In this issue of Blood, Terrier and colleagues report on the largest cohort of patients with type II mixed cryoglobulinemia.1 Steroids and rituximab appear to be the most effective therapy.
More than 100 patients were treated with rituximab and almost 100 patients were treated with an alkylating agent. A higher response rate was seen with rituximab and a corticosteroid compared with corticosteroids as a single agent or an alkylating agent with a corticosteroid. The end points were clinical, renal, and immunologic and not hematologic surrogates.1 Excluded from this report are those patients who had cryoglobulinemia associated with hepatitis.
By convention, when a precipitate is detected after storage for 7 days at 4°C, the precipitate after iced saline washing undergoes immunoelectrophoresis. There are 3 possibilities. The first is that all of the cryoglobulin is a monoclonal protein (type I). This cohort was excluded from the current report because patients with only a discrete monoclonal protein frequently have a B-cell lymphoid, lymphoplasmacytic or plasmacytic dyscrasia. In these patients, cytotoxic chemotherapy is standard.2 Many patients possessing type I cryoglobulins are found incidentally while screening patients with multiple myeloma who do not possess clinical signs or symptoms.
When immunoelectrophoresis reveals only polyclonal immunoglobulins, this is referred to as type III cryoglobulinemia. It is unclear in my mind whether this is an actual clinical entity with a specific clinical syndrome. The most challenging problem is when immunoelectrophoresis demonstrates a complex composed of both a monoclonal protein and polyclonal immunoglobulins, so-called “mixed” cryoglobulinemia (type II).3 In the majority of patients, the monoclonal protein is IgM, the polyclonal immunoglobulins are IgG; therefore, the mixed cryoglobulin has by definition high titer rheumatoid factor activity, and patients have been misdiagnosed as having rheumatoid vasculitis because of a failure to recognize the rheumatoid factor activity inherent in all mixed cryoglobulins.
Of 43 426 monoclonal gammopathies seen at Mayo Clinic over the 50-year period from 1960 to 2010, cryoglobulinemia was diagnosed in 461 (1.1%). In the Mayo Clinic experience, all mixed cryoglobulins had an IgM monoclonal protein 98% κ light chain, 2% λ light chain, and rheumatoid factor positivity was present in all type II patients in whom it was measured.4
The etiologies underlying the development of a type II mixed cryoglobulin are many. In Europe, hepatitis C is responsible for the majority, although patients with hepatitis B and CMV infections have also been recognized. The management of hepatitis-associated cryoglobulinemia starts with treatment of the underlying hepatitis C infection with a combination of pegylated interferon α2b weekly and oral daily ribavirin for 6 to 12 months.5 The conundrum dealt with by Terrier and colleagues in the current article is how to manage those patients who have a type II cryoglobulin not associated with a viral infection, either associated with an underlying rheumatic disorder such as progressive systemic sclerosis or Sjögren syndrome or when no specific etiology (“essential” mixed cryoglobulinemia) is found.
Many patients with type II cryoglobulins have been reported to have coexisting lymphoproliferative disorders including lymphoplasmacytic lymphoma and MALT lymphoma. The frequency of finding an underlying lymphoproliferative disease is, in part, dependent on how aggressively one searches for a clonal B-cell process. All patients with type II cryoglobulins have a serum monoclonal IgM protein; by definition, there should be a source of clonal B-cells responsible for the synthesis of the monoclonal protein. Older studies that depended on bone marrow histology for the finding of clonal low-grade lymphoma infiltration could easily overlook small numbers of clonal cells. With the advent of immunohistochemistry, flow and PET scanning,6 looking for nodal uptake of fluorodeoxyglucose, a higher proportion of patients can be recognized as having a low-grade lymphoma with low tumor burden. One deficiency of the current article is that it is based on registry data, and there was inconsistent analysis of the patients' bone marrow, which makes the reported frequency with which lymphoma coexists suspect.
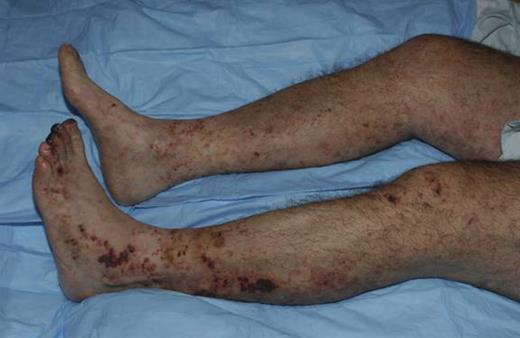
Cryoglobulinemia is a form of systemic vasculitis. The immune complex consisting of the monoclonal protein, polyclonal immunoglobulin, and complement deposits diffusely on endothelial surfaces, and the complement activation causes endothelial damage. The most common clinical manifestations are purpura, edema, neuropathy, and glomerulonephritis. In more advanced situations, livedo reticularis and cutaneous ulcers develop (see figure). Patients presenting with isolated purpura do not require systemic therapy. Purpura can be managed by support hose, and the purpura is primarily a cosmetic concern. Involvement of the kidneys leading to proteinuria and active urinary sediment manifests histologically as membranoproliferative glomerulonephritis or vasculitis-driven cutaneous ulcers require urgent therapy. Corticosteroids have been the mainstay of treatment of systemic vasculitis because of cryoglobulinemia and are effective. Responses can occur within 72 hours, but many patients become corticosteroid dependent. Rituximab was reported to be an effective therapy over 10 years ago,7 but up until this issue of Blood sufficient numbers of patients had not been treated to comparatively assess its efficacy.
The lymphocytes producing the IgM monoclonal protein are expected to strongly express CD20; thus the use of rituximab in this disorder is logical. At the Sixth International Workshop for Waldenström macroglobulinemia, it was reaffirmed that cryoglobulinemia was considered a variant of Waldenström macroglobulinemia characterized by IgM-mediated symptoms.8 The activity of rituximab in Waldenström macroglobulinemia is well-recognized, the expectation that rituximab would provide benefit in type II cryoglobulinemia is reasonable.9
Immunologic derangements have been described with cryoglobulinemia including a decreased percentage of naive B-cells and CD4-positive, CD25-positive, FOXP3-positive regulatory T cells. These immunologic abnormalities revert after rituximab therapy.10 It is unlikely that prospective randomized trials of the therapy of nonhepatitis-associated cryoglobulinemia will ever occur. It is reasonable to consider rituximab and corticosteroid the default standard of therapy for patients who do not have evidence of frank lymphoma and require therapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■