Abstract
Treatments for immune thrombocytopenic purpura (ITP) providing durable platelet responses without continued dosing are limited. Whereas complete responses (CRs) to B-cell depletion in ITP usually last for 1 year in adults, partial responses (PRs) are less durable. Comparable data do not exist for children and 5-year outcomes are unavailable. Patients with ITP treated with rituximab who achieved CRs and PRs (platelets > 150 × 109/L or 50-150 × 109/L, respectively) were selected to be assessed for duration of their response; 72 adults whose response lasted at least 1 year and 66 children with response of any duration were included. Patients had baseline platelet counts < 30 × 109/L; 95% had ITP of > 6 months in duration. Adults and children each had initial overall response rates of 57% and similar 5-year estimates of persisting response (21% and 26%, respectively). Children did not relapse after 2 years from initial treatment whereas adults did. Initial CR and prolonged B-cell depletion predicted sustained responses whereas prior splenectomy, age, sex, and duration of ITP did not. No novel or substantial long-term clinical toxicity was observed. In summary, 21% to 26% of adults and children with chronic ITP treated with standard-dose rituximab maintained a treatment-free response for at least 5 years without major toxicity. These results can inform clinical decision-making.
Introduction
Rituximab is a chimeric monoclonal antibody (mAb) directed against CD20, an antigen (Ag) expressed on the surface of B lymphocytes1,2 but not present on most plasma cells. Once rituximab binds to the CD20 Ag on B lymphocytes, its Fc domain facilitates both complement and Ab-dependent B-cell lysis and Fc receptor–mediated clearance.3
Rituximab was initially developed to treat non-Hodgkin B-cell lymphoma in the early 1990s, and was licensed for this indication in 1997 within the United States. Since that time, it has been widely used in the treatment of autoantibody-mediated disorders. The initial hypothesis for its effect was that removal of autoreactive B-cell clones would lead to amelioration of clinical disease by reducing the level of or even eliminating circulating autoantibody. The autoimmune disorders treated with rituximab in addition to immune thrombocytopenic purpura (ITP)4-18 include systemic lupus erythematosus (SLE),19,20 vasculitis,20 rheumatoid arthritis (RA),21 autoimmune hemolytic anemia,11,12,22 cryoglobulinemia,23 acquired factor VIII Abs,24 IgM polyneuropathies,25 and thrombotic thrombocytopenic purpura.26
As of 2011, at least 17 studies of rituximab treatment in children and adults with ITP accruing a minimum of 5 patients each had been reported, totaling 492 patients4-18,27-29 (Table 1). The overall response rates, complete and partial, to initial treatment with rituximab (375 mg/m2 × 4) in adults and children with ITP were both 57% (Table 1). Two times as many responses in adults, as defined in the original reports,4-18,27-29 were complete responses (CR: platelet count > 150 × 109/L, 38%) as opposed to partial responses (PR: platelet count 50-150 × 109/L, 19%). In the 2 studies that assessed duration of effect according to type of initial response, patients achieving CRs had a greatly increased likelihood of maintaining their response at least 1 year from initial treatment compared with patients who achieved PRs.6,27
Published reports of adults and children with ITP treated with rituximab
| Author, Y . | Patients . | Response to treatment, N (%) . | |||
|---|---|---|---|---|---|
| Complete, > 100 × 109/L or > 150 × 109/L* . | Partial, 50-100 × 109/L or 50-150 × 109/L* . | None, < 50 × 109/L . | Total N response . | ||
| Published reports of adults with ITP treated with rituximab | |||||
| Braendstrup et al, 20055 | 35 | 6 (17) | 6 (17) | 23 (66) | 12 (34) |
| Cooper et al, 20046 † | 57 | 18 (32) | 13 (23) | 26 (45) | 31 (54) |
| Giagounidis et al, 20027 | 12 | 5 (42) | 4 (33) | 3 (25) | 9 (75) |
| Narang et al, 20038 | 6 | 3 (50) | 2 (33) | 1 (17) | 5 (83) |
| Penalver et al, 20069 | 83 | 38 (46) | 7 (8) | 38 (46) | 45 (54) |
| Saleh et al, 200010 | 13 | 2 (15) | 1 (8) | 10 (77) | 3 (23) |
| Narat et al, 200511 | 6 | 4 (67) | 1 (17) | 1 (17) | 5 (83) |
| Shanafelt et al, 200312 ‡ | 11 | 5 (45) | 1 (10) | 5 (45) | 6 (55) |
| Wang et al, 200513 | 4 | 4 (100) | 0 (0) | 0 (0) | 4 (100) |
| Zaja et al, 200314 § | 15 | 6 (40) | 2 (13) | 7 (47) | 8 (53) |
| Alasfoor et al, 200915 | 12 | 10 (83) | 2 (17) | 0 (0) | 12 (100) |
| Medeot et al, 200816 | 26 | 14 (54) | 4 (15) | 8 (31) | 18 (69) |
| Parodi et al, 200918 | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) |
| Godeau et al, 200827 | 60 | 21 (35) | 15 (25) | 24 (40) | 36 (60) |
| Garcia-Chavez et al, 200728 | 18 | 5 (28) | 5 (28) | 8 (44) | 10 (56) |
| Pasa et al, 200929 | 17 | 2 (12) | 8 (47) | 7 (41) | 10 (59) |
| Total | 376 | 144/376 (38) | 71/376 (19) | 161/376 (43) | 215/376 (57) |
| Published reports of children with ITP treated with rituximab | |||||
| Bennettt et al, 20064 | 36 | 8 (22) | 3 (8) | 25 (70) | 11 (31) |
| Mueller et al, 200917 (follow-up study) | |||||
| Penalver et al, 20069 | 6 | 3 (50) | 1 (17) | 2 (33) | 4 (66) |
| Wang et al, 200513 | 24 | 15 (63) | 2 (8) | 7 (29) | 17 (71) |
| Alasfoor et al, 200915 | 2 | 1 (79) | 0 (14) | 1 (7) | 1 (93) |
| Parodi et al, 200918 | 48 | 25 (52) | 8 (17) | 15 (31) | 33 (69) |
| Total | 116 | 52/116 (45) | 14/116 (12) | 50/116 (43) | 66/116 (57) |
| Author, Y . | Patients . | Response to treatment, N (%) . | |||
|---|---|---|---|---|---|
| Complete, > 100 × 109/L or > 150 × 109/L* . | Partial, 50-100 × 109/L or 50-150 × 109/L* . | None, < 50 × 109/L . | Total N response . | ||
| Published reports of adults with ITP treated with rituximab | |||||
| Braendstrup et al, 20055 | 35 | 6 (17) | 6 (17) | 23 (66) | 12 (34) |
| Cooper et al, 20046 † | 57 | 18 (32) | 13 (23) | 26 (45) | 31 (54) |
| Giagounidis et al, 20027 | 12 | 5 (42) | 4 (33) | 3 (25) | 9 (75) |
| Narang et al, 20038 | 6 | 3 (50) | 2 (33) | 1 (17) | 5 (83) |
| Penalver et al, 20069 | 83 | 38 (46) | 7 (8) | 38 (46) | 45 (54) |
| Saleh et al, 200010 | 13 | 2 (15) | 1 (8) | 10 (77) | 3 (23) |
| Narat et al, 200511 | 6 | 4 (67) | 1 (17) | 1 (17) | 5 (83) |
| Shanafelt et al, 200312 ‡ | 11 | 5 (45) | 1 (10) | 5 (45) | 6 (55) |
| Wang et al, 200513 | 4 | 4 (100) | 0 (0) | 0 (0) | 4 (100) |
| Zaja et al, 200314 § | 15 | 6 (40) | 2 (13) | 7 (47) | 8 (53) |
| Alasfoor et al, 200915 | 12 | 10 (83) | 2 (17) | 0 (0) | 12 (100) |
| Medeot et al, 200816 | 26 | 14 (54) | 4 (15) | 8 (31) | 18 (69) |
| Parodi et al, 200918 | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) |
| Godeau et al, 200827 | 60 | 21 (35) | 15 (25) | 24 (40) | 36 (60) |
| Garcia-Chavez et al, 200728 | 18 | 5 (28) | 5 (28) | 8 (44) | 10 (56) |
| Pasa et al, 200929 | 17 | 2 (12) | 8 (47) | 7 (41) | 10 (59) |
| Total | 376 | 144/376 (38) | 71/376 (19) | 161/376 (43) | 215/376 (57) |
| Published reports of children with ITP treated with rituximab | |||||
| Bennettt et al, 20064 | 36 | 8 (22) | 3 (8) | 25 (70) | 11 (31) |
| Mueller et al, 200917 (follow-up study) | |||||
| Penalver et al, 20069 | 6 | 3 (50) | 1 (17) | 2 (33) | 4 (66) |
| Wang et al, 200513 | 24 | 15 (63) | 2 (8) | 7 (29) | 17 (71) |
| Alasfoor et al, 200915 | 2 | 1 (79) | 0 (14) | 1 (7) | 1 (93) |
| Parodi et al, 200918 | 48 | 25 (52) | 8 (17) | 15 (31) | 33 (69) |
| Total | 116 | 52/116 (45) | 14/116 (12) | 50/116 (43) | 66/116 (57) |
ITP indicates immune thrombocytopenic purpura.
Complete and partial response criteria were defined in the original reports for each patient series listed.
Patients from Stasi et al51 included here.
Actual patient count is 12, but one patient died only 6 days posttreatment and was removed from analysis.
Patients from Zaja et al52 included here.
Four studies16,18,27,30 (Table 1) preliminarily assessed the durability of response in patients with ITP. The largest study, with 60 initially treated adults, reported a 43% 1-year response rate and a 40% 2-year response rate.27 In considering these 4 studies, the numbers of responders with which to address long-term outcome are relatively few because only approximately one-half of the patients achieve any response and the duration of follow-up has been limited in these responders.
The study reported here focuses on the duration of response in patients who had already demonstrated initial responses to rituximab. Using a relatively large cohort of 138 responders, a relapse-free 5-year sustained response rate in both children and adults with refractory ITP was projected and clinical and immunologic correlates of long-term response were assessed.
Methods
Patients
A total of 138 patients, 72 adults and 66 children, younger than 18 years of age, from 7 centers (Weill Cornell Medical College, New York, NY; Hôpital Henri Mondor, Paris, France; Regina Apostolorum Hospital, Albano Laziale, Italy; Charité Universitiy Hospital, Berlin, Germany; University of Torino, Torino, Italy; Washington University School of Medicine, St Louis, MO; Boston Children's Hospital, Boston, MA) consented in accordance with the Declaration of Helsinki to participate in this institutional review board (IRB)–approved, long-term follow-up study. Because rituximab is not approved for the treatment of ITP in the United States and abroad, IRB approval had been obtained for the initial treatments as required by local authorities. Amendments with separate consents were obtained specifically for the follow-up study at each site. For all patients treated, rituximab was used as part of the local practice of the time for ITP patients who had failed at least one other therapy, with platelet count < 30 × 109/L. The primary treatment reports describing the great majority of these patients' initial responses to rituximab have been published.4,6,13,16,17,30,31
Treatment
All patients received the standard dose of rituximab (MabThera, Roche; Rituxan, Genentech; and Biogen Idec) of 4 weekly infusions of 375 mg/m2/infusion except children treated at Charité in Berlin (12 children) who received a single dose of 375 mg/m2 of rituximab.31 In patients receiving more than one course of rituximab, only response to the initial course of rituximab was considered. All patients had baseline platelet counts < 30 × 109/L. CRs were defined as platelet count > 150 × 109/L, and PRs were defined as platelet count 50-150 × 109/L. Relapse of ITP was defined as institution of new, platelet-directed treatment. Because of the retrospective nature of this study, it was not possible to adopt the recently described standard terminology and criteria for responses and duration of ITP.32
Eligibility
Adults with ITP were included in this report only if their responses to rituximab were documented to be ongoing (platelet count ≥ 50 × 109/L) 1 year from their first of 4 infusions without additional ITP treatment. In contrast, children who achieved a partial or complete response of any duration were included in this study. Consecutive patients were enrolled at each participating center. For the current long-term follow-up study, a specific patient history questionnaire was completed by telephone, chart review, and/or visit to the outpatient department. Only verified platelet counts were used to assess duration of response; extrapolation was not allowed.
Literature review
Patients included in the literature review (Table 1) were identified from peer-reviewed journals written in English indexed in PubMed (National Center for Biotechnology Information, National Institutes for Health) as of June 2009 (more recent reports have been limited and did not change the primary findings). Only reports of 5 or more patients were included; abstracts were not included.
Immunophenotyping
Serial immunophenotyping was performed on freshly drawn heparinized peripheral blood from a limited number of initially treated patients at a single center (Weill Medical College of Cornell University). Immunophenotyping was conducted as part of the initial, IRB-approved studies of rituximab treatment at this site. Flow cytometry (FACSCalibur; BD Pharmingen) was used to assess expression of cell-surface markers on whole blood. Two- and 3-color staining was carried out for 15 minutes at room temperature and samples were fixed with 1.5% paraformaldehyde. Specific lymphocyte subsets were identified using combinations of mAbs to lymphocyte surface Ags directly conjugated to the fluorochromes FITC, PE (both from BD Biosciences), and either peridinin chlorophyll protein A (PerCP) or PE- Cy-5 (Tri-Color; Caltag Laboratories). For each analysis, 15 000 events were acquired in the lymphocyte gating region using CellQuest software. The lymphocyte gating region was determined using the forward versus side light scatter gate and the fluorescent gate by CD45FITC/CD14PE (Leukogate; BD Biosciences). Gate purity always exceeded 95% and all data are shown as corrected values. T lymphocytes were assessed as CD3+CD4+ and CD3+CD8+, and B lymphocytes as CD3−/CD19+.
Statistical analysis
Kaplan-Meier analysis of response duration past 1 year in adults and children was performed using SPSS Version 19. A subject was considered censored for Kaplan-Meier survival analysis if the patient was lost to follow-up or died while still in remission.
Response rate in children.
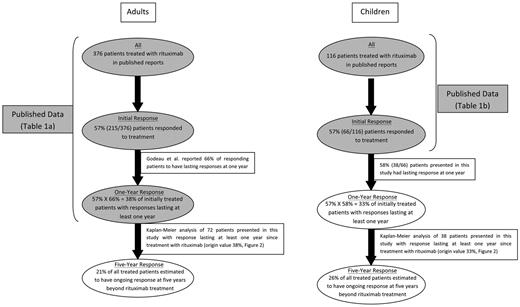
To compute the long-term response rate to rituximab in children, the initial overall response rate was taken from published reports of 116 children treated with rituximab (Figure 1).
Long-term response rates in adults and children treated with rituximab: estimates incorporating published reports and data from this report. Shaded ovals represent data derived from published reports (Table 1). Open ovals represent data presented in this report.
Long-term response rates in adults and children treated with rituximab: estimates incorporating published reports and data from this report. Shaded ovals represent data derived from published reports (Table 1). Open ovals represent data presented in this report.
Response rate in adults.
Published reports describing both the initial overall response rate to rituximab in adults and the 1-year response rate were used in computation of the long-term response rate in adults (Figure 1). Of 376 adults whose rituximab treatment outcomes were reported, 215 patients (57%) responded initially. Unfortunately, response duration was not available in many of the reported patients. In the largest available series assessing durability of response, 66% of initially responding adults, or 38% of all initially treated adults, were reported to have a response lasting at least 1 year.27
SPSS Version 19 was used to perform Cox regression analyses that explored associations between duration of response lasting > 1 year with age, sex, splenectomy, duration of prior ITP, current status of response, and type of initial response. In addition, the rate of B-cell return (absolute peripheral blood CD19+ cells) was investigated in 32 adults treated and followed at the Weill Cornell Medical College site. CD19+ B-cell counts (× 109/L) were measured at 50-day intervals in patients who relapsed 1 year after rituximab treatment (n = 14) and patients responding over 2.5 years without relapse (n = 18). Linear functions describing the mean rate of B-cell return in individual patients were used to compare the average linear rate of B-cell return in relapsers and responders > 2.5 years.
Results
Study population
A total of 138 patients, treated from 2000 to 2007 with rituximab 375 mg/m2 × 4 at 6 centers and 375 mg/m2 × 1 for children at one center, were included in this report. The 72 adults were drawn from a much larger number of treated patients; adults were only included if they had a platelet response to rituximab (platelet count ≥ 50 × 109/L) of at least 1 year's duration. The 66 children were also drawn from a larger group of treated patients, but were selected differently; all children who had achieved an initial PR or CR to rituximab of any duration were included.
Subject characteristics
The responder patient population at the time of the first rituximab infusion ranged from 2 to 78 years of age, including 66 children (median age 12.2 years) and 72 adults (median age 39.0 years); ∼ 60% were female (Table 2). The median duration of ITP before rituximab was 3 years for adults and 20 months for children. Twenty-three adults (32%) and 10 children (15%) had undergone splenectomy before rituximab treatment. Of 138 patients, 130 had previously received at least 2 different treatments for their ITP.
Baseline characteristics of the ITP patients with platelet responses to rituximab
| . | Adults . | Children . | Adults sustaining responses without relapsing . | Children sustaining responses without relapsing . |
|---|---|---|---|---|
| No. of patients | 72 | 66 | 46 | 32 |
| Median age, y (range)* | 39 (18-78) | 12 (2-17) | 35 (18-78) | 13 (2-17) |
| Female sex, n (%) | 47 (65) | 40 (61) | 29 (63) | 21 (66) |
| Median duration of ITP (range) | 3 y (1 mo-39 y) | 20 mo (1 mo-9 y) | 3 y (1 mo-39 y) | 20 mo (1 mo-9 y) |
| Splenectomized, n (%) | 23 (32) | 10 (15) | 13 (28) | 4 (13) |
| Type of initial response, n (%) | ||||
| Complete response (%) | 55 (76) | 45 (68) | 38 (83) | 26 (81) |
| Partial response (%) | 17 (24) | 21 (32) | 8 (17) | 6 (19) |
| . | Adults . | Children . | Adults sustaining responses without relapsing . | Children sustaining responses without relapsing . |
|---|---|---|---|---|
| No. of patients | 72 | 66 | 46 | 32 |
| Median age, y (range)* | 39 (18-78) | 12 (2-17) | 35 (18-78) | 13 (2-17) |
| Female sex, n (%) | 47 (65) | 40 (61) | 29 (63) | 21 (66) |
| Median duration of ITP (range) | 3 y (1 mo-39 y) | 20 mo (1 mo-9 y) | 3 y (1 mo-39 y) | 20 mo (1 mo-9 y) |
| Splenectomized, n (%) | 23 (32) | 10 (15) | 13 (28) | 4 (13) |
| Type of initial response, n (%) | ||||
| Complete response (%) | 55 (76) | 45 (68) | 38 (83) | 26 (81) |
| Partial response (%) | 17 (24) | 21 (32) | 8 (17) | 6 (19) |
ITP indicates immune thrombocytopenic purpura.
Children were included starting at the time of their response whereas adults were only included if their response duration was at least 1 year. This minimally biases the results of the median age towards an increased age at 1 year because children who relapse during the first year are removed but no adults are removed.
Response rate in children
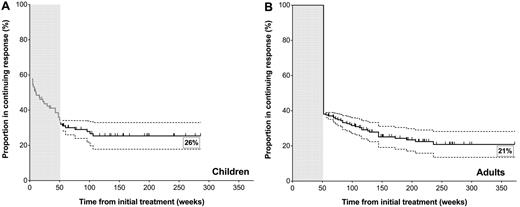
Of the 66 children who had an initial response to rituximab, 45 (68%) had CRs and 21 (32%) had PRs. Of these 66 responders, 38 (58%) sustained platelet counts ≥ 50 × 109/L for at least 1 year after rituximab treatment, including 28 of 45 of the initial CRs and 10 of 21 of the initial PRs; 1-year outcomes of the 12 children (treated at Charité in Berlin) receiving a single dose of 375 mg/m2 rituximab appeared similar to those of children receiving 4 treatments. Only 6 of the 38 children (2 CRs and 4 PRs) relapsed > 1 year from initial treatment, all within ∼ 2 years (Figure 2A). Therefore, children with a response lasting at least 1 year had a > 80% chance (32 of 38 children) of having their response last at least 2 years. Twenty children had ongoing responses of at least 2 years with a median follow-up of 3.1 year (range, 2.25-5.5 years); 6 of the patients had ongoing responses at 5 years. This trend was even more pronounced for children with CRs, only one of whom relapsed > 55 weeks from initial treatment.
Response duration in all children who responded to rituximab (however transiently) and adults whose response lasted at least 1 year since treatment. Kaplan-Meier estimates of response to rituximab past 1 year in (A) children (n = 38) and (B) adults (n = 72). Vertical lines along the Kaplan-Meier curve indicate the last follow-up of an ongoing response. Adult long-term response rate projection originated at 1 year, 38%, based on published reports (Figure 1). Children's long-term response rate projection originated at 1 year, 33%, based on published reports and data presented in this study (gray line; Figure 1). Error bars (dotted lines) represent the 95% confidence intervals of response projections.
Response duration in all children who responded to rituximab (however transiently) and adults whose response lasted at least 1 year since treatment. Kaplan-Meier estimates of response to rituximab past 1 year in (A) children (n = 38) and (B) adults (n = 72). Vertical lines along the Kaplan-Meier curve indicate the last follow-up of an ongoing response. Adult long-term response rate projection originated at 1 year, 38%, based on published reports (Figure 1). Children's long-term response rate projection originated at 1 year, 33%, based on published reports and data presented in this study (gray line; Figure 1). Error bars (dotted lines) represent the 95% confidence intervals of response projections.
Figure 2A incorporates the initial overall response rate derived from published reports (57%) and the 1-year response rate (33%) from the children in this study, yielding after subsequent relapses the estimated 5-year response rate (26%) of children with persistent-chronic ITP.
Response rate in adults
Seventy-two adults with a response to rituximab of at least 1 year's duration were included in this manuscript; 55 had CRs and 17 had PRs. The 46 adults with ongoing responses (64%; 38 CRs and 8 PRs) had a median follow-up of 3.79 years (range, 1.2-7.15 years).
Relapse of ITP was observed in 26 adults (36%; 17 CRs and 9 PRs) occurring at a median of 2.1 years (range, 1.1-4.5 years) from initial rituximab treatment (Figure 2B). In 22 of 26 of these patients (85%), relapse occurred between 1 and 3 years from initial treatment.
Based on published reports, 57% of adults with chronic ITP initially responded to rituximab (with either CR or PR); more than one-half of the responders had their response last at least 1 year, resulting in a 1-year response rate of 38%. As seen in this series, the 2-year response rate for all adults treated with rituximab was 31% and the 5-year response rate was 21%, illustrating the continuing (slow) rate of relapse (Figure 2B).
Relationship of clinical variables to response to rituximab
Splenectomy.
There was no difference in the projected 5-year outcomes in children and adults who had undergone splenectomy and those who had not. However, splenectomized patients tended to relapse earlier than nonsplenectomized patients; the only 4 adult patients who relapsed > 3 years from initial treatment had not undergone splenectomy.
Type of response.
PRs tended to relapse more often and earlier than CRs, even after a 1-year duration of response. After rituximab treatment, children with responses lasting at least 1 year demonstrated a 7% (2 of 28) relapse rate in those with CRs compared with 40% (4 of 10) in those with PRs (P < .05). Similarly, adults demonstrated a 31% (17 of 55) relapse rate in CRs compared with 53% (9 of 17) in PRs (P < .1).
Other clinical variables.
Neither age, sex, prior duration of ITP, nor response to other treatments of ITP was predictive of duration of response.
Laboratory variables.
In the long-term assessment of the subgroup of 32 adult patients followed at the Weill Cornell Medical College with serial measurement of lymphocyte subsets, B cells tended to reappear sooner in the peripheral blood and increased to higher levels in subjects who were going to relapse compared with those who maintained responses lasting > 2.5 years in duration (P < .05, Figure 3). None of the parameters of the baseline blood count (including the initial platelet count) or the starting lymphocyte subset profiles (eg, the CD3+ T cells, the CD4+ and CD8+ T-cell subsets, and the absolute B-cell number) had any relationship with duration of response to rituximab when considering only those patients whose response lasted at least 1 year.
B-cell repopulation after rituximab infusion in ITP patients with initial response > 1 year. Solid lines denote patients who relapsed after 1 year (n = 14), and dashed lines denote patients responding over 2.5 years without relapse (n = 18). Linear functions describing the mean rate of B-cell return in individual patients were used to calculate the average linear rate of B-cell return in those who relapsed (solid line) and in responders > 2.5 years (dashed line); dotted lines represent the SEM of these linear functions (P < .05). Curved solid and dashed trendlines represent the spline fit of the average number of CD19+ B cells (×109/L) in patients who relapsed after 1 year and those responding over 2.5 years (measured at 50-day intervals), respectively; curved trendlines were not used for statistical determinations.
B-cell repopulation after rituximab infusion in ITP patients with initial response > 1 year. Solid lines denote patients who relapsed after 1 year (n = 14), and dashed lines denote patients responding over 2.5 years without relapse (n = 18). Linear functions describing the mean rate of B-cell return in individual patients were used to calculate the average linear rate of B-cell return in those who relapsed (solid line) and in responders > 2.5 years (dashed line); dotted lines represent the SEM of these linear functions (P < .05). Curved solid and dashed trendlines represent the spline fit of the average number of CD19+ B cells (×109/L) in patients who relapsed after 1 year and those responding over 2.5 years (measured at 50-day intervals), respectively; curved trendlines were not used for statistical determinations.
Toxicity
Infusion-related reactions associated with rituximab were minimized by the addition of prednisone as premedication. Further, none of the 138 patients in this study experienced serious or severe long-term toxicity clearly related to B-cell depletion after rituximab. There was no evidence of an increased rate of serious or minor infections. Two 21-year-old women developed hypogammaglobulinemia 32 and 36 months after the first rituximab infusion. These 2 patients subsequently received substitutive treatment with monthly IV Ig infusions. One adult developed mild asymptomatic neutropenia > 4 years after rituximab treatment. A 29-year-old woman developed SLE with kidney involvement 36 months after the first rituximab infusion. The platelet counts remained normal in all 4 patients. No cases of progressive multifocal leukoencephalopathy (PML) or of hepatitis B reactivation were observed.
Discussion
Treatments of patients with chronic ITP which provide a curative effect without untoward toxicity or poor tolerability are highly desirable. They allow a patient to avoid the disadvantages of low platelet counts including continued platelet count monitoring, continued treatment, and possibly bleeding and/or fatigue. The use of rituximab has been an exciting development in ITP because patients may achieve complete responses lasting at least 1 year without additional treatment. However, robust data on the long-term response to rituximab are lacking for both children and adults.
This study specifically selected responders to rituximab to assess the duration of response 5 or more years from initial treatment. The estimates were derived separately for adults and children principally because ITP is hypothesized to reflect different pathologic processes in the 2 age groups. Further, reports describing the response to rituximab are substantially greater in adults. The estimated respective 21% and 26% 5-year sustained response rates help to provide clinicians and patients with realistic expectations of the long-term outcome of rituximab treatment.
Our data do not support the conclusion that a plateau in the incidence of relapse had been reached. Nonetheless, there are ongoing responses past the last relapse in 11 adults (beyond week 236) and in 20 children (beyond week 106). The longest ongoing response in this series was 7.15 years. The possibility of late relapses suggests that regular follow-up at no less than 2- to 3-month intervals is indicated for at least the first 3 years after the initial rituximab infusion in children, and possibly for 5 or more years in adults.
Existing long-term outcome data for patients with ITP treated with rituximab are limited to case reports and studies with limited numbers of long-term responders, except for a recent series providing 2-year follow-up in 24 responding adults.16-18,22,27,33 Of all other therapies for ITP, only splenectomy has been clearly shown to have a superior 5-year response rate, 60% to 65%.34,35 Data on long-term responses to rituximab in other autoimmune diseases are also very limited.20,36,37
Using a Cox regression model, long-term (> 1 year) responders achieving CR had significantly lower relapse rates than did those achieving PR. This trend had previously been observed within the first year after rituximab treatment in adults.6 Second, adult responders who later relapsed had their B cells return sooner and to higher levels than did those who never relapsed; this too was consistent with trends observed within the first year after treatment in adults (Figure 3).6 None of the other clinical variables analyzed substantially affected the long-term response rate. However, among patients who relapsed after 1 year from initial treatment, children and splenectomized patients tended to relapse sooner than did adults and nonsplenectomized patients. The children and splenectomized patients who relapsed did so within 2 years after treatment. The only other known clinical variable to affect response, not assessed in this study, is common variable immunodeficiency (CVID). Published data describing 33 patients with CVID and ITP receiving rituximab showed better long-term response rates than those with primary ITP.22
Why do infusions of rituximab and the resulting B-cell depletion lead to long-term immune tolerance in only 21% to 26% of patients? Autoreactive pre-B cells may persist in germinal centers38 and autoreactive pro-B cells and plasma cells may persist in the bone marrow; studies of B-cell depletion using a human-CD20 expressing mouse model support this “persistence” hypothesis.38 Additional studies are needed to understand why protracted depletion of B cells in the peripheral blood of patients with durable responses to rituximab is associated with dissolution of the autoimmune state. A recent report by Stasi et al demonstrated that B cells may be important in “sustaining” autoreactive T cells in patients with ITP.39 Prolonged depletion of B cells potentially facilitates “normalization” of T-cell subpopulations implicated in the persistence of the autoimmune condition in certain patients.39-42
Reported toxicities of rituximab use in ITP have included neutropenia, long-term infection, hematologic malignancies, and other isolated conditions.43,44 In SLE specifically, long-term use of rituximab has been linked to respiratory problems, hypogammaglobulinemia, and 2 cases of PML.45 Four cases of neutropenia, hypogammaglobulinemia, and SLE (32-48 months postinfusion in all 4 cases) were observed in the 138 patients in this study. ITP can precede other manifestations of Evans syndrome, CVID, and SLE. Especially in view of the late occurrence of complications in the 4 patients reported here, it is not clear how or whether they were related to rituximab. Hypogammaglobulinemia, a complication of rituximab treatment which may be more prevalent in very young patients treated for autoimmune hemolytic anemia,46 has also been infrequently reported in adults treated for ITP.47 Hypogammaglobulinemia could be a result of CVID developing subsequent to ITP.48 Although it appears that this complication is infrequent (2 cases in this study) and not associated with severe infection, survey of gammaglobulin levels after rituximab treatment may be warranted yearly for at least 3-5 years to assess the need for prophylactic IV Ig maintenance therapy.
Limitations
By using published reports to estimate initial overall response rates, we may have selected for a higher response rate based on publication bias. This would have resulted in an increased estimation of the long-term response rate. In addition, our determination of the 1-year response rate in adults is reliant to a limited extent on the single largest prospective, multicenter study assessing durability of response to ritixumab.27 However, the most robust approach to estimate the initial overall response rate in all patients and the 1-year response rate in adults was to use a publication-based summary rate; the numbers calculated reflect our experience as well as that of others, and are largely based on consecutive patient series from studies performed by the authors of this manuscript. Because the follow-up information is derived from 7 centers located on 2 continents, the study does not represent a uniform, single-center experience and depends on accurate reporting from all centers being condensed into a uniform format. An advantage of this, however, is that this report describes by far the largest number of responders to rituximab and is not dependent on the findings of any one center. Finally, randomized long-term studies of rituximab compared with standard of care (currently in progress) are needed determine whether spontaneous remission of ITP partially contributes to the estimated 5-year response rate attributed to rituximab in this study.
Small pilot studies of ITP attempted to enhance the rituximab-induced 21% to 26% long-term response rates either by adding cytotoxic agents to standard dose rituximab (R-CVP, similar to R-CHOP) or by doubling the dose of rituximab; both approaches failed to yield a longer-lasting response.49 Studies using the combination of dexamethasone and rituximab suggest additive effects, but more data are needed.50 Maintenance therapy with rituximab may also be effective if additive toxicities are not seen. Ultimately, further understanding of the mechanism of action of rituximab and its potential interactions with the immune system, and other treatments, is needed to safely maximize the number and duration of long-term responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the patients and nurses who made this work possible. They thank Christopher Yurchuck for invaluable assistance in the preparation and submission of this manuscript.
This work was partially supported by National Institutes of Health grant NIH1U01HL72196-01-05 (for the Transfusion Medicine and Hemostasis Network), the Children's Cancer & Blood Foundation, and Dana Hammond Stubgen.
National Institutes of Health
Authorship
Contribution: Vivek L. Patel designed the study, collected and analyzed data, and wrote the manuscript; M.M., R.S., B.G., J.K., E.N., T.T., U.R., S.S., and P.B. performed the clinical trials, contributed patient information, and reviewed the manuscript; S.Y.L. analyzed data and wrote and reviewed the manuscript; S.C.-R. performed laboratory testing, participated in data analysis and reviewed the manuscript; M.J.W. performed statistical analyses and reviewed the manuscript; N.M. and Vinay L. Patel collected data and reviewed the manuscript; M.L. performed statistical analyses and reviewed the manuscript; N.C. helped with the design of the study and reviewed the manuscript; and J.B.B. designed the study, performed the clinical trials, reviewed the data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: B.G. had consultancy within the past 2 years with Amgen France and LFB France (Laboratoire de fractionnement et de biotechnologie), and has received research funding from Amgen France and Roche France. N.C. received honorarium from GlaxoSmithKline (GSK), from Eisai for consultant work, and from GSK and Amgen for speaking at educational meetings. J.B.B. was a consultant for, and on the advisory board of, Amgen, GSK, Ligand, and Baxter; was as investigator for Amgen, GSK, Ligand, Biogen-Idec, Cangene, Genentech, Genzyme, Immunomedics, and Eisai Sysmex; and is a stockholder (family) in Amgen and GSK. The remaining authors declare no competing financial interests.
Correspondence: Vivek L. Patel, Department of Pediatrics, Division of Hematology/Oncology, Platelet Disorders Research and Treatment Program, Weill Medical College of Cornell University, 525 E 68th St, Rm P695, New York, NY 10065; e-mail: vivek.patel@med.einstein.yu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal