Abstract
The K blood group remains an important target in hemolytic disease of the newborn (HDN), with no immune prophylaxis available. The aim was to characterize the Th response to K as a key step in designing specific immunotherapy and understanding the immunogenicity of the Ag. PBMCs from K-negative women who had anti-K Abs after incompatible pregnancy, and PBMCs from unimmunized controls, were screened for proliferative responses to peptide panels spanning the K or k single amino acid polymorphism. A dominant K peptide with the polymorphism at the C terminus elicited proliferation in 90% of alloimmunized women, and it was confirmed that responding cells expressed helper CD3+CD4+ and “memory” CD45RO+ phenotypes, and were MHC class II restricted. A relatively high prevalence of background peptide responses independent of alloimmunization may contribute to K immunogenicity. First, cross-reactive environmental Ag(s) pre-prime Kell-reactive Th cells, and, second, the K substitution disrupts an N-glycosylation motif, allowing the exposed amino acid chain to stimulate a Th repertoire that is unconstrained by self-tolerance in K-negative individuals. The dominant K peptide was effective in inducing linked suppression in HLA-transgenic mice and can now be taken forward for immunotherapy to prevent HDN because of anti-K responses.
Introduction
K is the most immunogenic and clinically important Kell blood group Ag and a target for harmful alloantibody responses when there is either transfusion of mismatched blood, or incompatibility between mother and fetus.1 Maternal anti-K Abs that cross the placenta during an incompatible pregnancy can suppress fetal erythropoiesis and cause hemolytic disease of the newborn (HDN).2,3 Unlike the RhD blood group Ag, which was the most frequent blood group targeted in HDN until the advent of effective prophylaxis with anti-D Ab, there are no specific immune-based treatments to prevent or control K alloimmunization in K-negative women. The management of pregnancies affected by anti-K Abs therefore remains unsatisfactory and reliant on invasive procedures such as fetal blood sampling and transfusion.4 Advances in the understanding of immune tolerance now raise the prospect of effective immunotherapy,5-7 but the exploitation of such an approach first requires detailed characterization of the pathogenic anti-K response.
Definition of the molecular basis of the Kell Ags now provides the opportunity to analyze the immune responses they elicit. Kell is a 93-kDa type II glycoprotein peptidase that is expressed primarily on erythroid tissues and encodes at least 20 blood group Ags. K is carried by 9% of the white population and differs from the antithetical k because of a single nucleotide polymorphism, C to T, in exon 6 at position 698.8,9 This substitution results in the expression of methionine, rather than threonine, at residue amino acid 193 to create the K Ag.8,9 The loss of threonine also disrupts an N-glycosylation site at amino acid 191,9 but this is not thought to contribute directly to the formation of anti-K Ab binding sites. Characterization of the immune response to K may lead not only to a therapeutic benefit, but also help to explain the surprising immunogenicity of such blood groups that arise from only single amino acid substitutions.
Conventional IgG Ab responses are helper dependent,10 including all those studied that are specific for RBC Ags.11 It follows that the differentiation of B cells to produce IgG maternal anti-K alloantibodies is also likely to require stimulation from CD4+ Th cells responding to the same or a linked Ag. Unlike B cells, which often recognize conformational epitopes dependent on secondary or tertiary protein structure, Th cells are specific for short sequences of amino acids processed from the Ag and displayed bound to MHC class II molecules on the surface of APCs.5-7 Given that the K Ag is determined by a single amino acid substitution, the Th cells responsible for providing help for anti-K alloantibodies could well recognize peptide(s) spanning the polymorphic site. However, despite the advent of predictive algorithms, mapping experiments are required to identify Th epitope(s) definitively12,13 and, in the case of K, the precise register(s) of the M193 residue for optimum immunogenicity.
Peptide(s) that contain alloreactive Th epitope(s) spanning the K polymorphism would represent potential immunotherapeutics to prevent or switch off anti-K Ab responses. There is abundant evidence from rodent models,6,14-16 and support from early human trials,17,18 that damaging immune responses can be prevented or ameliorated by tolerogenic delivery of peptides containing dominant Th epitopes. Such tolerization of specific Th cells can be exploited to inhibit pathogenic Ab production, and the application of this approach to prevent HDN caused by anti-K would represent an important therapeutic advance. The aim of this work, therefore, was to characterize the Th cells that drive the alloresponse to K, and, in particular, to identify the epitope(s) that they recognize spanning the polymorphic site on the Kell protein and any features contributing to immunogenicity. It was then confirmed that a peptide containing the dominant epitope was capable of inducing linked suppression in a humanized mouse model of immunization.
Methods
Donors
Approval for the study was received from the Grampian Local and Regional Ethics Committee, and informed consent was obtained from all donors in accordance with the Declaration of Helsinki. PBMCs were isolated from 11 healthy K-negative women who had K alloantibodies in their sera as a result of incompatible pregnancy (Table 1). Control PBMCs were also obtained from 11 healthy K-negative unimmunized donors with no detectable anti-K serum alloantibodies (Table 1). None of the 22 individuals studied had Abs to any other blood group Ags. Blood samples for PBMCs and serum separation were taken by venepuncture, respectively, into lithium heparin and plain Vacutainers (BD Biosciences).
Details of K-negative alloimmunized and control donors
| Donor . | Sex . | Age, y . | Kell genotype . | HLA-DR genotype . | Anti-K Ab in serum, titer . |
|---|---|---|---|---|---|
| Alloimmune | |||||
| 1 | F | 43 | KEL*2, KEL*2 | DRB1*13, DRB1*14 | 40 |
| 2 | F | 54 | KEL*2, KEL*2 | DRB1*15, DRB1*07 | Positive† |
| 3 | F | 35 | KEL*2, KEL*2 | DRB1*01, DRB1*11 | 40 |
| 4 | F | 62 | KEL*2, KEL*2 | DRB1*03, DRB1*15 | 5120 |
| 5 | F | 42 | KEL*2, KEL*2 | DRB1*13, DRB1*04 | 40 |
| 6 | F | 38 | KEL*2, KEL*2 | NT | 1 |
| 7 | F | 38 | KEL*2, KEL*2 | DRB1*01, DRB1*01 | Positive† |
| 8 | F | 44 | KEL*2, KEL*2 | DRB1*04, DRB1*15 | 2560 |
| 9 | F | 53 | KEL*2, KEL*2 | DRB1*07, DRB1*07 | 160 |
| 10 | F | 53 | KEL*2, KEL*2 | DRB1*04, DRB1*15 | Positive† |
| Control | |||||
| 1 | F | 43 | KEL*2, KEL*2 | NT | Negative |
| 2 | M | 43 | KEL*2, KEL*2 | DRB1*01, DRB1*04 | Negative |
| 3 | F | 41 | KEL*2, KEL*2 | DRB1*07, DRB1*07 | Negative |
| 4 | M | 36 | KEL*2, KEL*2 | DRB1*04, DRB1*04 | Negative |
| 5 | M | 48 | KEL*2, KEL*2 | DRB1*03, DRB1*04 | Negative |
| 6 | F | 52 | KEL*2, KEL*2 | DRB1*07, DRB1*13 | Negative |
| 7 | F | 47 | KEL*2, KEL*2 | DRB1*02, DRB1*02 | Negative |
| 8 | M | 37 | KEL*2, KEL*2 | DRB1*01, DRB1*11 | Negative |
| 9 | M | 42 | KEL*2, KEL*2 | NT | Negative |
| 10 | M | 38 | KEL*2, KEL*2 | DRB1*03, DRB1*14 | Negative |
| Donor . | Sex . | Age, y . | Kell genotype . | HLA-DR genotype . | Anti-K Ab in serum, titer . |
|---|---|---|---|---|---|
| Alloimmune | |||||
| 1 | F | 43 | KEL*2, KEL*2 | DRB1*13, DRB1*14 | 40 |
| 2 | F | 54 | KEL*2, KEL*2 | DRB1*15, DRB1*07 | Positive† |
| 3 | F | 35 | KEL*2, KEL*2 | DRB1*01, DRB1*11 | 40 |
| 4 | F | 62 | KEL*2, KEL*2 | DRB1*03, DRB1*15 | 5120 |
| 5 | F | 42 | KEL*2, KEL*2 | DRB1*13, DRB1*04 | 40 |
| 6 | F | 38 | KEL*2, KEL*2 | NT | 1 |
| 7 | F | 38 | KEL*2, KEL*2 | DRB1*01, DRB1*01 | Positive† |
| 8 | F | 44 | KEL*2, KEL*2 | DRB1*04, DRB1*15 | 2560 |
| 9 | F | 53 | KEL*2, KEL*2 | DRB1*07, DRB1*07 | 160 |
| 10 | F | 53 | KEL*2, KEL*2 | DRB1*04, DRB1*15 | Positive† |
| Control | |||||
| 1 | F | 43 | KEL*2, KEL*2 | NT | Negative |
| 2 | M | 43 | KEL*2, KEL*2 | DRB1*01, DRB1*04 | Negative |
| 3 | F | 41 | KEL*2, KEL*2 | DRB1*07, DRB1*07 | Negative |
| 4 | M | 36 | KEL*2, KEL*2 | DRB1*04, DRB1*04 | Negative |
| 5 | M | 48 | KEL*2, KEL*2 | DRB1*03, DRB1*04 | Negative |
| 6 | F | 52 | KEL*2, KEL*2 | DRB1*07, DRB1*13 | Negative |
| 7 | F | 47 | KEL*2, KEL*2 | DRB1*02, DRB1*02 | Negative |
| 8 | M | 37 | KEL*2, KEL*2 | DRB1*01, DRB1*11 | Negative |
| 9 | M | 42 | KEL*2, KEL*2 | NT | Negative |
| 10 | M | 38 | KEL*2, KEL*2 | DRB1*03, DRB1*14 | Negative |
NT indicates not tested.
Donors having anti-K alloantibodies but levels too low for accurate Ab titer.
Ags and mitogens
Two panels of 15-mer peptides were synthesized (Pepceuticals Limited), corresponding to the Kell protein sequence spanning polymorphic residue amino acid 193, with either M193 (K) or T193 (k) included at every possible position (Table 2), together with a control peptide of the reverse sequence to 1M(179-193) (MRNFNLSTWKGSIRW). A glycosylated version of Kell peptide 1T(179-193), WRISGKWTSLNF-Asn(AcNH-b-Glc)-RT, was also synthesized by Cambridge Research Biochemicals using solid-phase chemistry, with a single Fmoc-Asn (Ac3-AcNH-b-Glc)-OH residue coupled manually. Peptides were checked for purity by mass spectrometry and used to stimulate PBMC cultures at a final concentration of 20 μg/mL.
Sequences of the Kell peptide panels
| Peptide . | Sequence . | Residues . |
|---|---|---|
| 1M | WRISGKWTSLNFNRM | 179-193 |
| 2M | RISGKWTSLNFNRML | 180-194 |
| 3M | ISGKWTSLNFNRMLR | 181-195 |
| 4M | SGKWTSLNFNRMLRL | 182-196 |
| 5M | GKWTSLNFNRMLRLL | 183-197 |
| 6M | KWTSLNFNRMLRLLM | 184-198 |
| 7M | WTSLNFNRMLRLLMS | 185-199 |
| 8M | TSLNFNRMLRLLMSQ | 186-200 |
| 9M | SLNFNRMLRLLMSQY | 187-201 |
| 10M | LNFNRMLRLLMSQYG | 188-202 |
| 11M | NFNRMLRLLMSQYGH | 189-203 |
| 12M | FNRMLRLLMSQYGHF | 190-204 |
| 13M | NRMLRLLMSQYGHFP | 191-205 |
| 14M | RMLRLLMSQYGHFPF | 192-206 |
| 15M | MLRLLMSQYGHFPFF | 193-207 |
| 1T | WRISGKWTSLNFNRT | 179-193 |
| 2T | RISGKWTSLNFNRTL | 180-194 |
| 3T | ISGKWTSLNFNRTLR | 181-195 |
| 4T | SGKWTSLNFNRTLRL | 182-196 |
| 5T | GKWTSLNFNRTLRLL | 183-197 |
| 6T | KWTSLNFNRTLRLLM | 184-198 |
| 7T | WTSLNFNRTLRLLMS | 185-199 |
| 8T | TSLNFNRTLRLLMSQ | 186-200 |
| 9T | SLNFNRTLRLLMSQY | 187-201 |
| 10T | LNFNRTLRLLMSQYG | 188-202 |
| 11T | NFNRTLRLLMSQYGH | 189-203 |
| 12T | FNRTLRLLMSQYGHF | 190-204 |
| 13T | NRTLRLLMSQYGHFP | 191-205 |
| 14T | RTLRLLMSQYGHFPF | 192-206 |
| 15T | TLRLLMSQYGHFPFF | 192-207 |
| Peptide . | Sequence . | Residues . |
|---|---|---|
| 1M | WRISGKWTSLNFNRM | 179-193 |
| 2M | RISGKWTSLNFNRML | 180-194 |
| 3M | ISGKWTSLNFNRMLR | 181-195 |
| 4M | SGKWTSLNFNRMLRL | 182-196 |
| 5M | GKWTSLNFNRMLRLL | 183-197 |
| 6M | KWTSLNFNRMLRLLM | 184-198 |
| 7M | WTSLNFNRMLRLLMS | 185-199 |
| 8M | TSLNFNRMLRLLMSQ | 186-200 |
| 9M | SLNFNRMLRLLMSQY | 187-201 |
| 10M | LNFNRMLRLLMSQYG | 188-202 |
| 11M | NFNRMLRLLMSQYGH | 189-203 |
| 12M | FNRMLRLLMSQYGHF | 190-204 |
| 13M | NRMLRLLMSQYGHFP | 191-205 |
| 14M | RMLRLLMSQYGHFPF | 192-206 |
| 15M | MLRLLMSQYGHFPFF | 193-207 |
| 1T | WRISGKWTSLNFNRT | 179-193 |
| 2T | RISGKWTSLNFNRTL | 180-194 |
| 3T | ISGKWTSLNFNRTLR | 181-195 |
| 4T | SGKWTSLNFNRTLRL | 182-196 |
| 5T | GKWTSLNFNRTLRLL | 183-197 |
| 6T | KWTSLNFNRTLRLLM | 184-198 |
| 7T | WTSLNFNRTLRLLMS | 185-199 |
| 8T | TSLNFNRTLRLLMSQ | 186-200 |
| 9T | SLNFNRTLRLLMSQY | 187-201 |
| 10T | LNFNRTLRLLMSQYG | 188-202 |
| 11T | NFNRTLRLLMSQYGH | 189-203 |
| 12T | FNRTLRLLMSQYGHF | 190-204 |
| 13T | NRTLRLLMSQYGHFP | 191-205 |
| 14T | RTLRLLMSQYGHFPF | 192-206 |
| 15T | TLRLLMSQYGHFPFF | 192-207 |
The Kell peptide panels consisted of 2 sets of 15-mer peptides spanning the K/k polymorphism. Peptides 1-15M and 1-15T include the K and k polymorphism, respectively (polymorphic site shown in bold).
The mitogen Con A (Sigma-Aldrich) and the nominal Ag Mycobacterium tuberculosis purified protein derivative (PPD; Statens Seruminstitut) were used as positive control T-cell stimuli and added to cultures at a final concentration of 10 μg/mL. PPD readily invokes recall T-cell responses in a high proportion of United Kingdom citizens19 as most have been immunized with the Bacilli Calmette-Guérin vaccine.
A conjugate immunogen was a gift from Prof R. Fraser and Dr R. Drake (Scottish National Blood Transfusion Service). It was made by cross-linking a synthetic peptide corresponding to Kell protein residues amino acids 184-197, and spanning the K polymorphic residue M193, to the carrier keyhole limpet hemocyanin (KLH). This conjugate was prepared for immunization by emulsification in IFA and 2% Tween 80 in saline.
Isolation and culture of PBMCs
PBMCs were isolated from fresh whole blood by density gradient centrifugation (Lymphoprep 1077; Nycomed). The viability of the PBMCs was > 90%, as confirmed by trypan blue staining. As previously described,13,19-21 PBMCs were cultured at a final concentration of 1.25 × 106 cells/mL in α modification of Eagle medium (α-MEM; Invitrogen) supplemented with 1% 2mM l-glutamine (Invitrogen), 2% 20mM HEPES buffer (Sigma-Aldrich), 2% penicillin streptomycin (Invitrogen), and 5% autologous sera. PBMCs were incubated with peptides or control stimuli for 5 days at 37°C in a humidified atmosphere of 5% CO2/95% air.
T-cell proliferation assay
The rate of proliferation of cell cultures was estimated by the incorporation of 3H-thymidine into newly synthesized DNA after 5 days of stimulation, as described previously.13,19-21 The proliferation by the cells was recorded as mean cpm ± SD of the triplicate samples, or as a stimulation index (SI) expressing the rate of mean cpm in stimulated versus unstimulated control cultures. An SI > 3 was interpreted as a proliferative response.21
Characterization of responding cells
The phenotype of the cells responding in cultures was determined using flow cytometry. Cells were stained with anti-CD3 PE Texas Red, anti-CD4 FITC, anti-CD71 PE (all from Beckman Coulter) and CFSE (Invitrogen). Stained cells were analyzed on a Beckman Coulter Epics XL flow cytometer with a minimum of 10 000 events counted. The flow cytometric data were processed using Expo V2 analysis software (Applied Cytometry Systems).
HLA-blocking Abs
Preparation of CD45RO+ and CD45RA+ depleted PBMCs
PBMCs depleted of CD45RO+ or CD45RA+ T cells were prepared as previously described.19,21-23 Briefly, PBMCs were incubated with murine mAb UCHL1 (specific for human CD45RO) or SN130 (specific for human CD45RA; gifts from Profs P. C. L. Beverley (University College London, London, United Kingdom) and G. Janossy (Royal Free Hospital, London, United Kingdom). Cells that bound mAb were removed by immunomagnetic separation using ferrous beads coated with Ab specific for mouse IgG (Biomag; PerSeptive Biosystems). Each depleted population contained < 10% cells expressing the respective CD45 isoform, as determined by flow cytometry (FACSCalibur).
Administration of Ag to HLA transgenic mice
All animal experiments were carried out under a project license granted by the United Kingdom Home Office and approved by the University of Aberdeen Local Ethical Committee. The generation of HLA-DR15 transgenic mice by Professor D. Altmann (Imperial College London) is described in detail elsewhere.24,25 Each mouse was positive for the genetic modification by PCR of DNA samples extracted from tail tips or ear punches, and transgenic expression of the human DR15 MHC class II molecule on APC was confirmed by flow cytometry. To induce responses, 100 μg of Kell-KLH conjugate or control unlinked KLH was injected subcutaneously in IFA to mice aged 12 weeks, followed at 14-day intervals by 2 IP boosters of the respective immunogen. For the induction of suppression, 100 μg/mL candidate dominant K1 peptide or control peptide with reverse sequence was administered to the nasal mucosa of HLA-DR15 transgenic mice either 2 weeks before immunization or 2 weeks after the last booster.
Measurement of murine T-cell responses
To measure murine T-cell responses, spleens were taken from immunized transgenic mice 2 weeks after the last boosting or suppressing treatment and single-cell suspensions were generated by homogenization. The splenocytes were incubated in α-MEM (Invitrogen) at a concentration of 2 × 106 cells/mL under conditions described above for isolation and culture of PBMCs, but supplemented with 0.5% complement inactivated murine serum and 50μM β-mercaptoethanol (Sigma-Aldrich).24 T-cell proliferation was estimated from the incorporation of 3H-thymidine in triplicate microtiter wells 5 days after stimulation. These experiments focused on splenocytes because T cells from a selection of lymph nodes demonstrated no consistent responsiveness to the immunization regimen.
Statistical analysis
All statistical analysis was carried out using SPSS 15.0 for Windows and SigmaStat 2.0 (Jandel Scientific). The χ2, Mann-Whitney U, Wilcoxon rank-sum, or the Student t test on log-transformed data, were used where appropriate. P < .05 was taken as being significant.
Results
Proliferative responses to the Kell peptide panels by PBMCs from alloimmune K-negative donors
The initial aim was to map the epitope(s) containing the M193 K polymorphism that are recognized by alloreactive Th cells from K-negative women who have produced anti-K alloantibodies after incompatible pregnancy with a K-positive fetus. PBMCs were obtained from 10 alloimmunized women (Table 1) and used to screen 2 panels of 15-mer linear peptides spanning the polymorphic region, with either M193 (K) or T193 (k) at each possible position (Table 2), for the ability to stimulate in vitro proliferation.
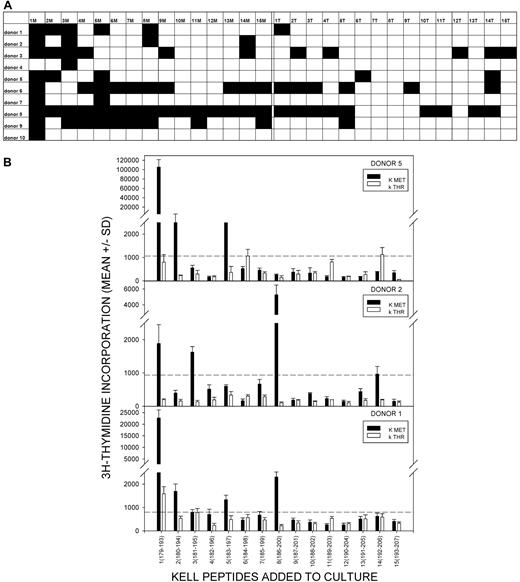
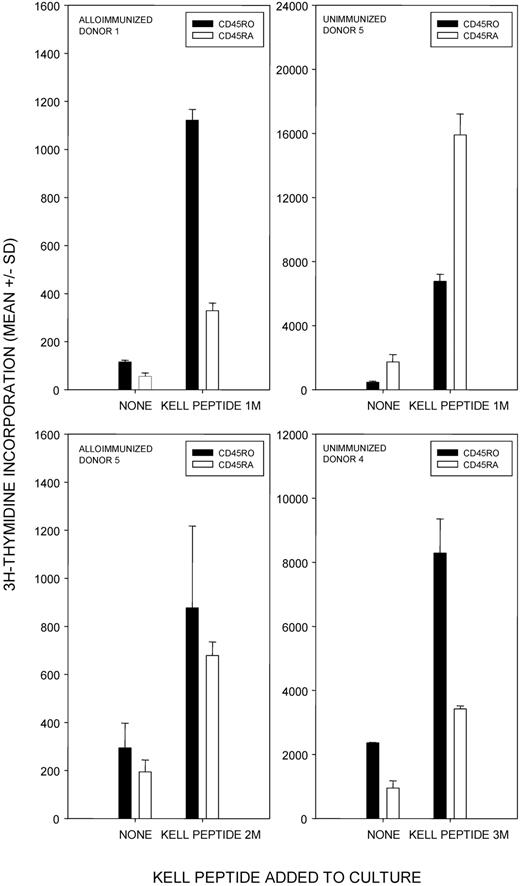
The results are summarized in Figure 1A, with representative proliferative responses from 3 anti-K alloantibody positive women illustrated in Figure 1B. Responses to one or more K peptides were observed in each alloimmune individual, and peptide 1M(179-193) with the polymorphic residue at the C terminus was the most commonly stimulatory sequence, eliciting proliferation in 90% of the donors. Furthermore, where multiple sequences were stimulatory, responses induced by the peptide 1M(179-193) were typically the strongest. Other, less dominant, peptides that elicited proliferation in several of the women included 3M(181-195), 5M(183-197), and 8M(186-200).
Proliferative responses to the panel of K and k Kell peptides by PBMCs from K-alloimmunized women are focused on K sequences. (A) Summary map of responses by PBMCs from 10 K-alloimmunized women to the panel of peptides spanning the M193 (K) and T193 (k) polymorphism. Filled boxes represent positive proliferative responses (SI > 3). K peptides elicited more responses than k (χ2 test P < .001), and K peptide 1M(179-193) was the predominant stimulatory sequence (90% donors were positive). (B) Representative proliferative responses to the Kell peptide panel by PBMCs from alloimmunized donors 1, 2, and 5. Dotted line denotes the level of proliferation taken as representing a positive response (SI > 3). These examples illustrate that, despite the predominance of responses to peptide 1M(179-193), other peptides can elicit proliferation, including k sequences. PBMCs from all donors proliferated significantly in response to the control recall Ag PPD.
Proliferative responses to the panel of K and k Kell peptides by PBMCs from K-alloimmunized women are focused on K sequences. (A) Summary map of responses by PBMCs from 10 K-alloimmunized women to the panel of peptides spanning the M193 (K) and T193 (k) polymorphism. Filled boxes represent positive proliferative responses (SI > 3). K peptides elicited more responses than k (χ2 test P < .001), and K peptide 1M(179-193) was the predominant stimulatory sequence (90% donors were positive). (B) Representative proliferative responses to the Kell peptide panel by PBMCs from alloimmunized donors 1, 2, and 5. Dotted line denotes the level of proliferation taken as representing a positive response (SI > 3). These examples illustrate that, despite the predominance of responses to peptide 1M(179-193), other peptides can elicit proliferation, including k sequences. PBMCs from all donors proliferated significantly in response to the control recall Ag PPD.
In addition to proliferating against K sequences, PBMCs from alloimmune donors also demonstrated some responsiveness, albeit significantly less commonly, to the panel of control k peptides (χ2 test P < .001, comparing total number of K and k peptide responses in all alloimmune donors). Unlike the K sequences, among which 1M(179-193) elicited strong proliferation by PBMCs from a large majority of the alloimmune women, there was no clear dominant k peptide.
Proliferative responses to the Kell peptide panels by PBMCs from control unimmunized K-negative donors
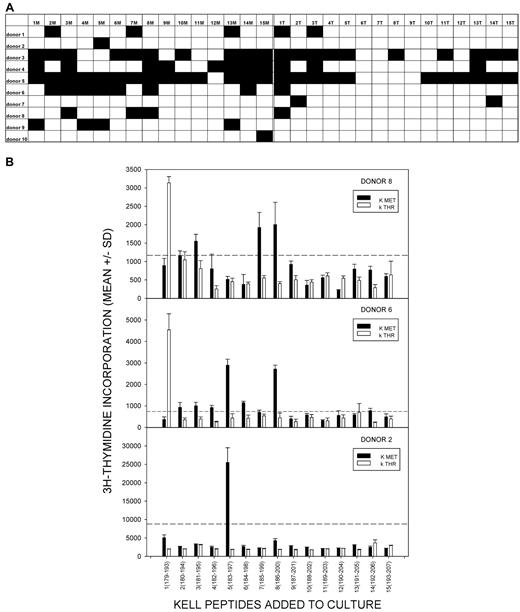
To test whether the proliferative responses to K peptides, particularly 1M(179-193), were associated with exposure to K-positive RBCs and the development of alloantibody, PBMC samples were also tested from 10 K-negative control unimmunized donors, none of whom had any detectable K alloantibodies in their sera (Table 1). PBMCs were again stimulated with the K and k peptide panels, and the proliferative responses are summarized in Figure 2A with representative examples shown in Figure 2B. Proliferation to the peptide panels was not uncommon in the control group, despite the lack of exposure to mismatched RBCs, but the pattern of responses was less focused than that seen in the alloimmunized donors. In particular, there was no dominant peptide that elicited responses by PBMCs from many of the control donors. K peptide 1M(179-193), which was the dominant sequence identified in the alloimmunized group, stimulated significantly less responsiveness in the control donors (Mann-Whitney U, P = .036). No other peptide demonstrated significant differences in stimulatory ability between the 2 donor groups.
Proliferative responses to the panel of K and k Kell peptides by PBMCs from unimmunized control donorsare not focused on particular sequences. (A) Summary map of responses by PBMCs from 10 unimmunized control donors to the panel of peptides spanning the M193 (K) and T193 (k) polymorphism. Filled boxes represent positive proliferative responses (SI > 3). There is no significant difference between the numbers of responses to K or k peptides and no peptide elicited responses in > 60% of donors. (B) Representative proliferative responses to the Kell peptide panel by PBMCs from unimmunized control donors 2, 6, and 8. Dotted line denotes the level of proliferation taken as representing a positive response (SI > 3). These examples illustrate that responses to peptide 1M(179-193) do not predominate, while other peptides can elicit proliferation, including k sequences. PBMCs from all donors proliferated significantly in response to the control recall Ag PPD.
Proliferative responses to the panel of K and k Kell peptides by PBMCs from unimmunized control donorsare not focused on particular sequences. (A) Summary map of responses by PBMCs from 10 unimmunized control donors to the panel of peptides spanning the M193 (K) and T193 (k) polymorphism. Filled boxes represent positive proliferative responses (SI > 3). There is no significant difference between the numbers of responses to K or k peptides and no peptide elicited responses in > 60% of donors. (B) Representative proliferative responses to the Kell peptide panel by PBMCs from unimmunized control donors 2, 6, and 8. Dotted line denotes the level of proliferation taken as representing a positive response (SI > 3). These examples illustrate that responses to peptide 1M(179-193) do not predominate, while other peptides can elicit proliferation, including k sequences. PBMCs from all donors proliferated significantly in response to the control recall Ag PPD.
Taken together, the results from alloimmunized and control donors demonstrate that PBMC responsiveness to one K sequence, 1M(179-193), is associated with alloimmunization with K, consistent with this peptide recapitulating a dominant Th epitope. However, there was a higher prevalence of background of responses, both to peptides incorporating the alternative k polymorphism, and among unimmunized control donors, than has been reported in studies of Th specificity for other blood groups.13,26
Characterization of lymphocytes that proliferated in response to Kell peptides
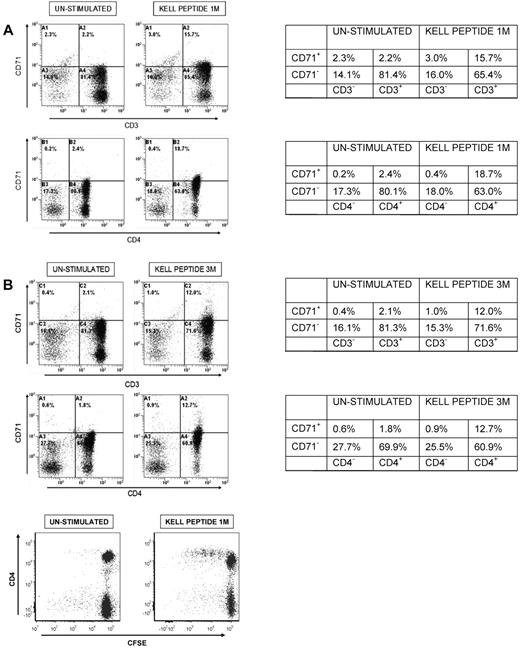
To test whether the PBMCs proliferating in response to Kell peptides were of the CD3+CD4+ Th phenotype, responding cultures were analyzed by flow cytometry, using the marker CD71 to identify activated cells. Figure 3A illustrates representative flow cytometric analyses of PBMCs from an alloimmune donor (n = 5), either resting, or responding to dominant K peptide 1M(179-193) or peptide 3M(181-195). As expected, proliferation is accompanied by expansion of the activated CD71+ population, and in all cases > 90% of these responding cells were CD3+CD4+. Similar results were obtained when stimulatory k peptides were tested (not shown), and when PBMCs from unimmunized control donors (n = 3) proliferated to K sequences (representative analyses Figure 3B). Flow cytometric analyses of responding PBMCs that had been stained with CFSE before stimulation with Kell peptides confirmed division of cells within the CD4+ population (Figure 3C).
Proliferation of PBMCs fromalloimmunized and unimmunized donorsin response to K peptides is mediated by cells with the CD3+CD4+ Th phenotype. Flow cytometric analyses of PBMCs from (A) alloimmunized donor 5 or (B) control donor 4 left unstimulated, or stimulated with K peptides 1M(179-193) or 3M(181-195) and stained for expression of CD3, CD4, and the activation marker CD71. Tables next to each pair of plots indicate the percentage of cells in quadrants. The results are representative of 5 alloimmunized and 3 control donors. In all cases, > 90% of the increase in the CD71+ activated population after peptide stimulation is accounted for by cells that are CD3+ and CD4+. (C) Flow cytometric analyses of CFSE-stained PBMC from control donor 5 left unstimulated, or stimulated with K peptide 1M(179-193) and stained for expression of CD4.
Proliferation of PBMCs fromalloimmunized and unimmunized donorsin response to K peptides is mediated by cells with the CD3+CD4+ Th phenotype. Flow cytometric analyses of PBMCs from (A) alloimmunized donor 5 or (B) control donor 4 left unstimulated, or stimulated with K peptides 1M(179-193) or 3M(181-195) and stained for expression of CD3, CD4, and the activation marker CD71. Tables next to each pair of plots indicate the percentage of cells in quadrants. The results are representative of 5 alloimmunized and 3 control donors. In all cases, > 90% of the increase in the CD71+ activated population after peptide stimulation is accounted for by cells that are CD3+ and CD4+. (C) Flow cytometric analyses of CFSE-stained PBMC from control donor 5 left unstimulated, or stimulated with K peptide 1M(179-193) and stained for expression of CD4.
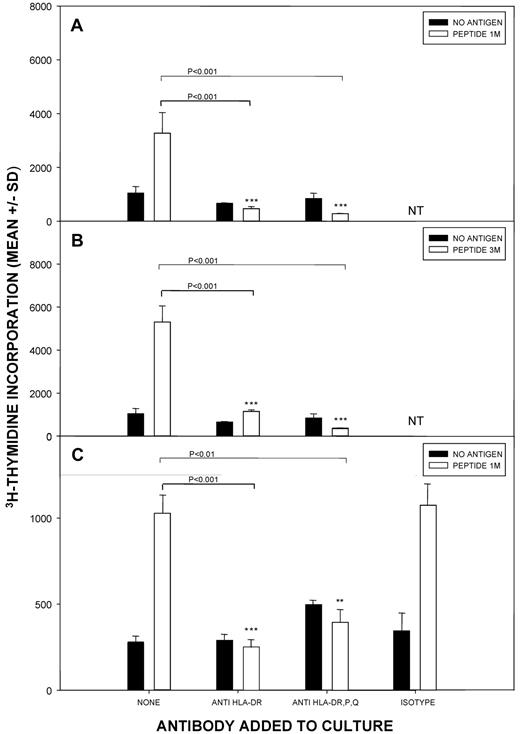
To further demonstrate that PBMCs responding to Kell peptides came from the helper subset which is restricted by MHC class II molecules, blocking Abs with pan-reactivity to HLA-DP, -DQ, and -DR, or specific for HLA-DR alone, were tested for their ability to inhibit proliferation. Representative results from alloimmunized (n = 4) and unimmunized control (n = 4) donors are depicted in Figure 4 and demonstrate that addition of the anti-HLA Abs to peptide-stimulated cultures markedly inhibited all proliferative responses to either 1M(179-193) or other K peptides. Similarly, consistent inhibitory effects were seen when proliferative responses to k peptides were investigated (results not shown). Addition of isotype-matched Ab with irrelevant specificity had no consistent or significant effects on proliferation to Kell peptides.
The proliferation of T cells against Kell peptides is dependent on HLA-class II molecules. Cultures of PBMCs from (A-B) alloimmunized donor 3 or (C) control donor 5 were stimulated with Kell peptide 1M(179-193) or 3M(181-195), and class II–restricted responses were blocked by addition of Ab against all HLA-DP, DQ, and DR molecules, or specific only for DR. Isotype control Ab was added in selected experiments. P < .05 was taken as significant inhibition (t test on log-transformed data). The results are representative of 4 alloimmunized and 4 control donors.
The proliferation of T cells against Kell peptides is dependent on HLA-class II molecules. Cultures of PBMCs from (A-B) alloimmunized donor 3 or (C) control donor 5 were stimulated with Kell peptide 1M(179-193) or 3M(181-195), and class II–restricted responses were blocked by addition of Ab against all HLA-DP, DQ, and DR molecules, or specific only for DR. Isotype control Ab was added in selected experiments. P < .05 was taken as significant inhibition (t test on log-transformed data). The results are representative of 4 alloimmunized and 4 control donors.
The above results indicate that the proliferative responses to Kell peptides, whether against the dominant sequence 1M(179-193), or other peptides, or by PBMCs from alloimmunized or control donors, are mediated by Th cells that are MHC class II restricted, largely by DR. When a web-based algorithm (www.imtech.res.in/raghava/propred)27 was interrogated for HLA-DR–restricted motifs spanning the M193 K polymorphism, several candidates were proposed. These included the core sequence amino acids 185-193, which was predicted as a relatively promiscuous binder to a panel of HLA-DR molecules, with W185 forming the key anchor residue in pocket one of the MHC class II binding groove, and K-specific residue M193 located at the C terminus, corresponding to the dominant K peptide 1M(179-193).
Background responses to Kell peptides by Th cells from unimmunized control donors
One explanation for the relatively high number of background responses to Kell peptides by PBMCs from the unimmunized control donors would be the detection of primary proliferation by naive Th cells in vitro, independent of Ag exposure in vivo. The experiments were designed to minimize this possibility because PBMC proliferation was assessed relatively early (on day 5 after stimulation), and cell culture was performed in microtiter plates. These conditions strongly favor recall, rather than primary, Th-cell responses.12,13,19 However, to confirm that any Th cells proliferating against Kell peptides in vitro had been activated in vivo, depletion experiments determined the isoform of the CD45 molecules they express because primary and recall responses are mediated by T cells bearing CD45RA and CD45RO, respectively.19,22,23 Results from alloimmunized and unimmunized individuals are illustrated in Figure 5 and show that the CD45RO+ subset containing previously activated T cells is a major contributor to Kell peptide-induced proliferation in vitro, regardless of the history of exposure to K-mismatched RBCs in vivo. The additional contributions made by the CD45RA+ subset to proliferation against Kell peptides are typical of in vitro responses to recall Ags13,19 and may reflect either naive T-cell production after the exposure of donors to Ag, the presence of T cells with low affinity that ignore Ag in vivo, or partial reversion of the memory population to the CD45RA+ phenotype.19 Thus, despite any proliferation by the CD45RA+ subset, the presence of responsive CD45RO+ T cells is a reliable indicator of prior priming in vivo.19,22,23
T cells from alloimmunized and unimmunized donors that proliferate in response to K peptides have previously been activated in vivo. Proliferative responses of CD45RO+ (previously activated/memory) and CD45RA+ (previously inactive/naive) T-cell fractions from alloimmunized donors 1 and 5 and unimmunized control donors 4 and 5 against selected Kell peptides. The results are representative of 4 alloimmunized and 4 control donors.
T cells from alloimmunized and unimmunized donors that proliferate in response to K peptides have previously been activated in vivo. Proliferative responses of CD45RO+ (previously activated/memory) and CD45RA+ (previously inactive/naive) T-cell fractions from alloimmunized donors 1 and 5 and unimmunized control donors 4 and 5 against selected Kell peptides. The results are representative of 4 alloimmunized and 4 control donors.
The confirmation of Th memory for particular Kell peptides in unimmunized individuals raises the possibility that the cells are primed in vivo by another Ag. Searches for homology between the sequence of Kell and microbial Ags have already reported several key matches28,29 and, to further illustrate the potential for such cross-reactivity, we used the basic local alignment search tool (BLAST)30 to interrogate the SwissProt database. The search revealed extensive homologies between the Kell protein sequences spanned by the peptide panels and many bacterial, parasite, viral, and fungal sequences (examples illustrated in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Effects of glycosylation on background Th responses to k self-peptides
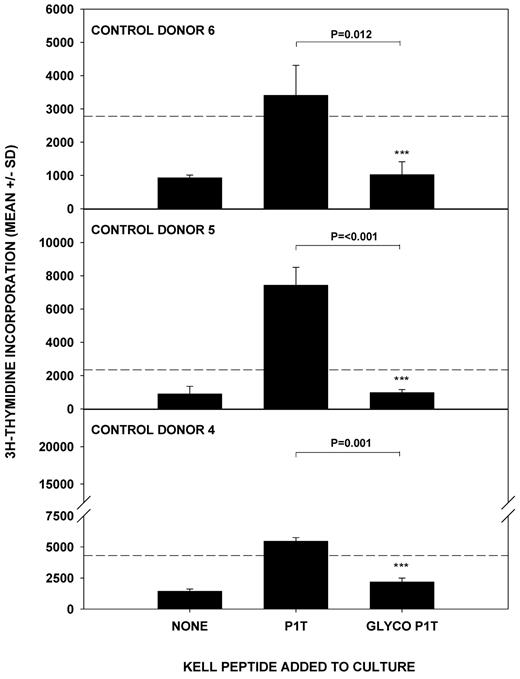
In addition to the background responses to K sequences exhibited by unimmunized control donors, and in contrast to parallel studies of other blood group Ags,13,26 Th cells from both the alloimmunized and unimmunized donors commonly proliferated against the k peptide panel. In such k-positive individuals, these responses potentially represent autoreactivity and a failure of self-tolerance. However, substitution of T193 for M193 is believed to disrupt an N-glycosylation consensus sequence at amino acid 191 of the Kell protein,9,31 which therefore displays carbohydrate residues at this position in the k, but not K, type. One explanation for the relatively high background of responses to k peptides is that self-tolerance is established to the glycosylated k sequence spanning T193 and is therefore less secure to the exposed amino acid chain of the synthetic k peptides used for epitope mapping, and of the homologous K sequence. To test this possibility, a glycosylated version of peptide 1T(179-193), with a nominal sugar residue at amino acid 191, was synthesized and compared with the unglycosylated sequence for the ability to stimulate in vitro proliferation by PBMCs from unimmunized control donors (n = 3). The results in Figure 6 demonstrate that the glycosylated form, which reflects the natural state of the sequence, does fail to elicit proliferative responses.
Glycosylation of k peptide determines the ability to stimulate T-cell proliferation. The k peptide 1T(179-193) was synthesized in glycosylated (GLYCO P1T) and unglycosylated (P1T) forms and used to stimulate T cells from unimmunized donors 4-6. P < .05 was taken as significant inhibition (t test on log-transformed data).
Glycosylation of k peptide determines the ability to stimulate T-cell proliferation. The k peptide 1T(179-193) was synthesized in glycosylated (GLYCO P1T) and unglycosylated (P1T) forms and used to stimulate T cells from unimmunized donors 4-6. P < .05 was taken as significant inhibition (t test on log-transformed data).
In vivo suppression established by dominant K peptide 1M(179-193)
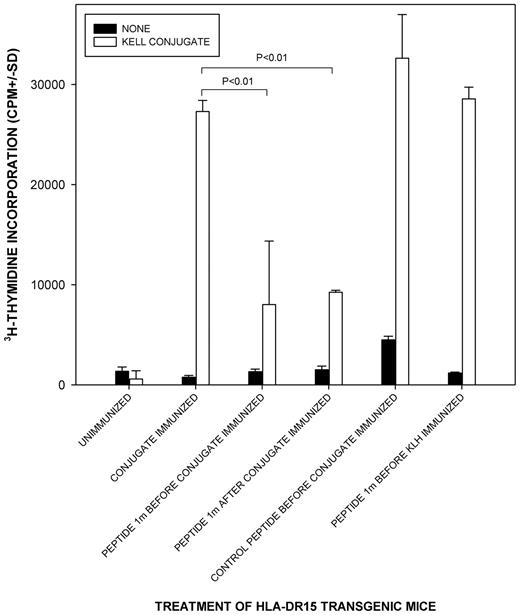
Despite the relatively common background responses to Kell sequences in unimmunized controls, in vitro screening identified K peptide 1M(179-193) as containing a dominant helper epitope. To confirm the dominance of 1M(179-193) in vivo, and its potential as a specific immunomodulator of responses, we sought to determine whether this peptide could induce suppression to T-cell epitopes on a carrier protein in mice immunized with a conjugate of KLH with a short sequence of the Kell protein spanning the K polymorphic site. We used a strain that had been genetically modified to express human, rather than murine, MHC class II molecules24,25 to model the presentation and restriction of peptides in humans. Mice transgenic for HLA-DR15 were chosen because DR was identified in Figure 4 as the predominant restricting locus for Kell peptides, and our typing of alloimmunized women with anti-K Abs revealed that DR15 was the most common allele they expressed (12 of 25 women were positive). Figure 7 demonstrates that when peptide 1M(179-193) was administered via the nasal mucosa (a recognized suppressive route)6,32 either before or after immunization, the T-cell response to the conjugate (including the linked KLH carrier protein) was significantly reduced. Although incomplete, the reductions are comparable with those seen in parallel work testing the suppressive properties of immunodominant peptides from other Ags including the RhD protein.24 The inhibition by 1M(179-193) was specific and not the result of any intrinsic immunosuppressive activity because treatment of mice with a control peptide of the reverse sequence failed to replicate the effect. Administration of 1M(179-193) did not reduce the response to immunization with the carrier protein KLH alone. An apparently spontaneous T-cell response to peptide 1M(179-193) in control unimmunized mice was also abrogated by the suppressive regimen (result not shown).
Tolerogenic administration of dominant K peptide 1m(179-193) to HLA-DR transgenic mice suppresses T-cell responsiveness to a Kell sequence immunogen and linked carrier protein. Proliferative responses of splenocytes against a Kell sequence-KLH conjugate when HLA-DR15 transgenic mice were given K peptide 1M(179-193) or control peptide with reverse sequence via the nasal mucosa either before or after immunization with the conjugate or the unlinked carrier protein KLH. P < .05 was taken as significant inhibition (t test on log-transformed data, n = 3-9/group). Splenocytes from all mice proliferated significantly in response to the control stimulus Con A.
Tolerogenic administration of dominant K peptide 1m(179-193) to HLA-DR transgenic mice suppresses T-cell responsiveness to a Kell sequence immunogen and linked carrier protein. Proliferative responses of splenocytes against a Kell sequence-KLH conjugate when HLA-DR15 transgenic mice were given K peptide 1M(179-193) or control peptide with reverse sequence via the nasal mucosa either before or after immunization with the conjugate or the unlinked carrier protein KLH. P < .05 was taken as significant inhibition (t test on log-transformed data, n = 3-9/group). Splenocytes from all mice proliferated significantly in response to the control stimulus Con A.
Discussion
This study determined, for the first time, the specificity of alloreactive Th cells that recognize the sequence of the Kell protein containing the M193 K polymorphism. The characterization of the helper response that drives anti-K Ab production in K-negative individuals reveals properties of the Ag that contribute to its immunogenicity, and now opens the way to develop novel strategies to prevent or treat alloimmunization, based on immunomodulation of specific T cells. Such approaches would be of clinical benefit given that current HDN prophylaxis targets only RhD allommunization, with no effective measures available to prevent alloresponses to other blood groups such as K.
Mapping of Th epitopes in women who had produced anti-K Abs after incompatible pregnancy identified several stimulatory Kell sequences, most notably the K peptide 1M(179-193), with the polymorphic residue M193 located at the C terminus. Phenotypic and functional analyses confirmed that the responses, including those against 1M(179-193), were mediated by CD3+CD4+ MHC class II–restricted Th cells. Several lines of evidence together support the view that the sequence 1M(179-193) recapitulates a dominant helper epitope that drives responses in vivo after exposure to K. First, Th proliferation to 1M(179-193) was detected in 90% of K-negative women who had produced anti-K Abs, and was typically the strongest evoked by any of the Kell peptides in these donors. Peptide 1M(179-193) was also the only Kell sequence to stimulate responses that were significantly stronger in the alloimmunized versus the unimmunized control group. Second, alloimmunized donor Th cells that were responsive to 1M(179-193) in vitro had been activated in vivo because the culture conditions were designed to minimize primary responses, and it was demonstrated that a major proportion of the responding cells was drawn from the CD45RO+ memory population. Finally, a dominant role for peptide 1M(179-193) was confirmed (and its potential for immunotherapy illustrated) by the ability of the sequence to induce tolerance in vivo to a linked carrier protein in a humanized mouse model24,25 of DR-restricted Th responses.
The use of blocking Abs specific for MHC class II molecules demonstrated that the predominant locus restricting responses to 1M(179-193) and other peptides was HLA-DR. The relatively high frequency of HLA-DR15 among K alloimmunized women suggests that this allele restricts many of the specific Th cells. Th epitopes from other blood groups, including RhD and HPA-1a, are also predominantly restricted by HLA-DR.13,26 Dominant peptide 1M(179-193) corresponded to one of a number of potential DR-binding motifs spanning the K M193 polymorphism that were predicted by computer algorithm, and was modeled with W185 forming the key anchor residue in pocket one of the MHC class II–binding groove, and K-specific residue M193 located at the C terminus. There are several explanations as to why the dominant Th-cell epitope requires M193 in this terminal position. It is possible that peptides extending beyond the polymorphism M193 either form motifs with high affinity for MHC molecules that do not restrict the alloreactive response, or create a secondary structure that directly interferes with TCR recognition.33 K peptides expressing M193 in other registers may represent further epitopes, but the responses they stimulated were similar in frequency in both alloimmunized and control donors.
The immunogenicity of blood groups such as K is striking, given that simple exposure to a foreign protein alone is typically insufficient for induction of immune responses, and, in the case of Ags arising from single amino acid polymorphisms, a highly homologous self sequence would be expected to tolerize many potentially reactive Th cells. Clinically important blood groups will therefore be predicted to exhibit features that enhance their ability to induce Th-cell responses, and the current work identifies 2 such characteristics in the case of K. First, the relatively high background of memory Th responses to K peptides among unimmunized control donors, compared with parallel work on other blood groups such as RhD and HPA-1a,13,26 now confirms that priming is common by environmental Ags cross-reactive with sequences spanning the Kell polymorphic site. The apparently spontaneous T-cell responses to peptide 1M(179-193) in HLA-DR transgenic mice is a further demonstration of such activation. It has long been recognized that certain bacteria—such as Escherichia coli or Shigella sonnei—are capable of expressing K-like Ags on their cell surface,29 and there are more recent suggestions of potential molecular mimicry between K Th epitopes and microbial peptides from Haemophilus influenzae, Bacteroides fragilis, and Vibrio species.28 Our own homology searches also illustrate that the potential for mimicry is extensive, and while it will be a major challenge to identify the particular environmental Ag(s) responsible for priming, animal models confirm that preexisting cross-reactive Th memory is a powerful factor in increasing the immunogenicity of both auto-34 and allo-28 RBC Ags. Such effects may be common in the induction of blood group responses. Their potency is illustrated by the finding that murine Ab responses to an RBC-bound complex of foreign Th- and B-cell determinants were increased 100- to 1000-fold after prior infection with virus expressing the helper epitope.28
The second characteristic of the K polymorphism that may enhance its ability to induce Th responses is the loss of the glycosylation motif encoded by the antithetical k sequence.9,31 The Kell protein displays carbohydrate residues at amino acid 191 in the k, but not K, type because substitution of T193 for M193 ablates the N-glycosylation site.9,31 Although this loss does not appear to affect Ab binding,9,31 there is growing evidence that posttranslational modifications such as glycosylation play an important role in T-cell recognition of Ags.35 There was a relatively high background of Th responses to self-k peptides among alloimmunized donors and unimmunized control volunteers when these peptides were synthesized by standard chemistry with no glycosylation, indicating a lack of tolerance to the primary amino acid sequence. However, we reasoned that self-tolerance would naturally be directed to the glycosylated form of the k sequence9,31 available in vivo, and this was borne out by the demonstration that addition of N-linked carbohydrate at residue 191 abolished background responses to k peptide. These results reveal a new mechanism to explain blood group immunogenicity, whereby an extended Th repertoire is available to drive K alloresponses because of failure of glycosylated k sequences to censor cells specific only for the underlying amino acid chain. Alloimmunization with the K blood group therefore challenges Th cells with an Ag that is “foreign” due not only to the single substitution, but also to the exposure an amino acid sequence that is cryptic in the homologous self-protein. This model has some parallel with immunogenicity of the platelet blood group HPA-1a, where the responsive Th repertoire is thought to be enhanced because the amino acid substitution in the antithetical HPA-1b sequence directly prevents binding to the restricting MHC molecule and thus induction of self-tolerance.26,36 However, although addition of carbohydrate to key residues may reduce the ability of peptides to bind restricting MHC molecules,37,38 in the case of k sequences, the glycosylation site N191 is not predicted to be such an anchor. We, therefore, consider it more likely that the N-glycosylation interferes with T-cell recognition of the MHC-peptide complex.
The therapeutic use of peptides containing dominant helper epitopes from target Ags to suppress damaging immune responses has proved very successful in rodent models.6,7,11,14-18 Although major hurdles remain in translating this approach to patients, most notably determining the optimum dosing regimen, human trials are ongoing in several diseases.39 In the field of transfusion medicine, the mapping of alloreactive helper epitopes on Rh proteins13 led to the development of a peptide product to suppress responses to the D Ag,24 which has now been modified to facilitate large-scale manufacture and is ready to enter clinical trials. The identification of peptide 1M(179-193) as containing an immunodominant epitope in the alloresponse to K, therefore, has implications for the future development of novel peptide immunotherapies. The administration of the immunodominant peptide 1M(179-193) via a suppressive route, such as the nasal mucosa, has the potential to inhibit alloresponses to the K Ag and therefore reduce or prevent K alloantibody production in susceptible individuals. Furthermore, the results in humanized mice encourage the possibility that such peptide immunotherapy could also have the potential to reverse fully established immune responses in individuals who have been previously alloimmunized with the K Ag. This would represent the first specific preventive measure for HDN because of anti-K responses and provide an approach that could be replicated for other blood groups encoded by single amino acid polymorphisms.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Scottish National Blood Transfusion Service.
Authorship
Contribution: J.S. performed research, analyzed data, and wrote the manuscript; L.S.C. and W.J.P. performed research and analyzed data; M.A.V. collected data; and S.J.U. and R.N.B. designed research, collected and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.J.U. and R.N.B. are co-inventers on patents covering the identification and therapeutic application of blood group derived peptides that are recognized by alloreactive Th cells, with the intention of securing commercial investment to fund clinical trials. The remaining authors declare no competing financial interests.
Correspondence: Prof Robert N. Barker, Section of Immunology and Infection, Division of Applied Medicine, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD United Kingdom; e-mail: r.n.barker@abdn.ac.uk.
References
Author notes
J.S. and L.S.C. are joint first authors.
S.J.U. and R.N.B. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal