Abstract
We report the relative efficacy of co-infusing 2 umbilical cord blood units (dUCB) compared with peripheral blood progenitor cells (PBPCs) from 8 of 8 or 7 of 8 HLA-matched unrelated donors. All patients received reduced-intensity conditioning (RIC) regimens. Four treatment groups were evaluated: 4-6 of 6 matched dUCB-TCF (n = 120; TCF = total body irradiation [TBI] 200 cGy + cyclophos-phamide + fludarabine), 4-6 of 6 matched dUCB-other (n = 40; alkylating agent + fludarabine ± TBI), and 8 of 8 (n = 313) and 7 of 8 HLA-matched PBPCs (n = 111). Compared with matched 8 of 8 PBPC transplantations, transplantation-related mortality (TRM), and overall mortality were similar after dUCB-TCF (relative risk [RR] 0.72, P = .72; RR 0.93, P = .60) but higher after dUCB-other RIC (hazard ratio [HR] 2.70, P = .0001; 1.79 P = .004). Compared with 7 of 8 PBPC transplantations, TRM (but not overall mortality) was lower after dUCB-TCF (RR 0.57, P = .04; RR 0.87 P = .41). The probabilities of survival after dUCB-TCF, dUCB-other RIC, and 8 of 8 PBPC and 7 of 8 PBPC transplantations were 38%, 19%, 44%, and 37%, respectively. With similar survival after 8 of 8, 7 of 8 matched PBPCs, and dUCB-TCF, these data support use of dUCB-TCF transplantation in adults with acute leukemia who may benefit from RIC transplantation urgently or lack a 7-8 of 8 unrelated donor.
Introduction
The relative efficacy of HLA 4-6 of 6 matched umbilical cord blood (UCB) compared with grafts from HLA 8 of 8 matched and mismatched unrelated donors in the treatment of adults with acute leukemia after myeloablative conditioning has demonstrated similar outcomes in multiple studies.1-6 However, few studies have focused on outcomes comparing the allogeneic HSC sources after reduced-intensity conditioning (RIC) which is of particular interest because a substantial proportion of older adults or less clinically fit patients are poor candidates for a myeloablative conditioning regimen. RIC has been shown to be an effective strategy permitting older and less fit patients access to potentially curative allogeneic HSCT.7-11 Single-center data indicate that UCB immune cells are sufficient for securing consistent engraftment after RIC.12-18 However, data on the relative efficacy of UCB after RIC compared with other donor HSC sources are limited.19,20 Because an increasing proportion of adults receive transplants with 2 UCB units rather than a single unit to achieve the desired cell dose (termed “double UCB” [dUCB]) transplantation, we report the results of a retrospective registry-based analysis comparing dUCB to 8 of 8 or 7 of 8 PBPC transplantation.
Methods
Data collection
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of more than 450 transplantation centers worldwide that provide detailed demographic, treatment, and transplantation outcome data on consecutive allogeneic and autologous HSCT recipients to a statistical center at the Medical College of Wisconsin and the National Marrow Donor Program (NMDP) Coordinating Center. Compliance is monitored by on-site audits. Patients are followed longitudinally until death or lost to follow-up. For the current analysis, only adults with acute leukemia who received transplants in the United States with partially HLA-matched UCB or mobilized PBPCs after RIC regimens between years 2000 and 2009 were eligible. Most patients received transplants between 2005 and 2009 (83% UCB and 70% PBPC transplantations). All patients provided written informed consent in accordance with the Declaration of Helsinki. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Of UCB recipients, 85% received a dUCB graft; for this reason, only dUCB recipients were included in this analysis. Patients who had received a single UCB unit or previous allogeneic transplantation were excluded. Of recipients of PBPCs, 318 donors had high-resolution HLA typing by molecular methods at HLA-A, HLA-B, HLA-C, and HLA-DRB1 (8 of 8 HLA match) and 111 had a single allele or Ag mismatch (7 of 8 HLA match). In contrast to PBPCs, HLA matching of UCB units considered matching at HLA-A, HLA-B (low resolution using molecular methods), and HLA-DRB1 (high resolution using molecular methods). Approximately 70% of dUCB transplantations were mismatched at 2 HLA loci. For this analysis, RIC was defined as a regimen that incorporated a busulfan (BU) total dose ≤ 8 mg/kg, low-dose melphalan (MEL) total dose ≤ 140 mg/m2, or low-dose total body irradiation (TBI) total dose 200 cGy.
Outcomes
Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) of ≥ 500 cells/mm3 for 3 consecutive days. Platelet recovery was defined as achieving ≥ 50 000 unsupported for 7 days. Grade II-IV acute and chronic GVHD was determined using standard criteria from each transplantation center.21,22 Transplantation-related mortality (TRM) was defined as death not related to leukemia recurrence. Relapse was defined as leukemia recurrence based on morphologic assessment and/or supported by reappearance of abnormalities in cytogenetic or molecular analyses. Leukemia-free survival (LFS) was defined as survival in a state of continuous complete remission (inverse of treatment failure; death from any cause or relapse). Overall mortality was defined as death from any cause.
Statistical analysis
Variables related to patients, disease, and transplantations were compared among the groups using the χ2 test for categorical variables. The probabilities of LFS and overall survival (OS) were calculated with the Kaplan-Meier estimator.23 Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, TRM, and relapse were calculated with the cumulative incidence estimator.23 The cumulative incidence estimator is used when there are 2 events for the outcome of interest, and the occurrence of one of the events precludes the occurrence of the other. For neutrophil and platelet recovery, and acute and chronic GVHD, death without the event was the competing event. For TRM, relapse was the competing event, and for relapse, TRM was the competing event. For the analysis of LFS, relapse or death from any cause (ie, treatment failure) was considered an event. In all analyses, data on patients without an event was censored at last follow-up. As there were significant differences in conditioning regimen among dUCB recipients, we first explored whether the type of regimen used for this graft source was associated with transplantation outcomes. Our analysis showed a significant difference in TRM and overall mortality risks after dUCB transplantation in those treated with total body irradiation (TBI) 200 cGy, cyclophosphamide and fludarabine (TCF), the predominant conditioning regimen relative to the other regimens (alkylating agent + fludarabine). While PBPC recipients were grouped as 8 of 8 or 7 of 8 HLA-matched, HLA match was ignored for dUCB recipients because most received 4 of 6 matched units. Therefore, for all analyses, outcomes were assessed for each of 4 groups: dUCB-TCF, dUCB-other regimens, and 8 of 8 HLA-matched PBPC and 7 of 8 HLA-matched PBPC transplantations.
Multivariate models were built using Cox proportional hazard-regression models24 for treatment failure, overall mortality, TRM, relapse, acute and chronic GVHD, and logistic regression models for neutrophil recovery at day 28. Models were built using the backward stepwise selection procedure and confirmed with the use of forward stepwise selection procedure. The proportional-hazards assumption was tested for each variable individually; when the assumption was violated, time-dependent covariates were constructed. A P value of .05 or less was considered significant. 95% confidence intervals (CI) were derived from log transformation.
The primary objective was to compare transplantation outcomes according to unrelated HSC sources. In all analysis, recipients of 8 of 8 or 7 of 8 HLA-matched PBPCs were compared with dUCB-TCF and dUCB-other regimens. Results are expressed as hazard ratio (HR) or odds ratio (OR) with 95% CIs. The variable for treatment group, the main effect term, was held in all steps of model building. Patient, disease, and transplantation variables considered were: patient age (< 40 vs ≥ 40 years), serologic status with respect to CMV in the recipient (yes vs no), disease (acute lymphoblastic leukemia [ALL] vs acute myeloid leukemia [AML]), disease status at transplantation (first complete remission [CR]) vs second/third CR vs not in remission), and year of transplantation (2000-2004 vs 2005-2009). There were no interactions between the variable for treatment group and other significant variables in all final models. There were no significant center effects detected using the random effects model.25 All P values are 2-sided. Analyses were done with SAS software (Version 9.1).
Results
Patients
Patients either had AML (n = 523) or ALL (n = 50). Patients' clinical characteristics by treatment group are summarized in the Table 1. There were differences in characteristics between the 4 treatment groups. Recipients of PBPC grafts were more likely to have AML. Recipients of dUCB-other regimens were younger compared with recipients of PBPCs or dUCB-TCF regimens and more likely to be CMV seropositive. Recipients of dUCB-TCF regimens were more likely to report performance scores 90-100 and more likely to be in first continuous CR; ∼ 90% of recipients were in CR at transplantation. There were significant differences in transplantation conditioning regimens used and in vivo T-cell depletion (ATG) was primarily used in combination with non-TBI regimens. Approximately one-third of PBPC recipients received low-dose TBI-containing regimens (200 cGy; N = 109) with others receiving an alkylating agent + fludarabine. In contrast, 75% of dUCB recipients received TCF. The remaining 25% received other regimens; 80% of these regimens included alkylating agent + fludarabine and almost all received ATG. Of the 75 centers that contributed patients to this study, 20 centers preformed dUCB and PBPC transplantations with 49 centers performing PBPCs only and 6 dUCB only. Among centers that performed PBPC transplantations, 42 centers contributed 1-4 patients; 16 centers, 5-10 patients; and 11 centers, > 10 patients. Among centers that performed dUCB transplantations, 21 centers contributed 1-4 patients; 3 centers, 5-10; and 2 centers, > 10 patients. One center contributed 89 of 121 cases in the dUCB-TCF group. UCB transplantations were more common after 2004. The median follow-up of surviving patients after 8 of 8 PBPCs was ∼ 3 years compared with 2 years after 7 of 8 PBPCs, dUCB-TCF, and dUCB-other regimen transplantations.
Patient, disease, and transplantation characteristics
| Variables . | 8/8 PBPCs, n (%) . | 7/8 PBPCs, n (%) . | dUCB-TCF, n (%) . | dUCB-other, n (%) . | P . |
|---|---|---|---|---|---|
| No. of patients | 313 | 111 | 121 | 40 | |
| Age at transplantation, y | |||||
| Median (range) | 59 (23-69) | 58 (21-69) | 55 (23-68) | 48 (21-67) | < .0001 |
| 21-29 | 6 (2) | 7 (6) | 5 (4) | 3 (8) | < .0001 |
| 30-39 | 14 (4) | 4 (4) | 5 (4) | 7 (18) | |
| 40-49 | 38 (12) | 12 (11) | 22 (18) | 12 (30) | |
| 50-59 | 108 (35) | 43 (39) | 52 (43) | 9 (23) | |
| 60-69 | 147 (47) | 45 (41) | 37 (31) | 9 (23) | |
| Patient CMV | .07 | ||||
| Positive | 174 (56) | 67 (60) | 82 (68) | 31 (78) | |
| Negative | 135 (43) | 43 (39) | 39 (32) | 9 (23) | |
| Not reported | 4 (1) | 1 (1) | 0 | 0 | |
| Performance score before transplantation | .04 | ||||
| < 90% | 123 (39) | 44 (40) | 33 (27) | 17 (43) | |
| 90%-100% | 166 (53) | 59 (53) | 85 (70) | 21 (53) | |
| Not reported | 24 (8) | 8 (7) | 3 (2) | 2 (5) | |
| Disease | < .0001 | ||||
| Acute myeloid leukemia | 294 (94) | 100 (90) | 99 (82) | 30 (75) | |
| Acute lymphoblastic leukemia | 19 (6) | 11 (10) | 22 (18) | 10 (25) | |
| Disease status prior to transplantation | < .0001 | ||||
| CR1 | 165 (53) | 53 (48) | 74 (61) | 10 (25) | |
| CR2 | 65 (21) | 19 (17) | 33 (27) | 17 (43) | |
| CR3 | 9 (3) | 6 (5) | 7 (6) | 2 (5) | |
| PIF, relapse | 74 (24) | 33 (30) | 7 (6) | 11 (28) | |
| Conditioning regimen | < .0001 | ||||
| TBI + cy + fludarabine ± atg | 0 | 0 | 121 (100)k | 0 | |
| TBI + fludarabine ± atg | 76 (24)a | 20 (18)f | 0 | 0 | |
| TBI + fludarabine + bu ± atg | 8 (3)b | 5 (5)g | 0 | 8 (20)l | |
| Busulfan + fludarabine ± atg | 119 (38)c | 46 (41)h | 0 | 2 (5)m | |
| Melphalan + fludarabine ± atg | 84 (27)d | 30 (27)i | 0 | 28 (70)n | |
| Cyclophosphamide + fludarabine ± atg | 26 (8)e | 10 (9)j | 0 | 2 (5)o | |
| Year of transplantation | .006 | ||||
| 2000-2004 | 96 (31) | 29 (26) | 25 (21) | 3 (8) | |
| 2005-2009 | 217 (69) | 82 (74) | 96 (79) | 37 (93) | |
| Median follow-up of survivors, mo (range) | 35 (6-96) | 25 (6-75) | 25 (5-97) | 25 (6-61) |
| Variables . | 8/8 PBPCs, n (%) . | 7/8 PBPCs, n (%) . | dUCB-TCF, n (%) . | dUCB-other, n (%) . | P . |
|---|---|---|---|---|---|
| No. of patients | 313 | 111 | 121 | 40 | |
| Age at transplantation, y | |||||
| Median (range) | 59 (23-69) | 58 (21-69) | 55 (23-68) | 48 (21-67) | < .0001 |
| 21-29 | 6 (2) | 7 (6) | 5 (4) | 3 (8) | < .0001 |
| 30-39 | 14 (4) | 4 (4) | 5 (4) | 7 (18) | |
| 40-49 | 38 (12) | 12 (11) | 22 (18) | 12 (30) | |
| 50-59 | 108 (35) | 43 (39) | 52 (43) | 9 (23) | |
| 60-69 | 147 (47) | 45 (41) | 37 (31) | 9 (23) | |
| Patient CMV | .07 | ||||
| Positive | 174 (56) | 67 (60) | 82 (68) | 31 (78) | |
| Negative | 135 (43) | 43 (39) | 39 (32) | 9 (23) | |
| Not reported | 4 (1) | 1 (1) | 0 | 0 | |
| Performance score before transplantation | .04 | ||||
| < 90% | 123 (39) | 44 (40) | 33 (27) | 17 (43) | |
| 90%-100% | 166 (53) | 59 (53) | 85 (70) | 21 (53) | |
| Not reported | 24 (8) | 8 (7) | 3 (2) | 2 (5) | |
| Disease | < .0001 | ||||
| Acute myeloid leukemia | 294 (94) | 100 (90) | 99 (82) | 30 (75) | |
| Acute lymphoblastic leukemia | 19 (6) | 11 (10) | 22 (18) | 10 (25) | |
| Disease status prior to transplantation | < .0001 | ||||
| CR1 | 165 (53) | 53 (48) | 74 (61) | 10 (25) | |
| CR2 | 65 (21) | 19 (17) | 33 (27) | 17 (43) | |
| CR3 | 9 (3) | 6 (5) | 7 (6) | 2 (5) | |
| PIF, relapse | 74 (24) | 33 (30) | 7 (6) | 11 (28) | |
| Conditioning regimen | < .0001 | ||||
| TBI + cy + fludarabine ± atg | 0 | 0 | 121 (100)k | 0 | |
| TBI + fludarabine ± atg | 76 (24)a | 20 (18)f | 0 | 0 | |
| TBI + fludarabine + bu ± atg | 8 (3)b | 5 (5)g | 0 | 8 (20)l | |
| Busulfan + fludarabine ± atg | 119 (38)c | 46 (41)h | 0 | 2 (5)m | |
| Melphalan + fludarabine ± atg | 84 (27)d | 30 (27)i | 0 | 28 (70)n | |
| Cyclophosphamide + fludarabine ± atg | 26 (8)e | 10 (9)j | 0 | 2 (5)o | |
| Year of transplantation | .006 | ||||
| 2000-2004 | 96 (31) | 29 (26) | 25 (21) | 3 (8) | |
| 2005-2009 | 217 (69) | 82 (74) | 96 (79) | 37 (93) | |
| Median follow-up of survivors, mo (range) | 35 (6-96) | 25 (6-75) | 25 (5-97) | 25 (6-61) |
PBPC indicates peripheral blood progenitor cell; CR, complete remission; PIF, primary induction failure; TBI, total body irradiation; and atg, antithymocyte globulin.
8/8 HLA-matched PBPC regimens: aTBI + fludarabine: 1 of 76 received ATG; bTBI + busulfan + fludarabine: 1 of 8 received ATG; cBusulfan + fludarabine: 78 of 119 received ATG; dMelphalan + fludarabine: 26 of 84 received ATG; and eCyclophosphamide + fludarabine: 1 of 26 received ATG.
7/8 HLA-matched PBPC regimens: fTBI + fludarabine: 3 of 20 received ATG; gTBI + busulfan + fludarabine: 1 of 5 received ATG; hBusulfan + fludarabine: 28 of 46 received ATG; iMelphalan + fludarabine: 14 of 30 received ATG; and jCyclophosphamide + fludarabine: none received ATG.
dUCB-TCF regimens: k38 of 120 patients received ATG.
dUCB-other regimens: lNone received ATG; m1 of 2 patients received ATG; n27 of 28 patients received ATG; 13 of 28 received thiotepa; and 1 of 2 patients received ATG.
Univariate analysis
The results of single-factor multivariate models for LFS, OS, transplantation-related mortality, relapse, and acute and chronic GVHD are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The results of single-factor multivariate models with variables that attained statistical significance and included in the final multivariate models are shown in Table 2.
Univariate analysis for LFS, OS, relapse, and GVHD
| . | Relative risk (95% CI) . | P . |
|---|---|---|
| Treatment failure (inverse of LFS) | ||
| Disease status (within 1 mo of HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 0.54 (0.22-1.33) | .18 |
| Relapse/PIF | 2.46 (1.33-4.52) | .004 |
| Disease status (> 1 mo after HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.29 (1.02-1.65) | .03 |
| Relapse/PIF | 1.32 (1.01-1.74) | .04 |
| Performance score (within 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 0.68 (0.50-0.90) | .007 |
| Performance score (> 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 1.13 (0.84-1.52) | .42 |
| Overall mortaiity | ||
| Disease status | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.23 (0.96-1.57) | .10 |
| Relapse/PIF | 1.50 (1.16-1.94) | .002 |
| Performance score (within 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 0.75 (0.59-0.96) | .02 |
| Performance score (> 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 1.26 (0.78-2.03) | .35 |
| Relapse | ||
| Disease status (within 1 mo of HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 0.44 (0.15-1.31) | .14 |
| Relapse/PIF | 2.43 (1.23-4.80) | .01 |
| Disease status (> 1 mo after HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.49 (1.09-2.02) | .01 |
| Relapse/PIF | 1.65 (1.18-2.31) | .004 |
| Grade II-IV acute GVHD | ||
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.73 (0.55-0.97) | .03 |
| Grade III-IV acute GVHD | ||
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.63 (0.42-0.95) | .03 |
| Chronic GVHD | ||
| CMV serostatus | ||
| Sero-positive | 1.00 | |
| Sero-negative | 1.30 (1.02-1.65) | .03 |
| Performance score | ||
| < 90 | 1.00 | |
| 90-100 | 0.78 (0.61-1.01) | .06 |
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.56 (0.43-0.73) | < .001 |
| . | Relative risk (95% CI) . | P . |
|---|---|---|
| Treatment failure (inverse of LFS) | ||
| Disease status (within 1 mo of HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 0.54 (0.22-1.33) | .18 |
| Relapse/PIF | 2.46 (1.33-4.52) | .004 |
| Disease status (> 1 mo after HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.29 (1.02-1.65) | .03 |
| Relapse/PIF | 1.32 (1.01-1.74) | .04 |
| Performance score (within 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 0.68 (0.50-0.90) | .007 |
| Performance score (> 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 1.13 (0.84-1.52) | .42 |
| Overall mortaiity | ||
| Disease status | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.23 (0.96-1.57) | .10 |
| Relapse/PIF | 1.50 (1.16-1.94) | .002 |
| Performance score (within 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 0.75 (0.59-0.96) | .02 |
| Performance score (> 4 mo of HCT) | ||
| < 90 | 1.00 | |
| 90-100 | 1.26 (0.78-2.03) | .35 |
| Relapse | ||
| Disease status (within 1 mo of HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 0.44 (0.15-1.31) | .14 |
| Relapse/PIF | 2.43 (1.23-4.80) | .01 |
| Disease status (> 1 mo after HCT) | ||
| CR1 | 1.00 | |
| CR2/CR3 | 1.49 (1.09-2.02) | .01 |
| Relapse/PIF | 1.65 (1.18-2.31) | .004 |
| Grade II-IV acute GVHD | ||
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.73 (0.55-0.97) | .03 |
| Grade III-IV acute GVHD | ||
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.63 (0.42-0.95) | .03 |
| Chronic GVHD | ||
| CMV serostatus | ||
| Sero-positive | 1.00 | |
| Sero-negative | 1.30 (1.02-1.65) | .03 |
| Performance score | ||
| < 90 | 1.00 | |
| 90-100 | 0.78 (0.61-1.01) | .06 |
| Year of transplantation | ||
| 2001-2004 | 1.00 | |
| 2005-2009 | 0.56 (0.43-0.73) | < .001 |
LFS indicates leukemia-free survival; OS, overall survival; CI, confidence interval; PIF, primary induction failure; and HCT, hematopoietic cell transplant.
Leukemia-free survival
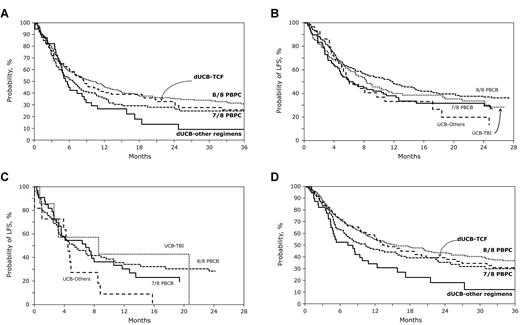
The 2-year LFS adjusted for disease status and performance score at transplantation was 31% (95% CI, 22%-41%) and 15% (95% CI, 2%-28%) after dUCB-TCF and dUCB-other regimens, respectively (Figure 1A). Corresponding LFS rates for 8 of 8 and 7 of 8 HLA-matched PBPC transplantations were 35% (95% CI, 30%-41%) and 29% (95% CI, 21%-38%), respectively (Figure 1A). The LFS for patients who received transplants in remission was 34% (95% CI, 25%-44%) and 20% (95% CI, 6%-39%) after dUCB-TCF and dUCB-other regimens, respectively (Figure 1B). Corresponding LFS rates for 8 of 8 and 7 of 8 HLA-matched PBPC transplantations were 37% (95% CI, 31%-44%) and 32% (95% CI, 22%-43%), respectively (Figure 1B). The LFS for patients who received transplants in relapse or primary induction failure was 43% (95% CI, 10%-73%) and 9% (95% CI, 1%-33%) after dUCB-TCF and dUCB-other regimens, respectively (Figure 1C). Corresponding LFS rates for 8 of 8 and 7 of 8 HLA-matched PBPC transplantations were 34% (95% CI, 24%-45%) and 30% (95% CI, 16%-46%), respectively (Figure 1C).
Survival after transplantation. (A) The probabilities of LFS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for disease status at transplantation and performance score. (B) The probabilities of LFS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations for patients who received transplants in CR. (C) The probabilities of OS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for patients who received transplants in relapse or primary induction failure. (D) The probabilities of OS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for disease status at transplantation and performance score.
Survival after transplantation. (A) The probabilities of LFS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for disease status at transplantation and performance score. (B) The probabilities of LFS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations for patients who received transplants in CR. (C) The probabilities of OS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for patients who received transplants in relapse or primary induction failure. (D) The probabilities of OS after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations adjusted for disease status at transplantation and performance score.
In multivariable analysis, risks of treatment failure (relapse or death; inverse of LFS) after dUCB-TCF and 8 of 8 HLA-matched and 7 of 8 HLA-matched PBPC transplantations were not significantly different (Table 3). Treatment failure, however, was higher after dUCB-other regimens compared with 8 of 8 HLA-matched but not 7 of 8 HLA-matched PBPC transplantations (Table 3).
Results of multivariate analysis
| . | Relative risk (95% CI) . | P . |
|---|---|---|
| Outcome for transplantation-related mortality, relapse, LFS, and OS | ||
| Transplantation-related mortality | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 0.92 (0.57-1.47) | .719 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 2.72 (1.63-4.52) | < .001 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.57 (0.33-0.96) | .035 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.68 (0.96-2.94) | .069 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.62 (1.08-2.43) | .020 |
| Relapse | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.26 (0.92-1.73) | .155 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 0.92 (0.53-1.59) | .756 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 1.15 (0.77-1.70) | .495 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.83 (0.46-1.52) | .553 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.10 (0.79-1.52) | .578 |
| Treatment failure (inverse of LFS) | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.13 (0.87-1.47) | .368 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.47 (1.01-2.13) | .046 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.88 (0.64-1.21) | .432 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.14 (0.76-1.72) | .057 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.28 (1.00-1.65) | .057 |
| Overall mortality | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.08 (0.81-1.43) | .602 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.78 (1.21-2.63) | .004 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.87 (0.55-1.21) | .409 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.44 (0.94-2.20) | .094 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.24 (0.95-1.62) | .117 |
| Outcome for acute GVHD and chronic GVHD | ||
| Grade II-IV acute GVHD | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.91 (1.39-2.62) | < .001 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.15 (0.66-2.02) | .616 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 1.44 (0.98-2.10) | .063 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.65 (0.48-1.58) | .869 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.33 (0.95-1.86) | .099 |
| Grade III-IV acute GVHD | ||
| 8/8 HLA-matched PBPCs | 1.00 | |
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.38 (0.81-2.33) | .727 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.48 (0.66-3.30) | .342 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.69 (0.39-1.22) | .197 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.74 (0.32-1.69) | .473 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 2.00 (1.26-3.19) | .004 |
| Chronic GVHD | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 0.43 (0.30-0.62) | < .001 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 0.71 (0.41-1.23) | .221 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.45 (0.30-0.67) | < .001 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.74 (0.41-1.34) | .327 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 0.96 (0.70-1.30) | .765 |
| . | Relative risk (95% CI) . | P . |
|---|---|---|
| Outcome for transplantation-related mortality, relapse, LFS, and OS | ||
| Transplantation-related mortality | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 0.92 (0.57-1.47) | .719 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 2.72 (1.63-4.52) | < .001 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.57 (0.33-0.96) | .035 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.68 (0.96-2.94) | .069 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.62 (1.08-2.43) | .020 |
| Relapse | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.26 (0.92-1.73) | .155 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 0.92 (0.53-1.59) | .756 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 1.15 (0.77-1.70) | .495 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.83 (0.46-1.52) | .553 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.10 (0.79-1.52) | .578 |
| Treatment failure (inverse of LFS) | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.13 (0.87-1.47) | .368 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.47 (1.01-2.13) | .046 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.88 (0.64-1.21) | .432 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.14 (0.76-1.72) | .057 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.28 (1.00-1.65) | .057 |
| Overall mortality | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.08 (0.81-1.43) | .602 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.78 (1.21-2.63) | .004 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.87 (0.55-1.21) | .409 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 1.44 (0.94-2.20) | .094 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.24 (0.95-1.62) | .117 |
| Outcome for acute GVHD and chronic GVHD | ||
| Grade II-IV acute GVHD | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.91 (1.39-2.62) | < .001 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.15 (0.66-2.02) | .616 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 1.44 (0.98-2.10) | .063 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.65 (0.48-1.58) | .869 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 1.33 (0.95-1.86) | .099 |
| Grade III-IV acute GVHD | ||
| 8/8 HLA-matched PBPCs | 1.00 | |
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 1.38 (0.81-2.33) | .727 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 1.48 (0.66-3.30) | .342 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.69 (0.39-1.22) | .197 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.74 (0.32-1.69) | .473 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 2.00 (1.26-3.19) | .004 |
| Chronic GVHD | ||
| dUCB-TCF vs 8/8 HLA-matched PBPCs | 0.43 (0.30-0.62) | < .001 |
| dUCB-other regimen vs 8/8 HLA-matched PBPCs | 0.71 (0.41-1.23) | .221 |
| dUCB-TCF vs 7/8 HLA-matched PBPCs | 0.45 (0.30-0.67) | < .001 |
| dUCB-other regimen vs 7/8 HLA-matched PBPCs | 0.74 (0.41-1.34) | .327 |
| 7/8 HLA-matched vs 8/8 HLA-matched PBPCs | 0.96 (0.70-1.30) | .765 |
CI indicates confidence interval; LFS, leukemia-free survival; OS, overall survival; and PBPC, peripheral blood progenitor cell.
Both performance score and disease status at transplantation were associated with treatment failure during the early posttransplantation period. Patients with performance scores < 90 were more likely to experience failure in the first 4 months after transplantation compared with those with scores 90-100 (relative risk [RR] 1.39, 95% CI 1.03-1.87, P = .03). We did not observe an effect of performance score beyond the 4-month period (RR 0.85, 95% CI 0.65-1.21, P = .48). Patients who received transplants in primary induction failure or in relapse at transplantation had a higher risk of treatment failure compared with patients who received transplants in first CR (RR 2.20, 95% CI 1.86-4.09, P = .01) and second CR (RR 4.27, 95% CI 1.71-10.75, P = .002).
Overall survival
The 2-year OS adjusted for disease status and performance score at transplantation were 37% (95% CI, 28%-48%) and 19% (95% CI, 4%-34%) for recipients of dUCB-TCF and dUCB-other regimens, respectively (Figure 1D). Corresponding OS rates for 8 of 8 and 7 of 8 HLA-matched transplantations were 44% (95% CI, 38%-50%) and 37% (95% CI, 27%-46%), respectively (Figure 1D). In multivariable analysis, overall mortality was not significantly different after dUCB-TCF, 8 of 8 HLA-matched and 7 of 8 HLA-matched PBPC transplantations (Table 3). However, overall mortality risks were higher after dUCB-other regimens compared with 8 of 8 HLA-matched but not 7 of 8 HLA-matched PBPCs (Table 2). Mortality risks were also higher after dUCB-other regimens compared with dUCB-TCF (RR 1.66, 95% CI 1.08-2.54, P = .021).
Performance score and disease status at transplantation were associated with overall mortality. Mortality risks were higher in patients with performance scores < 90 compared with those with scores 90-100 (RR 1.28, 95% CI 1.01-1.64, P = .05). Patients who received transplants in primary induction failure or in relapse at transplantation had a higher risk of mortality compared with patients who received transplants in first CR (RR 1.46, 95% CI 1.12-1.90, P = .006). The causes of death are shown in Table 4. Recurrent leukemia was the most common cause of death after dUCB-TCF, 8 of 8 and 7 of 8 PBPC transplantations. However, infections and organ failure were the most common causes of death after dUCB-other regimens.
Causes of death by treatment group
| . | 8/8 PBPCs (%) . | 7/8 PBPCs (%) . | dUCB-TCF (%) . | dUCB-other (%) . |
|---|---|---|---|---|
| No. of deaths | 189 | 75 | 70 | 31 |
| Graft failure | 2 (1) | 3 (4) | ||
| Recurrent leukemia | 100 (53) | 30 (40) | 35 (50) | 4 (13) |
| GVHD | 22 (12) | 9 (12) | 3 (4) | 5 (16) |
| Interstitial pneumonitis/ARDS | 5 (3) | 2 (3) | 5 (7) | |
| Infection | 25 (13) | 18 (24) | 11 (16) | 9 (29) |
| Organ failure | 21 (12) | 6 (8) | 5 (7) | 9 (29) |
| Pulmonary emboli | 3 (2) | 1 (1) | ||
| Hemorrhage | 4 (2) | 3 (4) | 3 (4) | |
| Metabolic (electrolyte abnormalities) | 2 (1) | |||
| Second malignant neoplasm | 3 (2) | 1 (3) | ||
| Accidental | 2 (6) | |||
| Not reported | 2 (1) | 6 (8) | 5 (7) | 1 (3) |
| . | 8/8 PBPCs (%) . | 7/8 PBPCs (%) . | dUCB-TCF (%) . | dUCB-other (%) . |
|---|---|---|---|---|
| No. of deaths | 189 | 75 | 70 | 31 |
| Graft failure | 2 (1) | 3 (4) | ||
| Recurrent leukemia | 100 (53) | 30 (40) | 35 (50) | 4 (13) |
| GVHD | 22 (12) | 9 (12) | 3 (4) | 5 (16) |
| Interstitial pneumonitis/ARDS | 5 (3) | 2 (3) | 5 (7) | |
| Infection | 25 (13) | 18 (24) | 11 (16) | 9 (29) |
| Organ failure | 21 (12) | 6 (8) | 5 (7) | 9 (29) |
| Pulmonary emboli | 3 (2) | 1 (1) | ||
| Hemorrhage | 4 (2) | 3 (4) | 3 (4) | |
| Metabolic (electrolyte abnormalities) | 2 (1) | |||
| Second malignant neoplasm | 3 (2) | 1 (3) | ||
| Accidental | 2 (6) | |||
| Not reported | 2 (1) | 6 (8) | 5 (7) | 1 (3) |
PBPC indicates peripheral blood progenitor cell; and ARDS, acute respiratory distress syndrome.
TRM and relapse
The 2-year cumulative incidences of TRM were 19% (95% CI, 11%-26%), 52% (95% CI, 33%-71%), 21% (95% CI, 16%-25%), and 28% (95% CI, 20%-37%) for recipients of dUCB-TCF, dUCB-other regimens, 8 of 8 and 7 of 8 HLA-matched transplantations, respectively (Figure 2). In multivariable analysis, there were no significant differences in risks of TRM for recipients of dUCB-TCF and 8 of 8 HLA-matched PBPC transplantations (Table 3). We observed lower TRM risks after dUCB-TCF transplantations compared with 7 of 8 HLA-matched transplantations. In contrast, TRM risks were similar in recipients of dUCB-other regimens compared with 7 of 8 HLA-matched transplantations and higher compared with 8 of 8 HLA-matched transplantations (Table 2). No other patient or disease characteristic was associated with TRM.
The probabilities of transplantation-related mortality after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations.
The probabilities of transplantation-related mortality after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations.
The 2-year cumulative incidences of relapse were 49% (95% CI, 38%-59%), 35% (95% CI, 19%-50%), 44% (95% CI, 38%-51%), and 44% (95% CI, 33%-54%) for recipients of dUCB-TCF, dUCB-other regimens, 8 of 8 and 7 of 8 HLA-matched PBPC transplantations, respectively. There was no difference in relapse risk by graft source (Table 3). Compared with patients who received transplants in first CR, relapse risk was higher in patients who received transplants in second or third CR (RR 1.49, 95% CI 1.09-2.04, P = .011) or in relapse or primary induction failure (RR 1.71, 95% CI 1.21-2.41, P = .002). Notably, patients who developed chronic GVHD had lower relapse risks compared with those without chronic GVHD (RR 0.55, 95% CI 0.38-0.79, P = .002), independent of graft type.
GVHD
The day-100 cumulative incidences of grade II-IV acute GVHD were 50% (95% CI, 41%-58%), 33% (95% CI, 19%-48%), 33% (95% CI, 28%-39%), and 40% (95% CI, 31%-49%) for recipients of dUCB-TCF, dUCB-other regimens, 8 of 8 HLA-matched and 7 of 8 HLA-matched PBPC transplantations, respectively. Corresponding cumulative incidences of grade III-IV acute GVHD were 17% (95% CI, 11%-25%), 18% (95% CI, 8%-31%), 14% (95% CI, 10%-18%), and 23% (95% CI, 16%-32%). In multivariable analysis, risks of grade II-IV but not grade III-IV acute GVHD were higher after dUCB-TCF compared with 8 of 8 HLA-matched PBPC transplantations (Table 3). Risks of grade II-IV acute GVHD (RR 1.46, 95% CI 1.10-1.93, P = .008) and grade III-IV acute GVHD were higher (RR 1.67, 95% CI 1.10-2.52, P = .02) for any transplantation in 2001-2004 compared with those in 2005-2009.
The 2-year cumulative incidences of chronic GVHD were 34% (95% CI, 25%-43%), 36% (95% CI, 22%-51%), 56% (95% CI, 50%-62%), and 54% (95% CI, 44%-64%) for recipients of dUCB-TCF, dUCB-other regimens, 8 of 8 and 7 of 8 HLA-matched PBPC transplantations, respectively. Risks of chronic GVHD were lower in recipients of dUCB-TCF compared with 8 of 8 and 7 of 8 HLA-matched recipients of PBPC transplantations. There were no differences in chronic GVHD risks between recipients of dUCB-other RIC and 8 of 8 and 7 of 8 HLA-matched transplantations (Table 3). Chronic GVHD risks were higher in the period 2001-2004 (RR 1.75, 95% CI 1.35-2.27, P = .0001) compared with the period 2005-2009.
Hematopoietic recovery
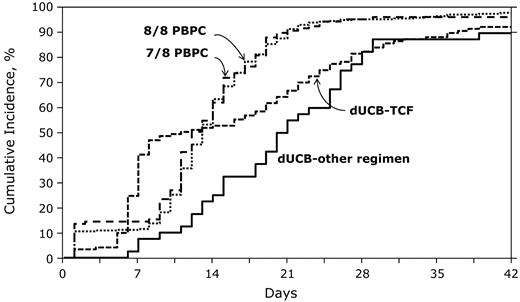
The day-28 cumulative incidences of neutrophil recovery were 83% (95% CI, 75%-89%) and 83% (95% CI, 69%-93%) after dUCB-TCF and dUCB-other regimens, respectively (Figure 3) with corresponding probabilities of 93% (95% CI, 87%-96%) and 90% (95% CI, 79%-97%) at day 42. By comparison, the day-28 cumulative incidences of neutrophil recovery were 93% (95% CI, 88%-97%) and 92% (95% CI, 83%-98%) after 8 of 8 and 7 of 8 HLA-matched PBPC transplantation, respectively, with the corresponding probabilities of 98% (95% CI, 96%-99%) and 96% (95% CI, 93%-99%) at day 42. The median time to neutrophil recovery after dUCB-TCF transplantation was 9 days and after dUCB-other regimens, 20 days. Corresponding times after 8 of 8 and 7 of 8 HLA-matched PBPC transplantations were 13 and 12 days, respectively. Compared with 8 of 8 HLA-matched PBPC transplantations, the likelihood of neutrophil recovery at day 28 was significantly lower after transplantation of dUCB-TCF (OR 0.21, 95% CI 0.10-0.46, P < .0001) and dUCB-other regimens (OR 0.16, 95% CI 0.07-0.38, P < .0001). Neutrophil recovery was also lower compared with 7 of 8 HLA-matched PBPC transplantations; OR 0.21, 95% CI 0.07-0.72, P = .013 and OR 0.16, 95% CI 0.05-0.58, P = .005 after dUCB-TCF and dUCB-other regimens transplantations, respectively. Primary graft failure was higher after dUCB transplantations compared with 8 of 8 and 7 of 8 PBPC transplantations (P < .001). Eleven of 114 (9%) patients in the dUCB-TCF group and 4 of 40 (10%) patients in the dUCB-other regimens group had primary graft failure (defined as failure to achieve neutrophil recovery or < 5% chimerism). In contrast, 8 (3%) of 313 patients in the 8 of 8 HLA-matched PBPC group and 3 (3%) of 111 patients in the 7 of 8 HLA-matched PBPC group had primary graft failure. Platelet recovery was lower after dUCB compared with PBPC transplantation. The 6-month cumulative incidences of platelet recovery (≥ 50 000/mm3) was 66% (95% CI, 58%-74%) and 58% (95% CI, 42%-72%) after dUCB-TCF and dUCB other regimen transplantations. Corresponding incidences of platelet recovery after 8 of 8 and 7 of 8 PBPC transplantations were 90% (95% CI, 86%-93%) and 89% (95% CI, 83%-94%).
The probabilities of neutrophil recovery after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations.
The probabilities of neutrophil recovery after dUCB-TCF, dUCB-other regimen, 8/8 PBPCs, and 7/8 PBPC transplantations.
Discussion
Increasing numbers of allogeneic transplantations in adults use RIC rather than a myeloablative therapy either because of advanced age, active infections, or presence of comorbidities at transplantation. The primary objective of this analysis was to assess the relative efficacy of dUCB compared with the standard of 8 of 8 HLA-matched PBPCs, the most commonly used sources of allogeneic hematopoietic cells in adults with acute leukemia treated with RIC. This analysis demonstrates the suitability of partially HLA-matched dUCB as an alternative to 8 of 8 HLA-matched PBPCs with the comparable results in the subset of dUCB patients treated with TCF. Higher TRM and lower OS and LFS were observed in recipients of dUCB transplantation if they were treated with alternative regimens. The majority of alternative regimens in the current analysis included busulfan, mephalan, or cyclophosphamide, fludarabine, and in vivo T-cell depletion with ATG. While TRM was higher in recipients of 7 of 8 compared with 8 of 8 HLA-matched PBPCs, OS and LFS were similar.
In the current analysis, most recipients of dUCB-TCF received transplants at a single center. However, we did not demonstrate a center effect. Furthermore, the relatively low rate of TRM after dUCB-TCF regimens was also observed in a recent registry European Group for Blood and Marrow Transplantation (EBMT) analysis26 and a multicenter phase 2 trial in the United States that tested the TCF regimen with dUCB in adults with leukemia and lymphoma.27 Higher TRM has also been observed in recipients of RIC regimens who also received in vivo T-cell depletion with ATG treated for hematologic malignancies.28 In this analysis, most recipients of dUCB-other regimens had also received ATG, which may explain the excess incidence of TRM. The relatively small numbers of dUCB-other regimens and the near universal inclusion of ATG prevent us from undertaking a more detailed analysis of this group of patients. While the benefit of TCF can only be proven by a prospective clinical trial in a homogenous patient population, a series of retrospective reports showing similar relative results suggest a benefit of TCF when considering a RIC regimen and dUCB for acute leukemia. In the meantime, RIC regimens other than TCF in recipients of dUCB transplantation for acute leukemia should be used cautiously. In contrast to the TCF regimen data that reflects the experience on a large number of patients, the data on the other regimens represent a small number of patients from individual institutions. While it could be argued that the significant contribution of single institution may have skewed results, no center effect was demonstrated as the outcomes of other center that used TCF and a results of recent prospective multicenter study27 yielded similar results. Notably, risk of relapse did not differ between stem cell sources. While dUCB transplantation has been associated with a lower risk of relapse in patients with acute leukemia compared with single UCB transplantation after a myeloablative conditioning,29 others observed lower risks after both myeloablative regimens and RIC but only in patients in first CR.30 However, in the current analysis, after adjusting for disease status at transplantation, relapse risk was not significantly different after dUCB and PBPC transplantation.
In our study, the risk of acute GVHD was higher after dUCB-TCF compared with MUD (matched unrelated donor) and MMUD (mismatched unrelated donor) in contrast to other studies that compared single4,6 and double UCB transplantation7 to unrelated adult donor transplantation. The observed higher acute GVHD was limited to grade II and occurred only before 2005. Most transplantations during the early period occurred at a single institution.27 In contrast to acute GVHD, the risk of chronic GVHD after dUCB-TCF or dUCB-other RIC was significantly lower than that after 8 of 8 and 7 of 8 HLA-matched PBPC transplantations. While quality-of-life data31 were not collected in this study, chronic GVHD has been shown to adversely effect quality of life, and chronic GVHD risks are significantly higher after 8 of 8 or 7 of 8 PBPC transplantations. Certainly, it is plausible that there are differences in quality of life among recipients of dUCB and PBPC transplantations and should be systematically studied in the future.
Consistent with most other reports, neutrophil recovery after dUCB transplantation was inferior to that after 8 of 8 and 7 of 8 PBPC transplantations. Neutrophil recovery correlates with cell dose of the graft and recipients of PBPC transplantation received doses in excess of that delivered with dUCB graft. It is noteworthy that TRM was not significantly different after dUCB-TCF and PBPC transplantations despite lower recovery rates in the former group. However, TRM was high for recipients of dUCB-other regimens implying that impaired neutrophil recovery is not the only explanation for excess TRM. The use of alkylating agents, fludarabine, and in vivo T-cell depletion as transplantation conditioning may have played a role in the observed excess TRM and lower OS in recipients of dUCB-other regimens.28 Another plausible explanation for the observed excess TRM in recipients of dUCB other regimens is that approximately one-third of these patients received transplants with active disease compared with ∼ 5% of dUCB-TCF recipients. Unfortunately, the relatively small numbers of patients in this group and the near universal use of in vivo T-cell depletion with non-TBI regimens prohibit us from further analysis. Another limitation of the current analysis is that we were not able to test for the effect of the “best” transplantation conditioning regimen in recipients of 8 of 8 HLA-matched PBPCs to those who received dUCB-TCF (ie, a regimen devoid of ATG); 45% of patients who received an alkylating agent and fludarabine also received ATG.
Certainly, it is acknowledged that a randomized trial is required to definitively address the relative risks and benefits of PBPCs versus dUCB in patients with a similar disease and conditioning regimen. However, until such a trial is accomplished, this carefully controlled analysis by a large registry support the use of dUCB grafts with TCF as a conditioning regimen as a suitable alternative for patients who may benefit from RIC HSCT and do not have a suitable unrelated volunteer donor in the time period transplantation is needed. Carefully planned prospective trials are needed to explore the effect of alkylating agent, fludarabine, and ATG-containing regimens for dUCB transplantations before widespread adoption of these regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, Health Resources and Services Administration (HHSH234200637015C), and the Office of Naval Research, Department of Navy, to the National Marrow Donor Program (N00014-10-01-0204).
The opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not reflect the views of the Office of Naval Research or the National Marrow Donor Program.
National Institutes of Health
Authorship
Contribution: C.G.B., M.E., and J.E.W. contributed equally to study design, data interpretation, and drafting the manuscript; K.W.A. performed the statistical analysis; F.R.A., K.K.B., R.E.C., C.C., M.J.L., R.J.S., D.J.W., and A.W. contributed to data interpretation and critically reviewed the manuscript; F.K. prepared the data file; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio G. Brunstein, MD, PhD, Blood and Marrow Transplant Program, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: bruns072@umn.edu.