Abstract
Cytotoxic T lymphocyte–associated antigen 4 (CTLA4) has been known to be a strong tolerance-inducing inhibitory receptor on T-cell surface. Systemic blocking of CTLA4 function with blocking antibodies has been regarded as an attractive strategy to enhance antitumor immunity. However, this strategy accompanies systemic autoimmune side effects that are sometimes problematic. Therefore, we developed a novel CTLA4 mutant that could be expressed in tumor antigen-specific T cells to enhance antitumor effect without systemic autoimmunity. This mutant, named CTLA4-CD28 chimera, consists of extracellular and transmembrane domains of CTLA4, linked with cytoplasmic CD28 domain. Overexpression of CTLA4-CD28 chimera in T cells delivered stimulatory signals rather than inhibitory signals of CTLA4 and significantly enhanced T-cell reactivity. Although this effect was observed in both CD4 and CD8 T cells, the effect on CD4 T cells was predominant. CTLA4-CD28 chimera gene modification of CD4 T cells significantly enhanced antitumor effect of unmodified CD8 T cells. Nonetheless, the gene modification of CD8 T cells along with CD4 T cells further maximized antitumor effect of T cells in 2 different murine tumor models. Thus, CTLA4-CD28 chimera gene modification of both tumor antigen-specific CD4 and CD8 T cells would be an ideal way of modulating CTLA4 function to enhance tumor-specific T-cell reactivity.
Introduction
Cytotoxic T lymphocyte–associated antigen 4 (CTLA4) is an inhibitory receptor expressed on T-cell surface that is induced by T-cell activation to negatively regulate excessive activation of T cells.1 CTLA4-deficient mice develop lethal lymphoproliferative diseases within 3 to 4 weeks of birth.2 CTLA4 deficiency or anti-CTLA4 antibody treatment ameliorated induction of peripheral T-cell tolerance3 and exacerbated autoimmune diseases4,5 in mouse models. Therefore, CTLA4 is thought to play a critical role in maintaining peripheral T-cell tolerance in vivo to prevent autoimmunity. Because tumor cells also were known to induce T-cell tolerance to tumor antigens to resist to antitumor immunity,6 CTLA4 blockade was believed to be able to break tumor-mediated T-cell tolerance, resulting in enhanced antitumor immunity. Indeed, systemic administration of anti-CTLA4 blocking antibody in preclinical murine tumor models led to efficient regression of several syngenic tumors.7-9 Thus, blocking CTLA4 in human malignancies has been regarded as a promising therapeutic target of antitumor immunotherapy.
Therapeutic efficacy of 2 fully human anti-CTLA4 antibodies is currently being tested for several malignancies in clinical trials.10 For metastatic melanoma, anti-CTLA4 monotherapy or combination therapy with other agents shows an overall response rate of 5% to 15%. A recent study indicated that combination therapy of anti-CTLA4 plus dacarbazine, a chemotherapeutic agent, could improve the overall survival compared with dacarbazine monotherapy in a phase 3 randomized clinical trial.11 However, a significant proportion of patients who received anti-CTLA4 therapy developed grade III or IV autoimmune side effects, such as enterocolitis, skin rash, hypophysitis, hepatitis, and uveitis.12 Although most of these side effects could be managed by steroid treatment, several patients experienced the serious conditions such as intestinal perforation.13 The more intriguing observation was that the degree of autoimmunity was correlated with treatment efficacy.12 Thus, systemic blockade of CTLA4 signaling leads to enhanced antitumor immunity at the expense of significant auto-immunity. Therefore, selective blockade of CTLA4 signaling in tumor-specific T cells would be an ideal means to enhance antitumor immunity without systemic autoimmune side effects.
Adoptive transfer therapy of tumor antigen-specific T cells would be a good example of tumor-specific immunotherapy.14 Basically, tumor antigen-specific T cells from peripheral blood or tumor tissues are cultured, expanded in the presence of antigenic peptide plus antigen-presenting cells in vitro, and reinfused into the patients. Recently, this strategy has been shown to be successful in treating some malignancies such as EBV-positive lymphoma15 and metastatic melanoma.16,17 Especially, adoptive T-cell therapy after lymphodepletion achieved 50% to 70% response rate in recent clinical trials on metastatic melanoma.17 However, adoptive T-cell therapy has not been generalized to other types of tumors yet, and this may be partly because of inactivation of the infused T cells by tumor-induced T-cell tolerance.18 Therefore, generation of tumor-specific T cells resistant to tumor-mediated T-cell tolerance may enhance therapeutic efficacy of adoptive T-cell therapy.
After initial success of adoptive T-cell therapy, ex vivo genetic modification of T cells before infusion of the cells is emerging as a promising tool to enhance the functionality of the T cells.19 A cutting-edge approach in this field is the introduction of tumor antigen-specific T-cell receptor (TCR) genes into polyclonal T cells from peripheral blood that increases the number of tumor-reactive T cells, obviating the need for lengthy in vitro culture and expansion of endogenous tumor-reactive T cells.20 Hybrid receptors composed of antitumor-antigen antibody linked with signaling portion of TCR complex, called chimeric antigen receptor (CAR), also have been used extensively for the same purpose.21 These 2 approaches are currently being evaluated in early clinical trials. In addition, genes coding for cytokines, chemokines, and antiapoptotic small interfering RNA have been used for modification of T cells to enhance survival and trafficking of the T cells.22-24
In this study, we attempted to selectively inhibit CTLA4 function in tumor-specific T cells by ex vivo genetic modification of T cells. To this end, we developed a novel CTLA4 mutant named CTLA4-CD28 chimera. This mutant was designed to deliver positive signal instead of the negative signal of CTLA4 and to compete with endogenous CTLA4 for binding to its ligand in a dominant-negative manner. Indeed, retroviral gene transfer of CTLA4-CD28 chimera to murine T cells enhanced cytokine production in vitro, and adoptive transfer of the gene-modified tumor antigen-specific T cells to tumor-bearing mice potentiated therapeutic efficacy of the T cells in 2 different syngenic tumor models. Thus, CTLA4-CD28 chimera gene modification of T cells provides a novel strategy to facilitate adoptive T-cell therapy by breaking CTLA4-mediated T-cell tolerance.
Methods
Mice and cell preparations
Pmel-1, OT-I, B6, and Thy1.1+ congenic B6 mice were obtained from The Jackson Laboratory. OT-II mice on a Rag1−/− background were from the Taconic Farms. All of the transgenic mice were on a B6 background. The mice were bred in a specific pathogen-free animal facility at the Research Institute National Cancer Center, Korea, and maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee. E.G7 lymphoma cells and B16-F10 (B16) melanoma cells (ATCC) were derived from B6 mice. Phoenix Eco and Phoenix GP cell lines were provided by Garry Nolan (Stanford University). For some experiments, CD4+CD25− T cells were purified by negative selection using CD4+CD25+ regulatory T-cell isolation kit (Miltenyi Biotec). The purity was ∼ 90% after selection.
Plasmid constructs
To generate CTLA4-CD28 chimera, the extracellular and transmembrane portion of murine CTLA4 (nucleotides 1-567) and the cytoplasmic portion of murine CD28 (nucleotides 529-657) were amplified by polymerase chain reaction (PCR) from the plasmids containing murine CTLA4 and CD28 cDNA (ImaGenes). The 2 amplified fragments were joined by blunt end ligation and were cloned into a cloning vector. Subsequently, CTLA4-CD28 chimera cDNA was cloned into pMIG-w retroviral expression vector25 (a gift from Yosef Refaeli, National Jewish Medical and Research Center). For CTLA4-decoy, the extracellular and transmembrane portion of CTLA4 (nucleotides 1-567) was amplified by PCR and was cloned into pMIG-w. All the sequences were confirmed by automated DNA sequencing.
Luciferase assay and Western blot
Jurkat T cells (1 × 107), mixed with retroviral expression plasmid, RE/AP luciferase plasmid (a gift from Arthur Weiss, University of California), and pRL-TK Renilla luciferase control plasmid for normalization (Promega), were electroporated at 250 V and 950 μF in a 0.4-cm-gap cuvette using Gene Pulser (Bio-Rad Laboratories) and allowed to recover for 24 hours before stimulation. For stimulation, a 96-well plate coated with goat anti–mouse IgG (5 μg/mL) plus anti–hamster IgG (5 μg/mL) overnight and washed and coated with anti-CD3 (1 μg/mL) along with normal hamster IgG or hamster anti–mouse CTLA4 (9H10; 2 μg/mL) for 2 hours at room temperature. Cells (1 × 105) were added to each well and incubated at 37°C for 6 hours followed by lysis. For some experiments, soluble anti-CD28 was added to the stimulation culture directly instead of plate-bound anti-CTLA4. Luciferase activity was measured with a luminometer using a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. Firefly luciferase activity was normalized to Renilla luciferase activity.
For Western blot analysis, the retroviral plasmids without luciferase plasmids were used in the above-mentioned transfection protocol. For stimulation of the transfected cells, the cells were treated either with mouse anti–human CD28 or with normal hamster IgG or hamster anti–mouse CTLA4 (2 μg/mL) for 10 minutes on ice, followed by cross-linking with goat anti–mouse IgG or anti–hamster IgG (5 μg/mL) for 10 minutes on ice. Then, cells were placed in a 37°C water bath for 30 minutes, and the reaction was stopped by adding ice-cold PBS. The cell lysates were subjected to SDS-PAGE, transferred to a nitrocellulose membrane (Millipore), and probed with anti–phospho-Akt or anti-Akt antibody (Cell Signaling Technology). HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were used to detect primary antibodies. Blots were visualized by chemiluminescence reaction using SuperSignal West Pico (Pierce Chemical).
Production and transduction of retrovirus
The retroviral plasmids and a plasmid encoding VSV-G cDNA (pMD.G) were transiently transfected into Phoenix GP cell line using Lipofectamine 2000 (Invitrogen). After 48 hours, the culture supernatant containing VSV-G pseudotyped retrovirus was harvested. Then, Phoenix Eco cell line was transduced with the retrovirus-containing supernatant overnight. After 3 to 5 days, green fluorescent protein (GFP)–positive Phoenix Eco cells were purified by a cell sorter (FACSAria; BD Biosciences) to generate stable cell lines for producing ecotropic retroviruses. The culture supernatant containing ecotropic retrovirus was harvested and then concentrated 10 fold using a centrifugal filter device (Amicon Ultra-15, 100-kDa cut-off; Millipore) for murine T-cell transduction.
For retroviral transduction of T cells, spleen cells from normal B6 mice or the transgenic mice were stimulated by plate-bound anti-CD3 (5 μg/mL; 145-2C11) and anti-CD28 (2 μg/mL; 37.51) antibodies or antigenic peptides. Twenty-four hours after stimulation, T cells were transduced with the concentrated retroviruses by centrifuging the cells at 1250g for 90 minutes (spin infection). This procedure was repeated once on the same day. During the spin infection, 6 μg/mL Polybrene (Sigma-Aldrich) was added to the culture supernatant, or the cells were transduced in a RetroNectin-coated plate (15 μg/mL; Takara) to enhance the transduction efficiency. Forty-eight hours after stimulation, the transduced T cells were transferred to a fresh medium containing 30 unit/mL mouse IL-2 (Invitrogen) and “rested” for 48 to 72 hours without further stimulation before functional analysis. After resting, more than 95% of the live cells were CD4 or CD8 T cells. Transduction efficiency was 50% to 90% as measured for GFP positivity for all cells tested (B6 splenocytes, OT-I, OT-II, Pmel-1, and purified CD4 T cells).
Cytokine ELISA, cell proliferation, and cell cytotoxicity assay (P-JAM test)
GFP-positive T cells purified by cell sorting (2 × 104/well) were stimulated with various concentrations of anti-CD3 antibody or antigenic peptides in the presence of irradiated splenocytes (2 × 105/well) for 48 hours. The cytokines in the supernatant were measured using ELISA sets (BD Biosciences) according to the manufacturer's instructions. To measure cell proliferation, 48-hour–stimulated cells were pulsed with [3H]thymidine for an additional 24 hours. Cells were harvested using a cell harvester, and radioactivity was counted in a Trilux 1450 scintillation counter (Wallac).
For cell cytotoxicity assay, the transduced Pmel-1 T cells were stimulated with 1μM hgp100 peptide in the presence of irradiated splenocytes for 48 hours. The various numbers of activated T cells were cocultured with B16 cells (1 × 104) for 20 hours and then washed off with PBS. The remaining B16 cells were pulsed with [3H]thymidine for 6 hours. Then, the cells were harvested and radioactivity was measured.
Adoptive cell transfer
B6 mice were injected subcutaneously with E.G7 cells (1-2 × 106) or B16 cells (1 × 105) on day 0. The retrovirus-transduced T cells were adoptively transferred into the mice on day 7. For the B16 melanoma models, the mice were lymphodepleted by nonmyeloablative (4 Gy) total body irradiation on the day of cell transfer. Tumor growth was measured using a caliper every 3 to 4 days, and their approximate sizes (square millimeters) were calculated using the following formula: length (millimeters) × width (millimeters) × π. The mice were euthanized when the tumor size exceeded 500 mm2. For IL-2 neutralization experiments, rat anti–mouse IL-2 antibody (clone JES6-1A12) and normal rat IgG were purchased from e-Bioscience and Sigma, respectively. For intracellular cytokine staining for the transduced T cells, the ex vivo–activated T cells were fixed and permeabilized (BD Cytofix/Cytoperm kit) and stained with PE-labeled anti–mouse IL-2 or IFN-γ (BD Biosciences).
Results
CTLA4-CD28 chimera enhances T-cell reactivity by positive conversion of the negative signal of CTLA4
To develop a dominant-negative mutant of CTLA4 to be expressed in T cells, we initially planned to generate a mutant with the intracellular signaling domain of CTLA4 deleted, a mutant that we called CTLA4 decoy. We expected that deliberate overexpression of this mutant in T cells would compete with endogenous CTLA4 for binding to its ligand, B7 on antigen-presenting cells (APCs), and hinder the negative signaling of endogenous CTLA4. However, this simple strategy turned out to have an innate pitfall, because T cells constitutively express a costimulatory molecule, CD28, on the cell surface that also binds to B7 on APCs (Figure 1A left). CD28-B7 engagement and the subsequent CD28 signaling are critical for initial T-cell activation on antigen recognition.26,27 Once T cells become activated, CTLA4 molecules are induced on the T-cell surface and compete with CD28 for binding to B7 to terminate stimulatory signal.1 In addition, B7-engaged CTLA4 delivers intracellular negative signal to inhibit further activation of T cells. Thus, overexpressed CTLA4 decoy in T cells would compete with CD28 before competing with CTLA4 and thereby would not activate but rather inhibit T-cell activation (Figure 1A middle). Hence, we decided to link intracellular stimulatory domain of CD28 with CTLA4 decoy to generate CTLA4-CD28 chimeric receptor (Figure 1A right). Conceptually, when overexpressed in T cells, CTLA4-CD28 chimera also may compete with endogenous CD28 for binding to B7. However, it will now deliver surrogate CD28 signal using intracellular CD28 domain, allowing the T cells to be activated properly. CTLA4-CD28 chimera will then compete with endogenous, newly up-regulated CTLA4 and further enhance the activation of T cells.
CTLA4-CD28 chimera delivers CD28 signals and enhances T-cell reactivity. (A) Conceptual design and mode of action of CTLA4-CD28 chimera (CTC28) and CTLA4-decoy (CTdc). + indicates stimulatory signal; −, inhibitory signal; and \\, abrogation of the signal. (B) Structure of retroviral constructs. EV is the empty retroviral vector (pMIG-w). CTC28 or CTdc cDNA was inserted in front of internal ribosomal entry site (IRES)–GFP cassette of pMIG-w. IRES-GFP cassette is used for tracing the virus-transduced cells. CTLA4 EC indicates extracellular domain of CTLA4 (amino acids 1-161); TM, transmembrane domain of CTLA4 (amino acids 162-189); CD28 CP, cytoplasmic domain of CD28 (amino acids 177-218); and LTR, long terminal repeat. (C-D) Jurkat T cells transiently transfected with the retroviral plasmids (10 μg), RE/AP luciferase plasmids (10 μg) and TK-Renilla luciferase plasmid (0.5 μg) were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 or with plate-bound anti-CD3 plus anti-CTLA4 or control IgG for 6 hours. Firefly luciferase activity was measured from cell lysates. Transfection efficiency was normalized with Renilla luciferase activity. (E) Jurkat T cells transfected with retroviral plasmids were stimulated with anti-CD28 or anti-CTLA4 cross-linked by secondary antibodies for 30 minutes. Cell lysates were immunoblotted with anti–phospho-Akt or anti-Akt. (F-G) GFP-positive T cells were sorted after retroviral transduction of activated splenic T cells from normal mice and 3-day resting in the absence of stimulation. Then, the cells were stimulated with various concentrations of anti-CD3 in the presence of irradiated splenocytes for 48 hours. [3H]Thymidine (1 μCi) was added to the culture for additional 24 hours to measure cell proliferation (F), or the supernatant was harvested to measure IFN-γ production by ELISA (G). (H) Transduced splenic T cells activated with anti-CD3 (1 μg/mL) in the presence of irradiated splenocytes for 48 hours as described in panels F and G were stained with APC-labeled anti-CD4 and PE-Cy5–labeled anti-CD8 along with PE-labeled anti-CTLA4 (intracellular staining), or anti-CD28 (surface staining), or relevant isotype control antibodies. Then, GFP-positive populations were analyzed by flow cytometry. (I) Transduced splenic T cells were stimulated with plate-bound anti-CD3 (1 μg/mL), anti-CD28 (2 μg/mL), or both in the presence or absence of anti-CTLA4 (10 μg/mL) cross-linked with plate-bound anti–hamster IgG (20 μg/mL). Seventy-two hours after stimulation, the supernatant was harvested to measure IFN-γ production by ELISA. Results are representative of 2 (E,H-I) or 3 independent experiments (C-D,F-G).
CTLA4-CD28 chimera delivers CD28 signals and enhances T-cell reactivity. (A) Conceptual design and mode of action of CTLA4-CD28 chimera (CTC28) and CTLA4-decoy (CTdc). + indicates stimulatory signal; −, inhibitory signal; and \\, abrogation of the signal. (B) Structure of retroviral constructs. EV is the empty retroviral vector (pMIG-w). CTC28 or CTdc cDNA was inserted in front of internal ribosomal entry site (IRES)–GFP cassette of pMIG-w. IRES-GFP cassette is used for tracing the virus-transduced cells. CTLA4 EC indicates extracellular domain of CTLA4 (amino acids 1-161); TM, transmembrane domain of CTLA4 (amino acids 162-189); CD28 CP, cytoplasmic domain of CD28 (amino acids 177-218); and LTR, long terminal repeat. (C-D) Jurkat T cells transiently transfected with the retroviral plasmids (10 μg), RE/AP luciferase plasmids (10 μg) and TK-Renilla luciferase plasmid (0.5 μg) were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 or with plate-bound anti-CD3 plus anti-CTLA4 or control IgG for 6 hours. Firefly luciferase activity was measured from cell lysates. Transfection efficiency was normalized with Renilla luciferase activity. (E) Jurkat T cells transfected with retroviral plasmids were stimulated with anti-CD28 or anti-CTLA4 cross-linked by secondary antibodies for 30 minutes. Cell lysates were immunoblotted with anti–phospho-Akt or anti-Akt. (F-G) GFP-positive T cells were sorted after retroviral transduction of activated splenic T cells from normal mice and 3-day resting in the absence of stimulation. Then, the cells were stimulated with various concentrations of anti-CD3 in the presence of irradiated splenocytes for 48 hours. [3H]Thymidine (1 μCi) was added to the culture for additional 24 hours to measure cell proliferation (F), or the supernatant was harvested to measure IFN-γ production by ELISA (G). (H) Transduced splenic T cells activated with anti-CD3 (1 μg/mL) in the presence of irradiated splenocytes for 48 hours as described in panels F and G were stained with APC-labeled anti-CD4 and PE-Cy5–labeled anti-CD8 along with PE-labeled anti-CTLA4 (intracellular staining), or anti-CD28 (surface staining), or relevant isotype control antibodies. Then, GFP-positive populations were analyzed by flow cytometry. (I) Transduced splenic T cells were stimulated with plate-bound anti-CD3 (1 μg/mL), anti-CD28 (2 μg/mL), or both in the presence or absence of anti-CTLA4 (10 μg/mL) cross-linked with plate-bound anti–hamster IgG (20 μg/mL). Seventy-two hours after stimulation, the supernatant was harvested to measure IFN-γ production by ELISA. Results are representative of 2 (E,H-I) or 3 independent experiments (C-D,F-G).
To test this hypothesis, we generated retroviral expression plasmids for CTLA4-CD28 chimera and CTLA4 decoy (Figure 1B). First, we tried to see whether CTLA4-CD28 chimera would deliver surrogate CD28 signal inside T cells using CD28 response element (RE/AP)–driven reporter assay in Jurkat T cells. When Jurkat cells transfected with empty plasmid plus RE/AP luciferase plasmid, were stimulated with agonistic anti-CD3 plus anti-CD28 antibody, luciferase activity greatly increased compared with that with anti-CD3 alone as reported previously,28 indicating that signals from endogenous CD28 were successfully delivered (Figure 1C). Likewise, when Jurkat cells were transfected with CTLA4-CD28 chimera plasmid plus RE/AP luciferase plasmid and stimulated with anti-CD3 plus cross-linked anti-CTLA4 antibody,29 a remarkable increase in luciferase activity was observed compared with anti-CD3 stimulation alone (Figure 1D). This result demonstrated that engagement of CTLA4-CD28 chimera with its extracellular ligand delivered CD28 signal inside the cell, consistent with our hypothesis. In contrast, transfection of the cells with CTLA4 decoy plasmid failed to enhance luciferase activity on anti-CD3 plus anti-CTLA4 treatment, suggesting that CTLA4 decoy did not transmit CD28 signal into the cells as expected. These effects were further examined by analysis of Akt phosphorylation that occurs immediately downstream of CD28 (Figure 1E second lane).28 When Jurkat cells transfected with CTLA4-CD28 chimera were stimulated with anti-CTLA4, Akt phosphorylation increased, whereas CTLA4 decoy-transfected Jurkat cells did not show any increase in Akt phosphorylation (Figure 1E). Thus, CTLA4-CD28 chimera converts negative signaling of CTLA4 to positive signaling similar to CD28 costimulation.
Next, we examined whether CTLA4-CD28 chimera enhances T-cell activation. CTLA4-CD28 chimera or CTLA4 decoy-encoding retrovirus was produced using retroviral expression plasmids generated as in Figure 1B and transduced into activated murine T cells. The retroviral plasmid was designed to coexpress GFP protein as an expression marker. Approximately 50% to 60% of the transduced T cells expressed GFP proteins as well as overexpressed CTLA4-CD28 chimera or CTLA4-decoy proteins (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The GFP-positive T cells were purified by cell sorting and stimulated with irradiated splenocytes plus anti-CD3 to measure proliferation and cytokine production. CTLA4 decoy-transduced T cells proliferated poorly and produced less IFN-γ compared with empty vector–transduced T cells (Figure 1F-G). In contrast, CTLA4-CD28 chimera–transduced T cells proliferated equivalently to control T cells, confirming that CTLA4-CD28 chimera delivered the surrogate costimulation to the T cells (Figure 1F). Moreover, these T cells showed a modest increase in cell proliferation in an early phase of T-cell activation (supplemental Figure 2). More strikingly, these T cells produced significantly greater amounts of IFN-γ than control cells (Figure 1G). The expression level of CTLA4-CD28 chimera was far higher than that of endogenous CTLA4 in activated T cells, suggesting that it may have a dominant-negative effect (Figure 1H). Consistently, when T-cell activation was inhibited by cross-linked anti-CTLA4 antibody, CTLA4-CD28 overexpression abrogated the inhibitory activity of anti-CTLA4 (Figure 1I). Overall, CTLA4-CD28 chimera fully compensated for the defect of CTLA4 decoy, and overexpression of CTLA4-CD28 chimera into T cells led to remarkable enhancement of T-cell activation, presumably through surrogate CD28 stimulation and dominant-negative effect on endogenous CTLA4.
CTLA4-CD28 chimera gene modification of CD8 T cells is not sufficient to suppress tumor growth
To test whether CTLA4-CD28 chimera overexpression in tumor antigen-specific T cells enhances their antitumor activity, we first transduced CTLA4-CD28 chimera-encoding retrovirus into CD8 T cells from the melanoma antigen-specific TCR transgenic mice called Pmel-1. Pmel-1 T cells recognize the tumor antigen gp100 that expresses in syngenic B16 melanoma cells.30,31 Although the transduced Pmel-1 T cells produced more IFN-γ than control Pmel-1 T cells in response to the antigenic peptide, they did not show any enhancement of cytolytic activity on B16 cells in vitro (Figure 2A-B). Consistently, when CTLA4-CD28 chimera–transduced Pmel-1 T cells were adoptively transferred to lymphodepleted B16 tumor-bearing mice in combination with high doses of IL-2,31 they did not enhance antitumor effect of the T cells in vivo (Figure 2C). Thus, in stark contrast to the data with polyclonal T cells from normal mice, CTLA4-CD28 chimera gene modification of Pmel-1 T cells did not show a huge enhancement of T-cell function.
Limited effects of CTLA4-CD28 gene modification on Pmel-1 T cells in vitro and in vivo. Empty vector–encoding (EV) or CTLA4-CD28 chimera–encoding (CTC28) retrovirus was transduced into activated Pmel-1 T cells with antigenic peptide, human gp10025-33 (KVPRNQDWL, 1μM). After 2 days of resting in the absence of antigen, GFP-positive T cells were purified by cell sorting. (A) GFP-positive T cells were stimulated with human gp10025-33 (1μM) in the presence of irradiated splenocytes for 48 hours, and the IFN-γ in the culture supernatant was measured by ELISA. (B) Forty-eight hour–stimulated GFP-positive T cells were cocultured with B16 melanoma cells for 24 hours. Cytotoxicity of the various ratios of T cells to B16 cells (killer/target ratio) were determined by P-JAM test. (C) GFP-positive T cells were expanded in the presence of IL-15 (10 ng/mL) and paramagnetic beads coated with anti-CD3 plus anti-CD28 for 7 days before adoptive transfer to the mice. B6 mice subcutaneously injected with B16 cells were lymphodepleted by total body irradiation (4 Gy) 7 days later, and then they were injected with expanded T cells (2 × 106) intravenously. Thereafter, recombinant human IL-2 (3 × 105 IU/head) was administered intraperitoneally twice a day for 3 days. Mean tumor size of at least 5 mice per group was recorded. Results are representative of 3 independent experiments.
Limited effects of CTLA4-CD28 gene modification on Pmel-1 T cells in vitro and in vivo. Empty vector–encoding (EV) or CTLA4-CD28 chimera–encoding (CTC28) retrovirus was transduced into activated Pmel-1 T cells with antigenic peptide, human gp10025-33 (KVPRNQDWL, 1μM). After 2 days of resting in the absence of antigen, GFP-positive T cells were purified by cell sorting. (A) GFP-positive T cells were stimulated with human gp10025-33 (1μM) in the presence of irradiated splenocytes for 48 hours, and the IFN-γ in the culture supernatant was measured by ELISA. (B) Forty-eight hour–stimulated GFP-positive T cells were cocultured with B16 melanoma cells for 24 hours. Cytotoxicity of the various ratios of T cells to B16 cells (killer/target ratio) were determined by P-JAM test. (C) GFP-positive T cells were expanded in the presence of IL-15 (10 ng/mL) and paramagnetic beads coated with anti-CD3 plus anti-CD28 for 7 days before adoptive transfer to the mice. B6 mice subcutaneously injected with B16 cells were lymphodepleted by total body irradiation (4 Gy) 7 days later, and then they were injected with expanded T cells (2 × 106) intravenously. Thereafter, recombinant human IL-2 (3 × 105 IU/head) was administered intraperitoneally twice a day for 3 days. Mean tumor size of at least 5 mice per group was recorded. Results are representative of 3 independent experiments.
CTLA4-CD28 chimera gene modification preferentially affects CD4 T cells over CD8 T cells
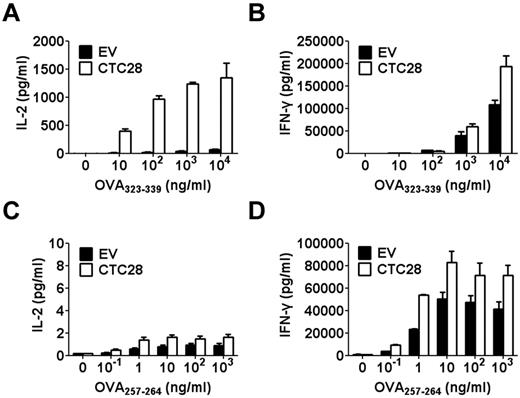
One of the differences between experiments in Figures 1G and 2 would be that T cells from normal mice were mixture of both CD4 and CD8 T cells, whereas Pmel-1 T cells are pure CD8 T cells. Therefore, we decided to check whether the tumor antigen-specific response of CD4 and CD8 T cells would be differentially regulated by CTLA4-CD28 chimera gene modification. To examine CD4 and CD8 T-cell responses to the same antigen, we adopted a new set of antigen-specific TCR transgenic mice called OT-I and OT-II. T cells from OT-I and OT-II mice are CD8 and CD4 T cells, respectively, that recognize ovalbumin peptides specifically. OT-I and OT-II T cells transduced with CTLA4-CD28 chimera–encoding retrovirus were stimulated with ovalbumin peptides in the presence of APCs, and production of cytokines was compared. CTLA4-CD28 chimera gene modification enhanced IFN-γ production in both OT-I and OT-II T cells (Figure 3B,D). IL-2 production of OT-I T cells also increased compared with control cells, although the amount of the cytokine produced was very small (Figure 3C). Strikingly, IL-2 production of OT-II T cells greatly increased (10-20-fold) compared with control cells (Figure 3A). Thus, although CTLA4-CD28 gene modification increased both CD4 and CD8 T cell responses, CD4 T-cell response was more affected by the gene modification than CD8 T-cell response, especially in terms of IL-2 production. This effect on CD4 T cells was only observed when they are activated by peptide and APCs and not by anti-CD3 and anti-CD28, suggesting that the ligand of CTLA4-CD28 chimera lies on the surface of APCs rather than on T cell themselves (supplemental Figure 3).
CTLA4-CD28 chimera gene modification preferentially affects CD4 T cells over CD8 T cells in vitro. EV or CTC28 retrovirus was transduced into activated OT-II or OT-I T cells with respective antigenic peptide, OVA323-339 (ISQAVHAAHAEINEAGR, 10 μg/mL) or OVA257-264 (SIINFEKL, 1 μg/mL). After 2 days of resting in the absence of antigen, GFP-positive T cells were purified by cell sorting. GFP-positive OT-II T cells (A-B) or OT-I T cells (C-D) were stimulated with various concentrations of antigenic peptides in the presence of irradiated splenocytes for 48 hours. IL-2 (A,C) or IFN-γ (B,D) secreted into the culture supernatant was measured by ELISA. Results are representative of 3 independent experiments.
CTLA4-CD28 chimera gene modification preferentially affects CD4 T cells over CD8 T cells in vitro. EV or CTC28 retrovirus was transduced into activated OT-II or OT-I T cells with respective antigenic peptide, OVA323-339 (ISQAVHAAHAEINEAGR, 10 μg/mL) or OVA257-264 (SIINFEKL, 1 μg/mL). After 2 days of resting in the absence of antigen, GFP-positive T cells were purified by cell sorting. GFP-positive OT-II T cells (A-B) or OT-I T cells (C-D) were stimulated with various concentrations of antigenic peptides in the presence of irradiated splenocytes for 48 hours. IL-2 (A,C) or IFN-γ (B,D) secreted into the culture supernatant was measured by ELISA. Results are representative of 3 independent experiments.
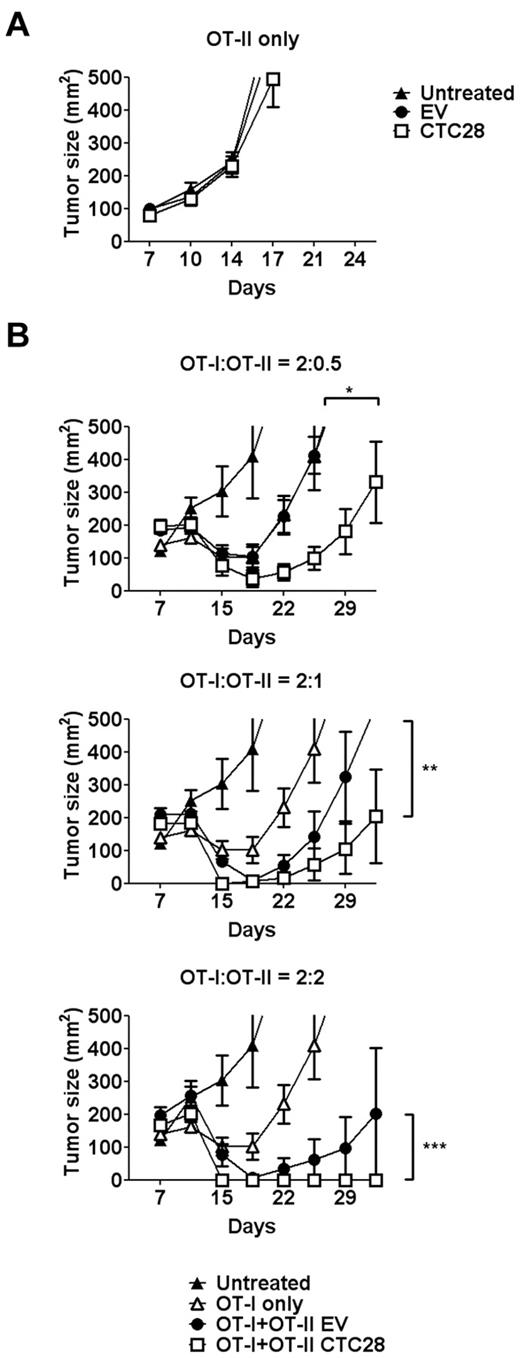
CTLA4-CD28 chimera gene modification enhances the therapeutic effect of both CD4 and CD8 T cells on model antigen-expressing tumors in vivo
It is well known that CD4 T cells are necessary for antitumor effect of CD8 T cells. Therefore, enhanced CD4 response to a tumor antigen can facilitate antitumor effect of CD8 T cells. Intrigued by the differential effect of CTLA4-CD28 chimera on CD4 T cells, we examined whether CTLA4-CD28 gene modification of CD4 T cells enhances antitumor effect of CD8 T cells, using the OT-I and OT-II T cells. To use ovalbumin as a model tumor antigen, syngenic EL4 lymphoma cell line transfected with ovalbumin cDNA (E.G7) was selected as a tumor model. When CTLA4-CD28–transduced OT-II T cells were adoptively transferred to E.G7-bearing mice, no tumor regression was observed, indicating that CD4 T cells cannot show antitumor effect in this model, without CD8 T cells (Figure 4A). Then, we injected OT-I T cells, alone or combined with varying numbers of the gene-modified OT-II T cells, to E.G7-bearing mice. OT-I T cells alone showed partial antitumor effect at the tested dose of T cells (Figure 4B). When the number of OT-II cells used was one fourth that of OT-I cells, empty vector–transduced OT-II cells did not show any additive effect on OT-1 T cells (Figure 4B top graph). In contrast, the gene-modified OT-II T cells further enhanced antitumor effect of OT-I T cells. When the number of OT-II T cells was increased, control OT-II T cells also showed enhanced antitumor effect in a dose-dependent manner (Figure 4B middle and bottom graphs). Again, the gene-modified OT-II T cells further enhanced antitumor effect, leading to the complete regression of tumors at the highest dose. Thus, CTLA4-CD28 gene modification of CD4 T cells enhanced CD4 help for the antitumor response of CD8 T cells.
CTLA4-CD28 chimera gene modification of antigen-specific CD4 T cells improves therapeutic effect of antigen-specific CD8 T cells in an antigen-expressing lymphoma model. (A) B6 mice were injected with E.G7 cells (1-2 × 106) subcutaneously. Seven days later, the mice were either left untreated or injected intravenously with EV-transduced OT-II T cells (EV; 2 × 106) or CTLA4-CD28 chimera–transduced OT-II T cells (CTC28; 2 × 106). (B) E.G7 tumor-bearing B6 mice as in panel A were either left untreated or injected intravenously with untransduced OT-I T cells (2 × 106) in combination with EV or CTC28 OT-II T cells in various ratios (0.5-2 × 106). The retrovirus-transduced T cells were rested for 48 hours in the absence of stimulus before adoptively transferred. Mean tumor size of at least 5 mice per group was recorded (*P = .0391, **P = .0078, ***P = .0078; Wilcoxon matched pairs test). Results are representative of at least 2 independent experiments.
CTLA4-CD28 chimera gene modification of antigen-specific CD4 T cells improves therapeutic effect of antigen-specific CD8 T cells in an antigen-expressing lymphoma model. (A) B6 mice were injected with E.G7 cells (1-2 × 106) subcutaneously. Seven days later, the mice were either left untreated or injected intravenously with EV-transduced OT-II T cells (EV; 2 × 106) or CTLA4-CD28 chimera–transduced OT-II T cells (CTC28; 2 × 106). (B) E.G7 tumor-bearing B6 mice as in panel A were either left untreated or injected intravenously with untransduced OT-I T cells (2 × 106) in combination with EV or CTC28 OT-II T cells in various ratios (0.5-2 × 106). The retrovirus-transduced T cells were rested for 48 hours in the absence of stimulus before adoptively transferred. Mean tumor size of at least 5 mice per group was recorded (*P = .0391, **P = .0078, ***P = .0078; Wilcoxon matched pairs test). Results are representative of at least 2 independent experiments.
Although CTLA4-CD28 gene modification affects CD4 T cells more than CD8 T cells in vitro, CD8 T-cell reactivity also increased by the gene modification (Figure 3D). Hence, we determined whether the gene modification of CD8 T cells in conjunction with CD4 T cells would further enhance antitumor activity of T cells. When the modified OT-I T cells were adoptively transferred to tumor-bearing mice without OT-II T cells, tumor regression was not enhanced just as with the modified Pmel-1 T cells (Figure 5A). However, in a condition that the gene-modified OT-II T cells showed a partial enhancement of antitumor activity of unmodified OT-I T cells, the gene modification of both OT-I and OT-II T cells further enhanced tumor regression (Figure 5B). Thus, double modification of CD4 and CD8 T cells maximized T cell–mediated antitumor effect.
Cotransfer of the gene-modified CD4 and CD8 T cells shows synergistic effect in an antigen-expressing lymphoma model. E.G7 tumor-bearing B6 mice as described in Figure 4 were either untreated or injected intravenously with EV-transduced or CTC28-transduced OT-I T cells (2 × 106) alone (A) or in combination with EV-transduced or CTC28-transduced OT-II T cells (1 × 106; B). Mean tumor size of at least 5 mice per group was recorded (*P = .0029; Wilcoxon matched pairs test). Results are representative of 2 (A) or 3 independent experiments (B).
Cotransfer of the gene-modified CD4 and CD8 T cells shows synergistic effect in an antigen-expressing lymphoma model. E.G7 tumor-bearing B6 mice as described in Figure 4 were either untreated or injected intravenously with EV-transduced or CTC28-transduced OT-I T cells (2 × 106) alone (A) or in combination with EV-transduced or CTC28-transduced OT-II T cells (1 × 106; B). Mean tumor size of at least 5 mice per group was recorded (*P = .0029; Wilcoxon matched pairs test). Results are representative of 2 (A) or 3 independent experiments (B).
To examine the reactivity of the gene-modified T cells in tumor-bearing mice, we isolated splenocytes from the mice into which the gene-modified OT-I and OT-II T cells had been infused, and we stimulated them ex vivo with ovalbumin peptides. The gene-modified OT-II T cells showed much higher percentages of both IL-2– and IFN-γ–producing cells than control OT-II T cells when analyzed by intracellular cytokine staining (Figure 6A). In addition, the total number of the gene-modified OT-II T cells increased compared with control OT-II T cells (Figure 6B). With the OT-I T cells, the percentage of the gene-modified OT-I T cells in the spleen was too low to allow analysis by intracellular cytokine staining (∼ 0.1%). Therefore, we purified total CD8 T cells and stimulated the equal numbers of the gene-modified and empty vector–modified OT-I T cells with ovalbumin peptide plus APCs in vitro, and we measured IFN-γ production by ELISA. Again, the gene-modified OT-I T cells produced larger amounts of IFN-γ than empty vector–modified OT-I T cells (Figure 6C). Thus, the gene-modified OT-I and OT-II T cells retained their enhanced reactivity in tumor-bearing mice as well as in vitro.
Adoptively transferred gene-modified CD4 and CD8 T cells retain enhanced reactivity to the antigens. Thy1.1-positive congenic B6 mice were subcutaneously injected with E.G7 cells (2 × 106). Seven days later, Thy1.2-positive retrovirus-transduced OT-I (1 × 107) and OT-II T cells (2 × 106) were adoptively transferred to tumor-bearing mice. After 3 days, splenocytes were isolated for the analysis. (A) Splenocytes were stimulated with OVA323-339 peptides (10 μg/mL) for 2 hours and further stimulated for additional 4 hours in the presence of GolgiStop solution. After fixation and permeabilization of the cells, CD4 and intracellular IL-2 or INF-γ were stained with antibodies. Representative flow cytometry profiles of GFP-positive cells from 4 separate experiments are shown. (B) Absolute numbers of retrovirus-transduced OT-II T cells (Thy1.2+CD4+) in the spleen were calculated from the flow cytometry analysis and live cell counting. Data were from 4 independent experiments using 3 mice per group. P values, Student t test. (C) CD8 T cells were purified using anti-CD8 microbeads. The defined number (2 × 104) of OT-I T cells (Thy1.2+CD8+) was stimulated with OVA257-264 in the presence of irradiated splenocytes for 48 hours. IFN-γ production was measured by ELISA.
Adoptively transferred gene-modified CD4 and CD8 T cells retain enhanced reactivity to the antigens. Thy1.1-positive congenic B6 mice were subcutaneously injected with E.G7 cells (2 × 106). Seven days later, Thy1.2-positive retrovirus-transduced OT-I (1 × 107) and OT-II T cells (2 × 106) were adoptively transferred to tumor-bearing mice. After 3 days, splenocytes were isolated for the analysis. (A) Splenocytes were stimulated with OVA323-339 peptides (10 μg/mL) for 2 hours and further stimulated for additional 4 hours in the presence of GolgiStop solution. After fixation and permeabilization of the cells, CD4 and intracellular IL-2 or INF-γ were stained with antibodies. Representative flow cytometry profiles of GFP-positive cells from 4 separate experiments are shown. (B) Absolute numbers of retrovirus-transduced OT-II T cells (Thy1.2+CD4+) in the spleen were calculated from the flow cytometry analysis and live cell counting. Data were from 4 independent experiments using 3 mice per group. P values, Student t test. (C) CD8 T cells were purified using anti-CD8 microbeads. The defined number (2 × 104) of OT-I T cells (Thy1.2+CD8+) was stimulated with OVA257-264 in the presence of irradiated splenocytes for 48 hours. IFN-γ production was measured by ELISA.
CTLA4-CD28 chimera gene modification of both CD4 and CD8 T cells potentiates the efficacy of adoptive T-cell therapy in a syngenic melanoma model
Because the experiments described were performed with a model tumor antigen and model tumor cells, we wanted to see whether CTLA4-CD28 chimera gene modification of both CD4 and CD8 T cells would produce similar enhancement of antitumor effect on authentic tumor-antigen–bearing syngenic tumors. B16 melanoma is one of such tumors because it has endogenous gp100 antigen that could be recognized by Pmel-1 CD8 T cells. However, B16 tumor has been reported to have poor immunogenicity and resistance to T-cell immunotherapy.30 Consistently, CTLA4-CD28 chimera–transduced Pmel-1 T cells could not cause regression of established B16 melanoma (Figure 2C). Nonetheless, addition of regulatory T cell–depleted polyclonal CD4 T cells to Pmel-1 T cell infusion along with viral vaccination has been shown to be effective in regression of the established B16 tumor in lymphopenic mice.32 Hence, we added CTLA4-CD28 chimera gene-modified polyclonal CD4 T cells (regulatory T cell–depleted) to CTLA4-CD28 chimera gene-modified Pmel-1 T cell therapy to check whether combined modification of CD4 and CD8 T cells enhances T cell–mediated antitumor effect. After lymphodepletion with irradiation, the B16 tumor-bearing mice were injected with unmodified or modified Pmel-1 and polyclonal CD4 T cells. In this experimental condition, simple addition of unmodified CD4 T cells to unmodified Pmel-1 T cells in the absence of vaccination did not enhance therapeutic effect of Pmel-1 T cells (Figure 7A). However, when both Pmel-1 T cells and CD4 T cells were modified, a dramatic reduction of tumor growth was observed. This antitumor effect also was demonstrated as prolonged survival in the longer time frame (Figure 7B). Consistent with this observation, the percentages and the numbers of the modified CD4 and CD8 T cells (Pmel-1) in the peripheral blood remarkably increased, compared with those of unmodified CD4 and CD8 T cells (Figure 7C-F). Expansion of CD4 T cells was greater than that of CD8 T cells, implying preferential effect of CTLA4-CD28 chimera on CD4 T cells (8.6-fold vs 3.7-fold; Figure 7E-F). Similar phenomena were observed in tumor-draining lymph nodes and tumor tissues (supplemental Figure 4). Noting that CD4 T cells were affected more by the gene modification than CD8 T cells, we checked whether gene modification of CD4 T cells alone can increase the antitumor effect of Pmel-1 T cells. As expected, the modified CD4 T cells substantially enhanced tumor regression. Additional gene modification of Pmel-1 T cells further enhanced tumor regression, although the degree of enhancement was rather small in this model (Figure 7G). Finally, because CTLA4-CD28 modification significantly increased IL-2 secretion in CD4 T cells (Figure 3A), we wondered whether IL-2 is essential for therapeutic effect of the gene-modified T cells in vivo. Consistent with this idea, on anti–IL-2 neutralizing antibody treatment in vivo, the therapeutic effect of gene modification was nearly abrogated (Figure 7H).
CTLA4-CD28 chimera gene modification of both CD4 and CD8 T cells potentiates the efficacy of adoptive T-cell therapy in a syngenic melanoma model. Seven days after B6 mice were subcutaneously injected with B16 melanoma cells, the mice were lymphodepleted by total body irradiation (4 Gy). Then, the mice were either left untreated or injected intravenously with the retrovirus-transduced CD4 and CD8 T cells. Regulatory T cell–depleted polyclonal CD4 T cells (CD4+CD25−) purified from normal B6 mice and Pmel-1 CD8 T cells were transduced with the retroviruses and rested for 48 hours before the transfer. Equal numbers of the transduced Pmel-1 T cells and CD4 T cells (2 × 106) were used for the adoptive transfer. (A) Mean tumor size of 5 mice per group was recorded. Results are representative of at least 2 separate experiments (*P = .0105; Wilcoxon matched pairs test). (B) Survival data of the mice from all the experiments are summarized (n = 10 for Pmel-1 EV only; n = 15 for all other groups; **P = .002; log-rank test). (C-F) Four weeks after the adoptive transfer, peripheral blood leukocytes were counted and analyzed by flow cytometry. The retrovirus-transduced CD4 or Pmel-1 T cells were identified as CD4+GFP+ or CD8+GFP+ cells. The representative flow cytometry profiles for CD4 T cells (C) and Pmel-1 T cells (D) are displayed. The absolute cell numbers of the transduced CD4 (E) and Pmel-1 T cells (F) were calculated from the 2 independent experiments. P values, Student t test. (G) B16 tumor-bearing mice generated as described in panel A were lymphodepleted and injected with various combinations of the retrovirus-transduced polyclonal CD4 and Pmel-1 T cells at 1:1 ratio (2 × 106). Mean tumor size of 5 mice per group was recorded. (H) B16 tumor-bearing mice generated as described in panel A were lymphodepleted and injected with EV-transduced polyclonal CD4 and EV-transduced Pmel-1 T cells or CTC28-transduced polyclonal CD4 and CTC28-transduced Pmel-1 T cells at 1:1 ratio (2 × 106). Anti–IL-2 antibody or control rat IgG (100 μg/head) was injected intravenously every 3 days beginning from the day of the cell transfer. Mean tumor size of 5 mice per group was recorded. Results are representative of 2 independent experiments (G-H; ***P = .0001; ****P = .0006; *****P = .0021; Wilcoxon matched pairs test).
CTLA4-CD28 chimera gene modification of both CD4 and CD8 T cells potentiates the efficacy of adoptive T-cell therapy in a syngenic melanoma model. Seven days after B6 mice were subcutaneously injected with B16 melanoma cells, the mice were lymphodepleted by total body irradiation (4 Gy). Then, the mice were either left untreated or injected intravenously with the retrovirus-transduced CD4 and CD8 T cells. Regulatory T cell–depleted polyclonal CD4 T cells (CD4+CD25−) purified from normal B6 mice and Pmel-1 CD8 T cells were transduced with the retroviruses and rested for 48 hours before the transfer. Equal numbers of the transduced Pmel-1 T cells and CD4 T cells (2 × 106) were used for the adoptive transfer. (A) Mean tumor size of 5 mice per group was recorded. Results are representative of at least 2 separate experiments (*P = .0105; Wilcoxon matched pairs test). (B) Survival data of the mice from all the experiments are summarized (n = 10 for Pmel-1 EV only; n = 15 for all other groups; **P = .002; log-rank test). (C-F) Four weeks after the adoptive transfer, peripheral blood leukocytes were counted and analyzed by flow cytometry. The retrovirus-transduced CD4 or Pmel-1 T cells were identified as CD4+GFP+ or CD8+GFP+ cells. The representative flow cytometry profiles for CD4 T cells (C) and Pmel-1 T cells (D) are displayed. The absolute cell numbers of the transduced CD4 (E) and Pmel-1 T cells (F) were calculated from the 2 independent experiments. P values, Student t test. (G) B16 tumor-bearing mice generated as described in panel A were lymphodepleted and injected with various combinations of the retrovirus-transduced polyclonal CD4 and Pmel-1 T cells at 1:1 ratio (2 × 106). Mean tumor size of 5 mice per group was recorded. (H) B16 tumor-bearing mice generated as described in panel A were lymphodepleted and injected with EV-transduced polyclonal CD4 and EV-transduced Pmel-1 T cells or CTC28-transduced polyclonal CD4 and CTC28-transduced Pmel-1 T cells at 1:1 ratio (2 × 106). Anti–IL-2 antibody or control rat IgG (100 μg/head) was injected intravenously every 3 days beginning from the day of the cell transfer. Mean tumor size of 5 mice per group was recorded. Results are representative of 2 independent experiments (G-H; ***P = .0001; ****P = .0006; *****P = .0021; Wilcoxon matched pairs test).
Collectively, these results show that poorly immunogenic, pre-established syngenic tumor can be efficiently regressed by adoptive transfer therapy with CTLA4-CD28 gene-modified CD4 and CD8 T cells through an IL-2–dependent mechanism.
Discussion
Selective inhibition of CTLA4 in antigen-specific T cells would be an ideal way of blocking CTLA4 function for antitumor immunotherapy. However, the complicated nature of receptor–ligand interactions of CD28/CTLA4 receptor family hampered the effective design of a dominant-negative mutant of CTLA4. Here, we report that CTLA4-CD28 chimeric receptor could overcome this hurdle and may act as a dominant-negative mutant of CTLA4.
CTLA4 mRNA is induced by TCR engagement and peaks in 1 to 2 days after stimulation.1 CTLA4 expression also is regulated posttranslationally. CTLA4 proteins usually reside in the intracellular vesicles and are mobilized to cell surface on TCR signaling. CD28 is expressed constitutively on T-cell surface and, on ligation with B7, delivers stimulatory signal by recruiting PI3K, Grb2, and Vav1 to the intracellular domain. CTLA4's inhibitory activity has been explained in 2 ways: (1) after T-cell activation, up-regulated CTLA4 competes with CD28 for binding to its ligand B7, thereby preventing CD28 from delivering stimulatory signals; and (2) CTLA4 itself delivers negative signals through recruitment of negative regulators such as SHP2 and PP2A.
Our observation that deletion of inhibitory cytoplasmic domain of CTLA4 did not prevent inhibitory function of CTLA4, but negated T-cell activation probably by competing with CD28 for binding to B7, is consistent with other previous reports.33,34 The idea of using CD28 cytoplasmic domain as a signaling motif for other receptors was successfully evaluated in the case of the second generation chimeric antigen receptor (CAR). The first generation CAR consisted of single chain Fv fragment of antibody fused with TCR ζ chain. Although this chimeric receptor could deliver activation signal to the T cells after engaging with target antigen, the persistence of the T cells could not be sustained.35 Insertion of CD28 cytoplasmic domain into the first generation CAR greatly improved cytokine secretion and persistence of T cells.35,36 Likewise, when we linked CD28 cytoplasmic domain with CTLA4 extracellular and transmembrane domains, this chimeric receptor successfully delivered CD28 signal inside the cell on cross-linking of extracellular CTLA4 domain with antibodies. This observation supported the idea that CTLA4-CD28 chimera converts negative signaling of CTLA4 to positive signaling. A similar phenomenon was demonstrated in 1 report in which switching of SHP2-binding motif of CTLA4 (GVYVKM) to the equivalent motif of CD28 (SDYMNM) converted inhibitory signal of CTLA4 to stimulatory signal for IL-2 production in a T-cell hybridoma.37
The fact that CTLA4-CD28 chimera constitutively expresses on the T-cell surface at high levels from the resting to activated state favors the possibility that it may outcompete endogenous CTLA4 for binding to B7. Another competition can occur intracellularly because intracellular CD28 domain also reportedly binds to SHP2 and PP2A. However, it is also possible that CTLA4-CD28 chimera can simply deliver strong CD28 signals that override negative signals of endogenous CTLA4 rather than directly compete with endogenous CTLA4, because binding affinity of CTLA4 to B7 is 100-fold higher than that of CD28. Although not mutually exclusive, these 2 possibilities are difficult to delineate, because the intracellular CD28 domain of the chimera are designed to deliver certain degree of stimulatory signals. Nonetheless, in a situation when endogenous CTLA4 inhibits T-cell activation, CTLA4-CD28 overexpression abrogates the inhibitory activity of CTLA4, suggesting that it acts as a dominant-negative mutant functionally (Figure 1I). Moreover, in vivo functional outcome of CTLA4-CD28 chimera–transduced T cells was very similar to that of CTLA4 knockout T cells as will be described in the next paragraph. Thus, it is very likely that CTLA4-CD28 chimera inhibits endogenous CTLA4 function as well as stimulates T cells through CD28 signaling.
CTLA4-CD28 chimera gene modification seems to affect CD4 T cells preferentially over CD8 T cells. The modified CD4 T cells showed a huge enhancement of IL-2 production compared with the modified CD8 T cells. In addition, the modified CD4 T cells expanded far better than the modified CD8 T cells in the T-cell therapy model of B16 melanoma (Figure 7E-F). These preferential effects exactly mirrored the results of previous studies with CTLA4−/− mice.38 CTLA4−/− mice developed skewing of CD4/CD8 ratio toward CD4 T cells over time. Depletion of CD4 T cells prevented lymphoproliferation, whereas CD8 depletion did not. Consistently, CD4 depletion prevented tissue infiltration of lymphocytes. A model more similar to ours would be the one that used Pmel-1 transgenic CTLA4−/− mice.39 Because Pmel-1 T cells recognize gp100 on normal melanocytes, the mice developed severe autoimmune vitiligo. The mice also showed polyclonal CD4 T-cell enrichment and elimination of CD4 T cells by crossing the mice with Rag1−/− mice abrogated vitiligo phenotype. Furthermore, Pmel-1 CTLA4−/− T cells failed to increase therapeutic efficacy on established B16 melanoma in spite of their enhanced ability to secrete IFN-γ, exactly what we observed with CTLA4-CD28 chimera–transduced Pmel-1 T cells. In our study, adding CTLA4-CD28–modified CD4 T cells to the CTLA4-CD28–modified Pmel-1 T cells significantly suppressed tumor growth. It would be interesting to see whether adding CTLA4−/− CD4 T cells to Pmel-1 CTLA4−/− T cells also would result in enhanced antitumor effect.
Nonetheless, the effect of CTLA4 on CD8 T cells should not be dismissed, because adoptive transfer of CTLA4−/− CD4 T cells plus the wild-type Pmel-1 T cells to Rag1−/− mice did not develop vitiligo.39 Thus, the absence of CTLA4 in Pmel-1 CD8 T cells was also important in developing autoimmunity. The importance of CTLA4 for inhibiting CD8 T-cell response also was demonstrated in other studies.40,41 Coherently, in our study, CTLA4-CD28 gene modification of both OT-II and OT-I T cells maximized antitumor effect. Collectively, inhibiting CTLA4 in both CD4 and CD8 T cells may be the most effective way to enhance antitumor immunity.
In our Pmel-1 model, we used polyclonal CD4 T cells for CTLA4-CD28 modification. This raises concerns regarding its possible autoimmune adverse effects, because CTLA4−/− mice die of autoimmune disease. Gross appearance of mice treated with the modified T cells was normal and the cell-transfer did not cause any lethality. However, when histologic examination was performed 2 months after transfer, a moderate degree of lymphocytic infiltration was observed in some organs, such as lung, stomach, and colon, but not in other organs, such as liver and kidney (data not shown). Thus, modification of polyclonal CD4 T cells elicited relatively mild chronic inflammation. The reason why the phenotype of the modified T cell–treated mice is weaker than that of CTLA4−/− mice may be that the number of transferred T cells is small and those cells would compete with endogenous T cells that restrict their hyperreactivity. Nonetheless, it would be much safer to use antigen-specific CD4 T cells for CTLA4-CD28 modification for the future application.
T-cell tolerance-resistant genetically modified T cells also have been studied targeting another immunosuppressive molecule, TGF-β. T cell–specific expression of dominant-negative TGF-β receptor type II(dnTGF-RII) in mice showed spontaneous autoimmunity42 and efficient rejection of engrafted tumors.43 Interestingly, although CD4 T cells were required for the antitumor activity of dnTGF-RII–expressing CD8 T cells, dnTGF-RII expression in CD4 T cells did not increase antitumor effect.43 Thus, antitumor effect of dnTGF-RII expression in T cells is a CD8-dominant phenomenon, quite opposite to CTLA4-CD28 chimera. Because the current adoptive T-cell therapy has focused on CD8 T cells, dnTGF-RII gene–modified antigen-specific cytotoxic T lymphocyte (CTL) therapy is being considered for clinical trials.44 Thus, tolerance breakage in tumor antigen-specific T cells would be one of attractive targets of gene modification of T cells.
Although CD4 T-cell therapy is just in its infancy, its value is increasingly appreciated.45 A recent success of antigen-specific CD4 T-cell therapy for metastatic melanoma may be a stepping stone for the future progress in this field.46 CD4 T cells have been known to be essential for CD8 T-cell response.47 Enhancement of the antitumor effect of CD8 T cells by CD4 T cells, also was demonstrated previously48 in addition to our OT-I and OT-II mice model. Therefore, combined CD4 and CD8 T-cell therapy is expected to increase the efficacy of T-cell therapy significantly. As a molecular mechanism of the combined T-cell therapy, the importance of IL-2 produced by CD4 T cells was suggested.49 In addition, IL-2 has been used to enhance CD8 T-cell antitumor reactivity in CTL therapy.16,30 Because CTLA4-CD28 gene-modified CD4 T cells produced a huge amount of IL-2 that was essential for the therapeutic effect of the modified T cells, the modified CD4 T cells may provide a consistent source of IL-2 in vivo to sustain appropriate antitumor CD8 T-cell reactivity in our model. Thus, coupled with the enhanced reactivity of the modified antigen-specific CD8 T cells, genetic modification of antigen-specific CD4 T cells using CTLA4-CD28 chimera would further improve the efficacy of the combined T-cell therapy without need for additional exogenous IL-2. In conclusion, we propose that CTLA4-CD28 chimera would serve as a useful tool to manipulate CTLA4 function ex vivo in antigen-specific T cells in the future.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Yosef Refaeli, Arthur Weiss, and Garry Nolan for providing valuable materials and Tae Sik Kim for providing technical expertise on cell sorting.
This work was supported by grants from the Innovative Research Institute for Cell Therapy (A062260) and the National Cancer Center (NCC-1010090), Republic of Korea.
Authorship
Contribution: J.H.S. designed and conducted experiments and wrote the manuscript; H.B.P., Y.M.O., D.P.L., J.E.L., and H.H.S. conducted experiments and analyzed data; S.J.L. and H.S.E. discussed and supervised in vivo tumor models; I.-H.K. and S.H.L. participated in study design; and K.C. designed and conceptualized the research, supervised the experiments, analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kyungho Choi, Research Institute National Cancer Center, Gyeonggi-do, 410-769, Republic of Korea; e-mail: kchoi@ncc.re.kr.

![Figure 1. CTLA4-CD28 chimera delivers CD28 signals and enhances T-cell reactivity. (A) Conceptual design and mode of action of CTLA4-CD28 chimera (CTC28) and CTLA4-decoy (CTdc). + indicates stimulatory signal; −, inhibitory signal; and \\, abrogation of the signal. (B) Structure of retroviral constructs. EV is the empty retroviral vector (pMIG-w). CTC28 or CTdc cDNA was inserted in front of internal ribosomal entry site (IRES)–GFP cassette of pMIG-w. IRES-GFP cassette is used for tracing the virus-transduced cells. CTLA4 EC indicates extracellular domain of CTLA4 (amino acids 1-161); TM, transmembrane domain of CTLA4 (amino acids 162-189); CD28 CP, cytoplasmic domain of CD28 (amino acids 177-218); and LTR, long terminal repeat. (C-D) Jurkat T cells transiently transfected with the retroviral plasmids (10 μg), RE/AP luciferase plasmids (10 μg) and TK-Renilla luciferase plasmid (0.5 μg) were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 or with plate-bound anti-CD3 plus anti-CTLA4 or control IgG for 6 hours. Firefly luciferase activity was measured from cell lysates. Transfection efficiency was normalized with Renilla luciferase activity. (E) Jurkat T cells transfected with retroviral plasmids were stimulated with anti-CD28 or anti-CTLA4 cross-linked by secondary antibodies for 30 minutes. Cell lysates were immunoblotted with anti–phospho-Akt or anti-Akt. (F-G) GFP-positive T cells were sorted after retroviral transduction of activated splenic T cells from normal mice and 3-day resting in the absence of stimulation. Then, the cells were stimulated with various concentrations of anti-CD3 in the presence of irradiated splenocytes for 48 hours. [3H]Thymidine (1 μCi) was added to the culture for additional 24 hours to measure cell proliferation (F), or the supernatant was harvested to measure IFN-γ production by ELISA (G). (H) Transduced splenic T cells activated with anti-CD3 (1 μg/mL) in the presence of irradiated splenocytes for 48 hours as described in panels F and G were stained with APC-labeled anti-CD4 and PE-Cy5–labeled anti-CD8 along with PE-labeled anti-CTLA4 (intracellular staining), or anti-CD28 (surface staining), or relevant isotype control antibodies. Then, GFP-positive populations were analyzed by flow cytometry. (I) Transduced splenic T cells were stimulated with plate-bound anti-CD3 (1 μg/mL), anti-CD28 (2 μg/mL), or both in the presence or absence of anti-CTLA4 (10 μg/mL) cross-linked with plate-bound anti–hamster IgG (20 μg/mL). Seventy-two hours after stimulation, the supernatant was harvested to measure IFN-γ production by ELISA. Results are representative of 2 (E,H-I) or 3 independent experiments (C-D,F-G).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/24/10.1182_blood-2011-09-380519/5/m_zh89991292240001.jpeg?Expires=1767698437&Signature=D7GCchy92OOHro1CdVWxe5GXFC9nOsAMqbbXY5L5IH8pUzTKZeWVsP-0RSjtHVwGy49lxCZD9HCAiTAyMB5UxoGGPiwS3FUFT5fVUUUgwXtI8VygS1s4V~ApsOaJwsjN7zi4v0YxwsfrvywSkuu54Frka5zbuBB9w0VXThy62IxdDn7lg~7wv4gq1tsKU-8-IIA3T~QPx5VpMbWn24ovNIawdg1FYQ-A1bRUGSXkYW-VGPkwnJFX00B0sFT-OpCw43q7x10YLetS6OMR~4iAOxQk292RfJCoVxtoUkwffnqHZ2eKUbPItElC~FGscc516pxT~NslnvGhmQbS643E6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal