reperfusion of infarct areas13 and thus had most likely occurred after dissolution of small thrombi and reconstitution of blood flow. Our data and those from Feys et al26 echo an earlier suggestion33 that routine measurement of troponin levels in TTP patients on admission may be worth considering. Furthermore, it is tempting to speculate that the heart is the first organ to be affected in the course of TTP onset.
Possible renal changes in mice were investigated by necropsy and pathohistology. The incidence of tubular necrosis in the acute phase was higher in rhVWF-treated animals than in untreated controls, but tubular regeneration was seen at late time points. However, thrombotic glomerular capillaries were absent and platelet aggregates were only found sporadically. The renal lesions are therefore most likely not a direct cause of thrombotic events, as described in humans. We also did not find any hyaline thrombi in brain tissues of rhVWF-treated ADAMTS13 KO mice. Some of the clinical symptoms observed in these mice might indicate neurologic deficits, including behavioral depression and cramps. However, these symptoms could also be caused by the poor overall condition of the animals because of lesions found in the heart. Human TTP patients also often show neurologic deficits in a wide range of severity. The presence of hyaline thrombi in the kidney and brain was noted in one individual animal in the baboon model for acquired TTP, but no clinical symptoms were observed.26
Because all mice included in the work presented survived the single-dose treatment with 2000 U/kg of rVWF and returned to normal after 14 days, the thrombi formed in the microvasculature did not lead to terminal organ failure despite significant occlusion of the capillaries. Based on the VWF:Ag levels measured, the portion of VWF that had not been entrapped in hyaline thrombi was reduced to 13% of the initial load within 24 hours, allowing newly formed platelets to compensate for the transient thrombocytopenia. The reversible nature of the rhVWF-induced effect resembles the pathogenic course of the ADAMTS13-inhibited baboons, which also exhibited a remarkable recovery on disappearance of the antibody.26
In summary, the clinical pathology of our mouse model resembles that of human TTP in many ways, including a progressive severe thrombocytopenia, increased LDH, and hemolytic anemia. The myocardial lesions are also consistent and represent the lesions observed in human patients diagnosed with TTP well. The frequent findings of lesions in the brain and kidneys of humans (but not in our model) might be because histopathologic examinations can only be conducted in humans by postmortem examination (ie, at the terminal stage of the disease), whereas we were looking at earlier and probably less severe events in the pathogenesis of TTP. It is worth noting in this context that microthrombi were also discernible in the brain and kidneys when ADAMTS13 KO mice were challenged with a higher rhVWF dose (4000 U/kg),18 indicating that the severity of the model (ie, thrombi prevalence exclusively in the heart or also in the brain and kidneys) can be adjusted by the rhVWF dose. Nonetheless, it remains entirely possible that quantitative differences exist between mice and humans in terms of the target organ affected. Alternatively, our mouse model may not reflect the situation in patients with TTP with regard to neurologic and renal symptoms.
Effect of rADAMTS13 in the rVWF-induced animal model of TTP
Patients with hereditary TTP are treated with fresh frozen plasma to supply the lacking enzyme with exogenous ADAMTS13. We explored the effect of human rADAMTS13 in our newly developed TTP model as a potential future substitute for human plasma, but obviously, other substances that interfere with VWF function could also be tested with ease. N-acetylcysteine, for instance, is proficient in reducing the size of VWF multimers by reduction of disulfide bonds.34 Another example is the aptamer ARC1779, which inhibits the interaction of VWF with its platelet receptor GpIba.35
When rhADAMTS13 (200 U/kg; Cmax of ∼ 2.5 U/mL) was administered prophylactically immediately before rhVWF (2000 U/kg; Cmax of 55 U/mL), none of the ADAMTS13-deficient animals showed clinical, hematologic, or pathologic signs of TTP. These data clearly indicate that rhADAMTS13 exerts a strong and expected protective effect, in line with the observed accelerated removal of the highest ULVWF multimers compared with untreated animals. The therapeutic efficacy of rhADAMTS13 was tested by administering 320 U/kg (Cmax ∼ 4.0 U/mL) up to 3 hours after rhVWF administration. TTP-related findings were only minor when rhADAMTS13 was administered 15 minutes after challenge with rhVWF, whereas after 180 minutes pathologic changes of various grades were noted, with the benefit of treatment being more evident in clinical than in histopathologic variables. After 30 minutes, the efficacy of treatment with rhADAMTS13 was in-between the early and the late time points. Thus, the incidence and severity of pathologic findings were reduced in a treatment interval-dependent manner. Whereas higher therapeutic doses of rhADAMTS13 than used here may result in a more pronounced dampening of the rhVWF-induced damage, the minimal effective prophylactic dose is likely to be lower than the one used in this study. It should be stressed, though, that preclinical models always have limitations in relation to human disease. The data generated here thus cannot be considered as the sole guidance for any therapeutic dosing of ADAMTS13 in potential future human applications.
In conclusion, we established a new mouse model of thrombotic microangiopathy showing symptoms caused by the presence of ULVWF multimers in the absence of ADAMTS13 in plasma, mimicking the situation in human TTP. Human rADAMTS13 acted as a prophylactic and therapeutic agent in this model of congenital TTP. Further work is required to determine whether this compound would also be of use in animal models of acquired TTP,26 as recent in vitro data seem to indicate.36
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all colleagues who additionally contributed to this study (B. Bischetsrieder, M. Resch, P. Leidenmuehler, W. Auer, D. Nehrbass, E. Farnleitner, E. Schweitzer, M. Vavra, A. Weber, M. Wolfsegger, A. Ghermai, H. Gritsch, B. Plaimauer, J. P. Lawo, M. Schuster, U. Berting, S. Rumpold, A. Peham, and S. Kubik), and E. Langdon-Neuner for editing the manuscript.
Authorship
Contribution: A.S. designed the protocol, performed the animal experiments, collected, analyzed, and interpreted data, and wrote the manuscript; K.W. performed pathologic and histopathologic assessment, collected, analyzed, and interpreted data, and wrote the manuscript; C.P. performed histology and immunohistologic staining and wrote the manuscript; B.D. and H.P.S. interpreted data and reviewed the manuscript for scientific content; W.H. and E.-M.M. supervised research, interpreted data, and reviewed the manuscript for scientific content; and H.R. and F.S. interpreted data, wrote the manuscript, and reviewed the manuscript for scientific content.
Conflict-of-interest disclosure: All authors are employees of Baxter Innovations GmbH.
Correspondence: Eva-Maria Muchitsch, Department of Preclinical Pharmacology & Toxicology, Baxter Innovations GmbH, Industriestrasse 67, 1221 Vienna, Austria; e-mail: eva_muchitsch@baxter.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal