In this issue of Blood, Follenzi and colleagues demonstrate that transplantation of the bone marrow cells into hemophilia A mice partially restored factor VIII (FVIII) production and protected hemophilia A mice from bleeding challenge.1 Surprisingly, in recipient hemophilia A mice, the donor BM-derived hepatocytes or endothelial cells were rare; the donor-derived mononuclear cells and mesenchymal stromal cells (MSCs) contributed to major factor VIII gene expression and activity.
Deficiency of blood coagulation FVIII results in hemophilia A, a serious bleeding disorder. The synthesis of FVIII has been found in many different tissues of mammals.2 However, it has been controversial for a long time which site(s)/cell types produce the biologically functional FVIII. Recent evidence showed that liver transplantation corrected hemophilia A; liver is recognized as the primary site of FVIII synthesis with immunohistochemical staining showing FVIII expression in both hepatocytes and liver sinusoidal endothelial cells (LSECs). Transplantation of LSECs in peritoneal cavity corrected the phenotype of hemophilia A mice,3 indicating LSECs are capable of synthesizing functional FVIII protein in the liver, whereas transplantation of hepatocytes did not correct murine hemophilia3 (see figure). Whether hapatocytes can produce functional FVIII in the liver environment compared with their inability to do this in the peritoneal cavity will need to be further studied. It is known that secreted FVIII would be unstable unless associated with von Willebrand factor (VWF). In the liver, hapatocytes are in close vicinity to LSECs where VWF is produced, which may promote FVIII/VWF complex formation once FVIII is secreted from hepatocytes. Notably, gene therapy protocols targeting hepatocytes were successful in correcting hemophilia A.4 FVIII mRNA was also detected in multiple tissues2 besides liver, including spleen, kidney, pancreas, muscle, and most other organs. In addition, high residual FVIII plasma levels were observed in dogs or people transplanted with hemophilia donor livers,5 indicating that extrahepatic sources also contribute to circulating levels of FVIII.
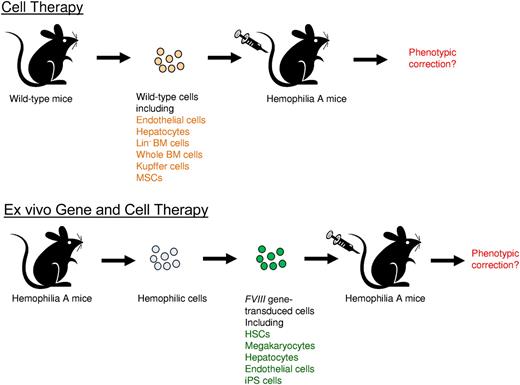
Correction of hemophilia A phenotype in mice after adoptive transfer of cells isolated from wild-type mice or FVIII gene-transduced cells isolated from hemophilia A mice. Top panel is the illustration of adoptive cell therapy, and the bottom panel is the illustration of ex vivo gene and cell therapy.
Correction of hemophilia A phenotype in mice after adoptive transfer of cells isolated from wild-type mice or FVIII gene-transduced cells isolated from hemophilia A mice. Top panel is the illustration of adoptive cell therapy, and the bottom panel is the illustration of ex vivo gene and cell therapy.
Here, Follenzi et al performed a detailed and thorough investigation concerning where FVIII gene expression is distributed after transplantation of normal bone marrow cells into hemophilia A mice.1 These interesting data help the understanding of FVIII expression sites besides hepatocytes and LSECs and promote potential development of adoptive cell therapy for treatment of hemophilia A. Nevertheless, this article challenges previous dogma in the field. Previous studies in humans and dogs have shown that bone marrow transplantation did not improve production of FVIII.6 However, most of these studies were carried out a long time ago with suboptimal allograft tolerance and other limitations. More canine transplantation studies will be required to resolve this important issue.
Follenzi and colleagues took advantage of the hemophilia A mouse model and recent development of sensitive FVIII assays to re-evaluate the potential of BM transplantation for correcting hemophilia A. Whole bone marrow cells were freshly isolated from wild-type mice and transplanted into recipient hemophilia A mice (see figure). A majority of the treated mice (∼ 70%) had average 8% to 12% circulating FVIII activity and survived bleeding challenge with correction of hemophilia. Using cells containing reporter genes, donor bone marrow cells were found in multiple organs, including BM, liver, spleen, lungs, heart, and kidneys. In the liver, a majority of the donor-derived cells were located in vascular spaces, and identified as CD11b+ Kupffer cells (KCs) or mononuclear cells. However, donor BM-derived hapatocytes and LSECs were extremely rare. They further identified MSCs in BM cell fractions also contributed significantly to FVIII expression. Subsequently, it was demonstrated that transplantation of KCs or MSCs respectively isolated from wild-type mice (see figure) diminished the mortality of recipient hemophilia A mice from excessive bleeding, indicating that these cells indeed produce a significant amount of functional FVIII.
It should be noted that in a previous study FVIII mRNA was detected in a hepatocytes and LSECs, but not in KCs in the murine liver.7 The study by Follenzi et al also contradicts with a previous study where lineage negative murine bone marrow cells were isolated and transplanted into hemophilia A mice with their livers perturbed by acetaminophen.8 It was reported that the engrafted cells transdifferentiated into liver cells including both hepatocytes and endothelial cells (see figure) and produced approximately 20% normal levels of FVIII activities that persisted for more than a year without detection of anti-FVIII inhibitory antibodies.8 Nonetheless, in the study by Follenzi et al, whole BM cells were used and none of these cells transdifferentiated into hepatocytes or endothelial cells with or without the perturbation of acetaminophen or other monocrotaline. It is unclear whether different selection pressures exerted on the different types of transplanted cells (lineage negative BM cells vs whole BM cells) led to the discrepancy between these 2 studies.
Despite the controversies, these carefully performed experiments by Follenzi et al shed revealing light on FVIII biology and expression. Recently it was shown that subsets of endothelial cells synthesize FVIII and store it with VWF in Wiebel-Palade bodies (WPBs); however, WPB-derived FVIII remained bound to VWF strings after secretion, which impairs functional recruitment of platelets.9 In addition, multiple transcripts of FVIII were produced in endothelial cells by complex alternative splicing.10 It will be important to examine the biologic functions of FVIII expressed in different cell types and their influence in downstream clotting activities. Furthermore, previous ex vivo gene therapy studies using gene transfer vectors targeting hematopoietic stem cells (HSCs), megakaryocytes, endothelial progenitor cells, and induced pluripotent stem (iPS) cells achieved therapeutic levels of FVIII gene expression4 (see figure). Identification of BM-derived cell types that contribute to the production of biologically functional FVIII will increase the therapeutic potential of the BM cells in adoptive cell therapy as well as ex vivo and in vivo gene therapy for the treatment of hemophilia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health