In this issue of Blood, Stephen et al report significant strides forward in mapping out the peptide determinants of alloimmunization to the K antigen and have taken initial steps to translate these findings into therapeutic interventions.1
The “K” antigen, carried by the Kell glycoprotein, is the most immunogenic blood group antigen for which we do not routinely match a priori. Alloantibodies against K can cause substantial problems, both in the context of incompatible transfusion and in hemolytic disease of the fetus and newborn. Unlike immunization to RhD during pregnancy, which can be mostly prevented by prophylactic use of anti-D immune globulin, no such interventions are available for K. Thus, study of the particulars of immune responses to K is of substantial clinical importance.
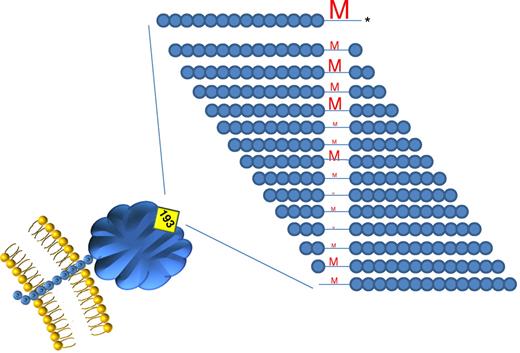
Schematic of T-cell proliferative responses to Kell peptides (surrounding K polymorphism) in subjects previously immunized to K. The size of the letters indicates the number of individuals responding. The peptide with the 193 methionine in the C-terminal position (*) was highly immunodominant and stimulatory in 9 of 10 alloimmunized subjects.
Schematic of T-cell proliferative responses to Kell peptides (surrounding K polymorphism) in subjects previously immunized to K. The size of the letters indicates the number of individuals responding. The peptide with the 193 methionine in the C-terminal position (*) was highly immunodominant and stimulatory in 9 of 10 alloimmunized subjects.
K differs from its antithetical antigen “k” by a single amino acid at position 193 (Methionine [in most cases] to Threonine). Thus, unlike most antigens (eg, microbial pathogens, vaccines, etc), the K antigen differs by a single amino acid between donor and recipient. Accordingly, T-cell epitopes for K are restricted to peptides containing the variant amino acid presented by MHC II. However, for a given peptide sitting in the MHC II pocket, the position of the K polymorphism can vary (MHC II typically presents peptides from 15-24 amino acids long), meaning a wide variety of different K-containing peptides derived from the Kell glycoprotein may be presented in MHC II in different registers. This situation leads to the possibility that multiple CD4+ T-cell epitopes for K may exist in a given individual. Moreover, given the wide variety of HLA within human populations, any given individual's MHC II molecules may present different peptides containing K. Finally, in some cases, an individual's MHC II may be incapable of presenting any peptide containing the K polymorphism, making them incapable of generating an antibody response. HLA mapping of humans making anti-K has shown HLA type to be fairly promiscuous for the K antigen,2 suggesting that a wide variety of individual HLA types can present K-containing peptides in an immunogenic fashion; however, this does not mean they each present the same peptide. Moreover, a predilection for certain HLA types has been found among K-immunized individuals (in particular, HLA-DRB1*11 and HLA-DRB1*13).3 The particular importance of elucidating the peptide/MHC biochemistry of CD4+ T-cell responses to K antigen is in the generation of basic understanding, the development of analytic reagents to study anti-K CD4+ T cells, and the potential development of peptide based therapeutics.
Here, Stephen and colleagues have used a cohort of K negative women, previously immunized to K (most likely during pregnancy), to perform detailed peptide mapping studies.1 Lymphocytes were stimulated with a panel of peptides spanning the K polymorphism, which tested the K polymorphism in each of the different potential positions within the peptide (15 amino acids total). Two important observations were derived from this approach. First, for most alloimmunized patients, more than 1 peptide containing the K allele was stimulatory (albeit the same single highly immunodominant peptide was found in 90% of the subjects; see figure). Second, proliferation of T cells was also seen in control patients who did not have detectable anti-K. The T-cell responses were less focused around any immunodominant peptide in the unimmunized group; however, they were significantly present above background, the expanding cells had a phenotype consistent with CD4+ helper T cells, and the responses were HLA dependent. This raises the very real possibility that part of the strong immunogenicity of K is due to existing immunity, likely as a result of exposure to environmental antigens and/or pathogens. Consistent with this, it has been previously reported that K exhibits protein homology to a number of microbial peptides that may stimulate T cells cross-reactive to K peptides,4 and additional homologies are reported by Stephen et al.1 In such cases of immunization to homologous peptide, anti-K CD4+ T cells would be present, but no anti-K immunoglobulin would be detected until exposure to authentic K antigen that linked the B-cell and T-cell epitopes.
The report by Stephen et al proceeds to provide a proof-of-principle demonstration (in a murine model) that nasal administration of Kell-derived peptide can take advantage of nasal tolerance pathways to reduce systemic responsiveness to subsequent immunization. This approach provides a pathway to inducing antigen-specific tolerance to the K antigen. Although untested thus far for the K antigen in the setting of transfusion or pregnancy (either in mice or humans), there is solid theory and data to support feasibility of peptide-based tolerance strategies in general. However, several potential problems exist with such approaches. First, whereas tolerance may be achieved in the majority of recipients, one always risks the potential of inadvertently inducing an immune response in some. Second, the feasibility presupposes that the variant amino acid (in this case K vs k polymorphisms) is the only polymorphism differing between donor and recipient. It has been appreciated that additional polymorphisms exist in the proteins that carry blood group antigens, which have not been observed by immunohematology, as they do not constitute epitopes for antibody binding on the surface of RBCs.5 However, from a T-cell epitope standpoint, it does not matter if the polymorphism elicits an antibody response, so long as it can be presented by MHC II. In such a case, linked recognition would allow helper T cells specific to such epitopes to promote antibodies against K.5 This represents only a theoretical concern, as cross-tolerance between positionally distinct peptides on the same molecule may occur, but it is unclear whether such would be the case.
In aggregate, the current work by Stephen and colleagues represents a major advancement in our understanding of human immunology in the area of immunization to the K antigen on RBCs. In addition to generating basic knowledge in the K/k system, these studies pave the way for both further mechanistic investigational biology and also as a platform to initiate translation of peptide based therapeutics.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal