It has been known for more than 130 years that mammalian red cells lack a nucleus and, thus, differ fundamentally from the red cells of fish, birds, and reptiles that maintain their nucleus caged in a network of intermediate filaments.1 However, the process of erythroblast enucleation has remained provocative and poorly understood. In this issue of Blood, Konstantinidis et al provide evidence that erythroblast enucleation is a more complex and multistep process than previously thought, involving sequential actions of tubulin and filamentous actin, as well as lipid raft formation.2
Pioneering studies of late-stage murine erythroblasts indicated that a filamentous actin ring forms between the nascent reticulocyte, which contains the majority of the cytoplasm, and the nascent “extruded nucleus” or pyrenocyte, which consists of the nucleus surrounded by a cell membrane and thin rim of cytoplasm3 (see figure). Subsequent studies indicated that microtubules as well as an actin ring regulated by Rac GTPases may be involved in erythroblast enucleation.4,5 Recently, a role for nonmuscle myosin IIB in both cytokinesis and enucleation of human erythroblasts has been postulated.6 Other investigators have found no functional role for microtubules during enucleation, but rather have provided evidence that membrane vesicle trafficking is important for enucleation and stressed the differences between cytokinesis and enucleation.7,8
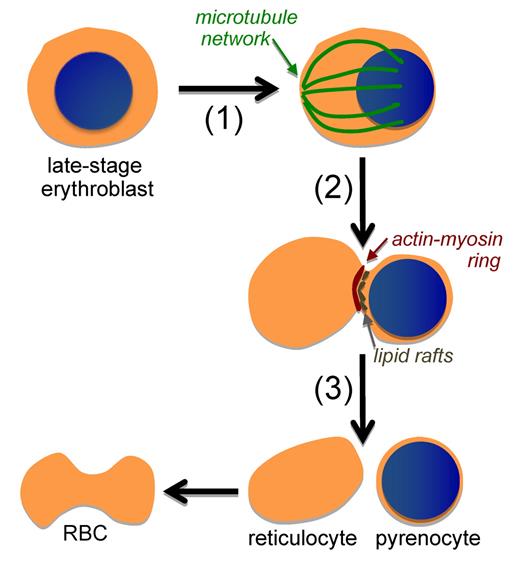
Red blood cell (RBC) formation in mammals requires the enucleation of late-stage erythroblasts. This process is now recognized to be a multistep one that includes (1) eccentric positioning of the nucleus associated with a microtubule network, (2) the pinching of the erythroblast associated with a localized actin-myosin ring and lipid rafts, as well as endocytic vesicle formation (not shown), and (3) the formation of 2 cells: a pyrenocyte that is rapidly engulfed by macrophage cells and a reticulocyte that continues to mature into a biconcave RBC.
Red blood cell (RBC) formation in mammals requires the enucleation of late-stage erythroblasts. This process is now recognized to be a multistep one that includes (1) eccentric positioning of the nucleus associated with a microtubule network, (2) the pinching of the erythroblast associated with a localized actin-myosin ring and lipid rafts, as well as endocytic vesicle formation (not shown), and (3) the formation of 2 cells: a pyrenocyte that is rapidly engulfed by macrophage cells and a reticulocyte that continues to mature into a biconcave RBC.
The process of erythroblast enucleation has been particularly difficult to study because it normally occurs very rapidly, taking less than 10 minutes to complete, and because enucleation rates can be notoriously low when erythroblasts are differentiated in vitro. Konstantinidis et al make innovative use of a novel technology, imaging flow cytometry,9 to visualize and investigate erythroblasts undergoing these evanescent events. They provide evidence that a tubulin network forms before enucleation and is associated with eccentric polarization of the nucleus within the erythroblast. Subsequently, an actin/myosin ring, mediated by Rac GTPases, is visualized and found to be partially necessary for efficient enucleation to occur. Taking cues from the study of cytokinesis, the authors also find that lipid rafts are distributed between the nascient reticulocyte and pyrenocyte and that inhibition of lipid raft organization decreased enucleation rates, suggesting a functional role for lipid rafts in the enucleation process.
The studies presented here argue for several similarities between the processes of cytokinesis and enucleation, because both involve the physical action of microtubules, as well as an actin-myosin ring, and result in the generation of 2 daughter cells. Future studies are needed to continue to define not only the similarities but also the differences between cytokinesis and erythroblast enucleation. What are the specific myosin isoforms present in erythroblasts and how do they physically interact with actin? Are actin/myosin polymerization and lipid raft formation connected to endocytic vesicle trafficking? Is there a role for mitochondria, which have also been found to line up at the junction between the nascent reticulocyte and pyrenocyte, in the enucleation process?10 Finally, can studies of other enucleating cell types, such as keratinocytes and lens epithelium, shed light on the mechanisms by which embryonic, fetal, and postnatal mammalian erythroblasts generate both pyrenocytes destined for a rapid demise and reticulocytes destined to remodel their membrane cytoskeleton and digest away their remaining organelles to become a fully functional red cell?
Conflict-of-interest disclosure: The author declares no competing financial interests. ■