Abstract
The therapeutic GVL effect after allogeneic stem cell transplantation is limited by the development of GVHD. The ultimate aim of current research is to separate the 2 processes in a meaningful fashion. The IFNs are a pleiotropic group of cytokines that were originally recognized because of their ability to interfere with viral replication. However, it is now established that these cytokines play an important role in orchestrating both innate and adaptive immunity. Multiple studies have investigated the effects of both types I and II IFN on GVHD and GVL in preclinical transplant models. The results indicate variable effects that are dependent on the period of activity within the developing immune response, the presence and type of pretransplant conditioning and the differential mechanisms, and IFN sensitivity of immune pathology within individual target organs during GVHD. This Perspective discusses the current literature on the IFNs and their potential modulation within clinical transplantation, focusing particularly on enhancing the therapeutic GVL effects.
Introduction
Stem cell transplantation (SCT) has been routinely used as curative therapy for hematologic malignancies for more than 3 decades. Although autologous SCT is now principally used as therapy for myeloma, non-Hodgkin lymphoma, and Hodgkin lymphoma, allogeneic SCT is more widely used to treat acute and chronic leukemia, myelodysplasia, and advanced lymphoid malignancies failing other modalities. Allogeneic SCT is limited by the consequences of donor immune activation in response to recipient alloantigens, leading to the development of GVHD. GVHD occurs in the majority of recipients of unmanipulated (T cell–replete) grafts1 and may develop early (in the first 100 days) after SCT (acute) or thereafter (chronic), typically with divergent presentations. The therapeutic potential of stem cell transplantation lies within the GVL effect whereby residual recipient-type malignancy is eradicated by donor NK cells and/or T cells. GVHD and GVL responses are closely related immune-mediated processes and are controlled in large part by cytokines. Our understanding of the IFNs in GVHD and GVL has greatly expanded in the last 5 years, opening the possibility of manipulation for therapeutic gain.
Pathophysiology of acute GVHD and GVL
Acute GVHD is initiated by recipient tissue inflammation in response to the transplant conditioning or preparative regimen, used typically to eradicate malignancy and inhibit rejection of the transplanted allodisparate graft. This results in production of proinflammatory cytokines, typically IL-1, IL-6, and TNF.2 The resulting inflammatory environment leads to activation of recipient cells capable of presenting alloantigen to incoming donor T cells. These antigen-presenting cells (APCs) can include both hematopoietic and nonhematopoietic cells.3,4 Acute GVHD is absolutely dependent on this interaction between APC and donor αβ T cells.5 Although T-cell depletion protects from GVHD, it can also increase graft rejection, inhibit GVL effects, and delay immune reconstitution. Consequently, recipients of allogeneic SCT have a lower risk of relapse than those of syngeneic SCT or T cell–depleted allogeneic SCT.6 In murine models where donor and recipients are MHC mismatched, both CD4+ and CD8+ T cells contribute to GVHD, although MHC class II responses dominate. In clinical patients where donors and recipients are predominantly HLA matched, GVHD is mediated by both CD4+ and CD8+ T cells, but MHC class I responses tend to dominate. The final effector phase of GVHD involves the infiltration and damage of target tissues by alloreactive cells. Although the expression of alloantigens in GVHD is not tissue specific, acute GVHD tends to occur predictably in particular organs (ie, gastrointestinal [GI] tract, skin, liver, and lung). This effect is multifactorial but is related to the differential presence of functional APCs,3 the induction of tissue-homing specific integrins on donor T cells,7 organ-specific inflammation,8 and, importantly, the differential effects of inflammatory cytokines on target tissues of which IFN is the archetype. In conjunction with cytotoxic T cell–mediated host tissue damage, the proinflammatory cytokines IFN-γ and TNF are also major contributors to GVHD. It is the tissue damage resulting from both direct T-cell cytotoxicity and proinflammatory cytokine responses that results in the clinical manifestations of GVHD. The most commonly affected organs after acute onset of GVHD are skin (81% of patients), GI tract (54%), and liver (50%).9 Epithelial apoptosis in these organs results in a loss of function and subsequent clinical symptoms, such as diarrhea, cholestatic hyperbilirubinemia, and maculopapular skin rashes.10
Therapeutic GVL responses are mediated by donor NK cells and T cells, and the antigenic targets are principally minor histocompatibility antigens (mHAs), although hematopoietic-specific and leukemia-specific responses also occur.11 Recipient mHAs are predominantly presented directly to donor CD8+ T cells by recipient hematopoietic-derived APCs within the first few weeks after transplantation, before their replacement by donor-derived APCs.12 These donor-derived APCs must subsequently cross-present recipient mHAs to donor CD8+ T cells. Both CD4+ and CD8+ T cells are important in GVL responses, with CD8+ T cells mediating direct cytotoxicity against leukemic cells, whereas CD4+ T cells are capable of both direct cytotoxicity (if the leukemia is MHC class II positive) and providing helper function to donor CD8+ T cells (via cytokines, such as IL-2 and the licensing of APCs). Importantly, recipient rather than donor APCs are most important for the induction of T-cell responses mediating both GVHD and GVL.3,13 After recognition of target antigen on leukemia cells, cytotoxic effector populations can mediate damage via multiple different pathways, including secretion of perforin and granzymes or through Fas-FasL, TNF, and/or TRAIL.14 Although T cells deficient in both Fas ligand and perforin have been demonstrated to exert GVL activity in vivo,15 these molecules are clearly required for meaningful GVL activity.16
The generation of GVHD and an effective GVL response is thus a complex process requiring the interaction of an APC presenting relevant mHAs with a donor T cell and subsequent differentiation and acquisition of effector function. The IFNs are key molecules in orchestrating this immune response.
The IFNs
The IFNs were discovered by Issaacs and Lindenmann in 1957 when they were investigating viral interference, and they have subsequently been defined by this ability.17 It was not until 1978 that they were able to be purified, analyzed, and characterized.18 They are recognized as a key component of innate immunity and the first line of defense against viral infection. There are 3 distinct types (type I, type II, and type III), which are distinguished based on their structure, cognate receptors, and biologic activities. The type I IFNs are well characterized and are the hallmark IFNs important in defense against viral infection. In contrast, the type II IFNs are better known for mediating immune responses to a broad range of intracellular pathogens. The type III IFNs are more recently described and not well characterized; however, they have similar downstream effects as type I IFNs. Therefore, although IFNs were initially described because of their antiviral properties, they exhibit multiple distinct additional immunologic properties.
Type I IFN
The type I IFNs encompass a large family of cytokines that include a single IFN-β isotype, more than 13 IFN-α isotypes, and multiple other less-described subtypes.19 Most cell types can be induced to secrete type I IFN in response to viral infection. The major pathway through which this occurs is activation of the cytosolic receptors retinoic acid-inducible gene I and melanoma differentiation-associated gene 5 by double-stranded RNA.20 Type I IFN production is induced after activation of 5 of the 11 TLRs.21 However, the most potent producers are plasmacytoid dendritic cells (DCs),22 which preferentially express TLR7 and TLR9 that recognize single-stranded RNA and CpG-rich DNA, respectively.23 After engagement of these receptors, the MyD88-IFN regulatory protein (IRF7) pathway is crucial for type I IFN production, with extended retainment of CpG and MyD88/IRF7 complexes in endosomal vesicles responsible for the ability of plasmacytoid DCs to induce high levels of type I IFN.24 All type I IFNs signal through the same receptor, which is composed of 2 subunits, IFNAR1 and IFNAR2.25 Importantly, the mouse has a type I IFN system comparable with that in humans, with multiple cytokine subsets and both IFNAR1 and IFNAR2 components of the receptor,25 which is expressed on essentially all cells.26 After signaling through the type I IFN receptor, downstream responses are transmitted through the kinases tyrosine kinase 2 and JAK1, which recruits STAT1 to form the STAT1-STAT2 heterodimer complex that dissociates and migrates into the nucleus.19 In the nucleus, this heterodimer associates with IFN regulatory factor 9 to form the IFN-stimulated gene factor 3 that binds to the IFN-stimulated response element promoter sequence to activate transcription of the IFN-inducible genes.19
Type I IFN signaling is important in both the innate and adaptive immune responses. These effects were initially studied in the context of viral infection, with signaling resulting in antiproliferative responses in conjunction with up-regulation of MHC class I expression.27 During the adaptive immune response, type I IFN signaling is vital for the expansion and differentiation of effector cytotoxic T lymphocytes (CTLs).28 The injection of synthetic dsRNA (polyinosinic acid/polycytidylic acid) to induce type I IFN augments the response of TCR transgenic CD8+ T cells to directly presented antigen. Increased levels of type I IFN have also been shown to promote cross-presentation of exogenous recipient mHAs to CD8+ T cells by APCs, a process that is independent of CD4+ T-cell help.29 Bystander activation of T cells can also occur in the presence of high levels of type I IFN, as proliferation is observed without up-regulation of CD69 and CD25.30 More recently, there has been renewed interest in the role of type I IFN in immunoediting and tumor clearance. Early NK antitumor responses are dependent on type I IFN signaling.31 Homeostatic numbers of NK cells in mice with a deficiency of both the AR1 and AR2 components of the IFNAR are reduced, but the cytotoxicity activity of naive NK cells is similar.31 However, IL-2–mediated activation and subsequent killing are defective in mice lacking the type I IFN receptor.31 This suggests that, although type I IFN signaling does not affect the baseline activity of NK cells, it serves to enhance antitumor responses after activation. Type I IFN signaling is also important for CD8 T-cell responses against tumor. The induction of type I IFN with CpG via the TLR9 receptor in combination with DC-targeted vaccines effectively activates tumor-specific CD8+ T cells, leading to prolonged survival in tumor models, and increased serum levels of IFN-α are observed.32 Further evidence for the importance of type I IFN in antitumor function demonstrates that they play a role in immunoediting.33 IFNAR1-deficient mice are more susceptible to induced sarcomas, and tumors arising in these mice have an “uneditited” phenotype, as they are rapidly rejected via a T cell–dependent mechanism when transplanted into wild-type mice.33 Interestingly, tumor rejection occurs via effects on hematopoietic cells, rather than via direct effects on tumor cells.33 Therefore, type I IFN contributes to both innate and adaptive arms of the antitumor immune response.
In conjunction with effects observed through NK and CD8 T cells, type I IFNs also modulate DC function. Type I IFN promotes the maturation of DCs34 and enhances cross-presentation to CD8 T cells by CD8α+ DC and subsequent rejection of tumor.35 The mechanism by which IFN-β administration induces beneficial outcomes in multiple sclerosis patients has been investigated in experimental autoimmune encephalitis.36 These studies demonstrate that type I IFN signaling down-regulates the Th17-mediated inflammatory response by inhibiting osteopontin expression in DCs, resulting in derepression of the potent inhibitory cytokine IL-27.37,38 The ability of type I IFN signaling to reduce IL-17 production has also been documented in patients with ulcerative colitis, with treatment over a period of 12 weeks resulting in a significant decrease in IL-17A mRNA expression in colonic biopsies.39 Therefore, type I IFN signaling in DCs modulates subsequent cellular and cytokine differentiation responses.
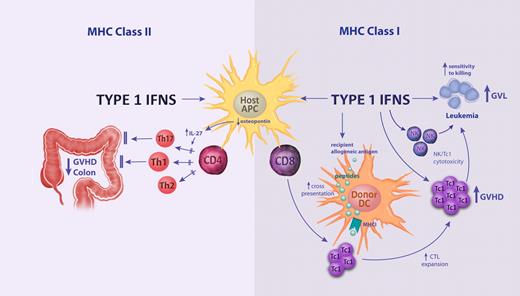
IFN-α was routinely used to treat patients with chronic myeloid leukemia in the pre-tyrosine kinase inhibitor (ie, imatinib, dasatinib, nilotinab) era, with responses thought primarily to relate to antiproliferative effects.40,41 IFN-α administration is now undergoing a renaissance as combination therapy with tyrosine kinase inhibitors in an effort to promote leukemia stem cell cycling and render these cells sensitive to the latter agents. The finding that IFN-α administration in vivo induces the transition of HSCs from a dormant stage into active cell cycle was surprising, considering the antiproliferative activity observed in in vitro culture systems.42 The increased proliferation of HSCs results in increased susceptibility to elimination by antiproliferative chemotherapeutic drugs. Clinical studies investigating the effects of IFN-α administration after allogeneic SCT demonstrated enhancement of both GVHD and GVL responses.43,44 The effects of IFN-α and mechanism of disease control were recently addressed by our group in preclinical models. This study demonstrated differential effects on donor CD4+ and CD8+ T cells45 (Figure 1). Type I IFN signaling indirectly inhibits donor CD4+ T cells, putatively via recipient APCs, resulting in alleviation of GVHD. In contrast, signaling enhanced donor CD8+ T-cell responses and increased susceptibility of host cells to killing by donor CD8+ T cells, both promoting GVHD and improving protective GVL responses. A mild reduction in hepatic GVHD histopathology in the absence of type I IFN signaling has also been demonstrated in a system of GVHD directed toward minor histocompatibility antigens only, although in these studies the overall effects of type I IFN on GVHD outcomes was limited.46 Clinical data from the 1990s demonstrated a detrimental effect of type I IFN administration before transplantation, with increases in severe GVHD.47,48 This is consistent with MHC class I–restricted effects on GVHD in clinical transplantation where donor and recipients are HLA-matched. It was also recently demonstrated that type I IFN enhances the cross-presentation of exogenous recipient antigens by donor APCs within MHC class I after SCT.49 The contribution of cross-presentation to GVL responses was not investigated in our studies because of the models used being MHC mismatched, but this effect would be expected to enhance therapeutic GVL responses in patients.
The differential effects of type I IFN signaling on MHC class I and MHC class II–dependent GVHD and GVL responses. Signaling on host hematopoietic tissue decreases donor CD4 T-cell proliferation and differentiation via inhibitory effects on recipient APCs, resulting in reduced T-cell differentiation and GVHD of the colon. The inhibition of Th17 differentiation has been associated with inhibition of APC-derived osteopontin, which promotes IL-27 secretion. The signaling of donor APC by type I IFN promotes cross-presentation (ie, the presentation of exogenous recipient allogeneic peptides within MHC class I) and the expansion of donor CTLs. This effect is further amplified by direct signaling to donor NK and CD8 T cells, which together promote cytotoxicity (eg, mediated by perforin/granzyme) against GVHD target tissue and residual recipient leukemia (ie, GVL). Finally, the type I IFNs enhance the susceptibility of residual leukemia to CD8 and NK cell–mediated cytotoxicity, further amplifying GVL responses.
The differential effects of type I IFN signaling on MHC class I and MHC class II–dependent GVHD and GVL responses. Signaling on host hematopoietic tissue decreases donor CD4 T-cell proliferation and differentiation via inhibitory effects on recipient APCs, resulting in reduced T-cell differentiation and GVHD of the colon. The inhibition of Th17 differentiation has been associated with inhibition of APC-derived osteopontin, which promotes IL-27 secretion. The signaling of donor APC by type I IFN promotes cross-presentation (ie, the presentation of exogenous recipient allogeneic peptides within MHC class I) and the expansion of donor CTLs. This effect is further amplified by direct signaling to donor NK and CD8 T cells, which together promote cytotoxicity (eg, mediated by perforin/granzyme) against GVHD target tissue and residual recipient leukemia (ie, GVL). Finally, the type I IFNs enhance the susceptibility of residual leukemia to CD8 and NK cell–mediated cytotoxicity, further amplifying GVL responses.
Although these data suggest there would be improved tumor clearance after treatment with IFN-α before transplantation, clinical SCT data in the chronic myeloid leukemia setting combined with our own data suggest that the detrimental effects of GVHD overwhelm this protective response. Because extensive data indicate that type I IFN signaling after SCT promotes GVL responses, the most effective therapeutic use of type I IFN is probably in SCT patients at a high risk of relapse. In this setting, type I IFN would be predicted to increase the sensitivity of malignancy to killing while enhancing the donor NK and CD8+ T-cell responses that mediate GVL. Whether newer pegylated versions of type I IFN will be more efficacious in this setting will be a critical issue.
Type II IFN
IFN-γ is the only type II IFN. It is produced by CD4+ Th1 lymphocytes, CD8+ T cells, NK cells, B cells, NKT cells, and APCs, with production stimulated by the secretion of IL-12 and IL-18, principally by APCs.50 IFN-γ signals through the IFN-γR, which is expressed on most cell types and consists of 2 subunits, IFN-γRα and IFN-γRβ.51 The type II IFN receptor interacts with JAK1 and JAK2 after ligand-dependent rearrangement of the receptor subunits, resulting in phosphorylation of STAT1. Homodimers of STAT1 form and translocate into the nucleus to bind IFN-γ–activated site elements and initiate transcription.51 A regulator of the JAK-STAT signaling pathway important for both type I and type II IFN signaling is the suppressor of cytokine signaling proteins, which inhibit signaling.52
IFN-γ has diverse functions that are involved in both innate and adaptive immunity and host defense. During the innate immune response, IFN-γ is important for the activation of macrophages, resting NK cell activity, and up-regulation of MHC class II on the macrophage cell surface.53,54 Consequently, mice deficient in the IFN-γR demonstrate increased susceptibility to multiple intracellular pathogens.53,54 After initiation of the adaptive immune response, IFN-γ regulates Th1 differentiation and function and subsequently inhibits Th2 differentiation. Signaling directly on CD8 T cells stimulates development and increases abundance during acute viral infection.55 Paradoxically, IFN-γ has also been implicated in down-regulation of the immune responses and T-cell homeostasis, with signaling inducing apoptosis in activated CD456,57 and CD858 T cells. Furthermore, production of IFN-γ by alloreactive CD4+ Treg is important for their function, and generation of CD4+ Treg is impaired in IFN-γ–deficient mice.59 During the immunosurveillance phase of tumor immunity, IFN-γ protects against the development of both induced and spontaneous tumors in a variety of models.60 Studies indicate that γδ T cells are an important source of IFN-γ during antitumor responses.61 However, although increased innate immune cell recruitment, MHC class I expression on APCs, and increased Th1 development inhibit tumor growth, the more recently identified functions of IFN-γ–like enhancement of Treg development and decreased neutrophil recruitment may enhance tumor development.62 Although type I and type II IFNs exhibit distinct immunologic properties, interaction between their respective signaling pathways was recently demonstrated in the context of Listeria monocytogenes infection. This study demonstrated that type I IFN signaling in macrophages and DCs resulted in down-regulation of the IFN-γR and subsequent responsiveness to IFN-γ.63
In the SCT setting, rapid IFN-γ release occurs by NK and NKT cells activated early after transplantation by interaction with activating or inhibitory killer inhibitory receptors. In the case of NKT cells, this cytokine secretion can occur within hours of TCR ligation by CD1 days loaded with glycolipid (relative to days for conventional T cells responding to peptide within MHC), and this appears critical for the licensing of recipient APCs and the subsequent control of GVHD and GVL effects.64,65 γδ T cells are also implicated in activation of host APCs via a cell contact– dependent mechanism, resulting in increased IFN-γ production by stimulated T cells and amplification of GVHD.66
Early studies investigating the effects of IFN-γ production by the donor graft on transplant outcome yielded contradictory results. When donor cells unable to produce IFN-γ were transplanted into nonirradiated recipients, there was a delay in GVHD mortality, which was attributed to enhanced Th2 differentiation67 and impairment of CTL function68 relative to recipients of WT grafts. In contrast, enhanced GVHD was observed in lethally irradiated recipients of IFN-γ−/− grafts or after IFN-γ neutralization.69,70 Consistent with this, the administration of recombinant IFN-γ provided protection from GVHD in CD4-dependent systems.71 Mechanistic studies revealed that in this setting IFN-γ signaling reduced proliferation and IL-2 production from donor T cells in response to allogeneic APCs. A subsequent study demonstrated that, although IFN-γ signaling attenuates CD8-dependent GVHD, it enhances therapeutic GVL responses in the absence of CD4 T cells.72 These results suggest that recipient conditioning regimen alters the overall effect of IFN-γ signaling on recipient versus the donor graft. In addition, the location and timing of IFN-γ production may impact effects observed.73 Subsequently, studies investigating links between IFN-γ polymorphisms and GVHD development have also yielded conflicting results.74 Because of inconsistent data obtained using donors unable to produce IFN-γ or broad neutralization/administration of cytokine, investigations were completed by our group using both IFN-γ−/− and IFN-γR−/− mice as donors and recipients. Surprisingly, IFN-γ did not protect from GVHD via direct effects on donor T cells, as had been previously concluded. Rather, IFN-γ was required to act within the recipient to protect from GVHD.75 These studies revealed that IFN-γ signaling on donor cells augmented GVHD by enhancing Th1 expansion and subsequent pathogenic responses in the GI tract. Furthermore, IFN-γ is directly cytotoxic to the GI tract. In our hands, this effect of IFN-γ as a mediator of GVHD within the GI tract (and ultimately mortality) was more penetrant than other inflammatory cytokines, such as TNF or IL-6, but there are important caveats. Critically, an overall protective (rather than pathogenic) effect of IFN-γ signaling was observed because of direct signaling on lung parenchyma, which prevents donor T-cell migration and expansion in that organ and subsequent idiopathic pneumonia syndrome (Figure 2). These effects have since been confirmed by 2 groups,76,77 and IFN-γ has been shown to up-regulate PDL-1 expression on lung parenchyma, putatively resulting in donor T-cell apoptosis at this site. In conjunction with this direct signaling being responsible for protection from GVHD, IFN-γ is also an important mediator of the protective effects of exogenous IL-12 and IL-18 when administered early after bone marrow transplantation (BMT).78,79 In this setting, IFN-γ induces Fas-dependent apoptosis of donor CD4 T cells. Further studies indicated that IFN-γ production by the donor only is required for protection from GVHD by IL-12.70 Interestingly, donor-derived IFN-γ has been shown to be responsible for the cytopenias and marrow suppression associated with GVHD.80 This effect is mediated by IFN-γ signaling in recipient tissue via an as yet unknown intermediary. It is important to note that IFN-γ plays a role in the induction of immunomodulatory molecules that are both suppressive (eg, indoleamine-2-3-dioxygenase and nitric oxide synthase) and pathogenic (eg, TNF family members).73 Therefore, the effects of IFN-γ production and signaling after SCT are highly dependent on variables, such as conditioning regimen, the balance of intermediate molecules invoked, and the GVHD target organs involved. Whereas cause-and-effect studies in clinical BMT are extremely difficult, multiple studies using both genotyping81 and mRNA analysis82,83 have correlated cytokine induction to severe acute and chronic GVHD. Unfortunately, these studies were too small or did not attempt to dissect organ-specific effects.
The differential effects of IFN-γ on GVHD target organs and GVL. After conditioning induced tissue damage, NK and NKT cells are activated through activating receptor ligands (eg, NKG2D) and inhibitory receptors (eg, killer inhibitory receptors [KIR]) and TCR ligation (in the case of NKT cells), resulting in rapid IFN-γ release and licensing of recipient APCs (ie, up-regulation of CD40 and CD80/86). Transplanted donor T cells are activated after interaction with these host APCs to undergo expansion and Th1/Tc1 differentiation. IFN-γ production by donor T cells augments gut damage via (1) promoting Th1 and Tc1 differentiation and subsequent cytolysis against target tissue (eg, via perforin/granzyme), (2) direct cytopathic effects of the cytokine itself on the GI tract, and (3) priming monocytes/macrophages such that they secrete quantities of TNF and lymphotoxin after signaling by TLR ligands that are themselves directly cytopathic to the GI tract. In contrast to these pathogenic effects, IFN-γ secretion by donor T cells is critical in the lung parenchyma to induce PDL1 expression, which subsequently inhibits donor T-cell survival and infiltration within this organ, preventing the development of idiopathic pneumonia syndrome (IPS). IFN-γ also promotes GVL effects via (1) the enhancement of CTL expansion and function (eg, perforin/granzyme-dependent killing) and (2) acting on leukemia cells directly (eg, to up-regulate MHC expression and death receptors), rendering them more susceptible to T cell–mediated cytolysis.
The differential effects of IFN-γ on GVHD target organs and GVL. After conditioning induced tissue damage, NK and NKT cells are activated through activating receptor ligands (eg, NKG2D) and inhibitory receptors (eg, killer inhibitory receptors [KIR]) and TCR ligation (in the case of NKT cells), resulting in rapid IFN-γ release and licensing of recipient APCs (ie, up-regulation of CD40 and CD80/86). Transplanted donor T cells are activated after interaction with these host APCs to undergo expansion and Th1/Tc1 differentiation. IFN-γ production by donor T cells augments gut damage via (1) promoting Th1 and Tc1 differentiation and subsequent cytolysis against target tissue (eg, via perforin/granzyme), (2) direct cytopathic effects of the cytokine itself on the GI tract, and (3) priming monocytes/macrophages such that they secrete quantities of TNF and lymphotoxin after signaling by TLR ligands that are themselves directly cytopathic to the GI tract. In contrast to these pathogenic effects, IFN-γ secretion by donor T cells is critical in the lung parenchyma to induce PDL1 expression, which subsequently inhibits donor T-cell survival and infiltration within this organ, preventing the development of idiopathic pneumonia syndrome (IPS). IFN-γ also promotes GVL effects via (1) the enhancement of CTL expansion and function (eg, perforin/granzyme-dependent killing) and (2) acting on leukemia cells directly (eg, to up-regulate MHC expression and death receptors), rendering them more susceptible to T cell–mediated cytolysis.
In conjunction with effects on immune reconstitution and T-cell activation after transplantation, IFN-γ also mediates GVL effects through direct interaction with tumor cells. This may result in the induction of apoptosis, suppression of proliferation, and increased sensitivity to cytotoxic T-cell killing via the up-regulation of Fas and MHC.84 However, further studies using tumor that does not express IFN-γR in models of BMT indicated that IFN-γ still promotes GVL effects, even in the absence of direct signaling by the cytokine with leukemic cells.85 Therefore, IFN-γ improves GVL via both direct effects on leukemic cells and improving antileukemia responses because of effects on T-cell priming and differentiation (Figure 2). It is tempting to consider that IFN-γ administration may prevent GVHD while enhancing GVL (ie, IFN-γ inhibits the infiltration of alloreactive cells into some GVHD target tissues while promoting CTL expansion and activity, particularly within the lymphohematopoietic system where leukemia predominantly resides). However, the known pathogenic effects of the cytokine, particularly within the GI tract, would suggest this as an unwise therapeutic strategy in patients.
Type III IFN
Type III IFNs were discovered in 2003 with the group composing 3 subtypes in humans, IFN-λ1, IFN-λ2, and IFN-λ3 or IL-29, IL-28A, and IL-28B, respectively. Mice only express IFN-λ2 and IFN-λ3. Type III IFNs signal through a distinct receptor complex consisting of the IFN-λR1 and IL10-R2 subunits,86 with signaling activating a similar set of transcription factors to that seen after type I IFN signaling.87 There is different expression of the receptors for type I and type III IFN, with most cell types expressing the type I IFN receptor whereas the IFN-λR1 is highly restricted to cells of epithelial origin, suggesting that this cytokine system may have evolved to play an important role in the prevention of viral invasion through the skin and mucosal surfaces.88
Similar to type I IFN, the expression of type III IFN is induced by signaling through pattern recognition receptors, such as TLRs.89 Although the cell types responsible for type III IFN require clarification, preliminary studies demonstrate limited production in the CNS compared with the liver.88 Initial studies suggested redundancy between the type I and type III IFN systems. Whereas recipients lacking receptors to both type I IFN and type III IFN are more susceptible to respiratory viral infections compared with those lacking type I IFN signaling only, mice lacking only type III IFN receptor display only slightly enhanced susceptibility to infection, unlike type I IFN receptor-deficient mice, which are extremely susceptible.90 However, the site of infection appears to be an important factor toward the redundancy of type III IFN, as mice lacking the type III IFN receptor are highly susceptible to rotavirus infection.91 Therefore, despite expression of the type III IFN receptor in both the lung and small intestine, type III IFN appears to play a nonredundant role in antiviral infections only in the GI tract.
There has been extensive interest in the potential of type III IFN as an alternative to type I IFN for the treatment for chronic hepatitis C infection. Although IFN-α is a potent antiviral agent, the broad expression of the type I IFN receptor results in responses in many organs apart from the target tissue. Thus, serious complications include the development of neutropenia and lymphopenia resulting from IFNAR1 expression in hematopoietic cells,92 depression resulting from CNS effects,93 and constitutional symptoms (fevers, chills, myalgias). The limitation of type III IFN receptor expression to epithelial cells suggests that such complications may be avoided by type III IFN administration. Furthermore, 3 independent genome-wide association studies have identified single nucleotide polymorphisms in the IL-28B gene region to be associated with response of patients with chronic hepatitis C to pegylated IFN-α treatment.94,95 Therefore, IFN-λ is currently being investigated as an alternative therapeutic agent to type I IFNs, with results indicating that IFN-λ elicits antiviral activity without significant side effects.96,97 However, the lack of IFN-λ expression on hematopoietic cells suggests it is unlikely that IFN-λ administration would result in comparable GVL effects to type 1 IFN. However, it will be interesting to investigate the role of IFN-λ in GVHD, particularly considering the surprising colon-specific GVHD observed in recipients of BMT who lack the IFNAR1.45
In conclusion, the broad range of outcomes and complex interactions after signaling with the various IFNs has resulted in conflicting studies observed in multiple experimental models and disease states. Although there has been limited investigation into cross-regulation between the IFNs, there is evidence that this does occur.63 Therefore, the ability of blocking one type of IFN to effect signaling and production of the other groups requires consideration when interpreting studies. Furthermore, the general lack of cross-species reactivity seen with many cytokines, including the IFNs, severely limits the ability of xenogeneic models of GVHD to provide informative data if the cytokine in question is capable of mediating significant end-organ damage and should probably be avoided in this setting.
Within SCT, the effects of IFN-γ are complex, highly temporally and conditioning dependent, with both beneficial and detrimental effects seen that are organ specific. Therefore, it will be important for future preclinical studies to use more sophisticated reagents that remove the ability of individual cell populations to produce or respond to IFN-γ. Ideally, this will involve inducible systems so that these issues can be more clearly delineated without the potential influence of developmental abnormalities that may be seen in knockout animals. Given current information, the exogenous administration of IFN-γ or its direct inhibition after clinical SCT is likely to result in highly unpredictable and potentially undesirable effects. However, promising preclinical results have been demonstrated by the inhibition of downstream signaling pathways of IFN-γ, such as Stat-1,98 or signaling pathways potentially upstream of IFN-γ, such as the Jak2 pathway,99 and this may be a viable alternative clinical strategy.
The role of type III IFNs in transplant outcome remains to be explored, but it is probable that, like type I IFN, target organ-specific effects will again be seen. In contrast, the type I IFNs have clear potential for immunomodulation to control relapse based on their ability to promote GVL effects. However, the administration of type 1 IFNs will be best tolerated in a setting temporally distant from conditioning where suppressive MHC class II–dependent effects dominate. Thus, the most appropriate timing would appear to be late after BMT, perhaps in conjunction with donor lymphocyte infusions, such that effects on antigen cross-presentation within donor DCs and direct effects on T cells may be invoked in the absence of the inflammatory milieu seen after conditioning and/or GVHD. Even then, the side effects, including the exacerbation of GVHD, will probably limit the use of this cytokine to patients at very high risk of relapse. Thus, future prospective clinical studies will need to initially focus on the ability of exogenous type I IFN administration to improve GVL and limit relapse in very high risk settings.
Acknowledgments
R.J.R. is a Leukemia Foundation Queensland PhD Scholarship Recipient. G.R.H. is a National Health and Medical Research Council Australia Fellow and a Queensland Health Senior Clinical Research Fellow.
Authorship
Contribution: R.J.R. and G.R.H. reviewed the literature and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Road, Herston, QLD, 4006, Australia; e-mail: geoff.hill@qimr.edu.au.

![Figure 2. The differential effects of IFN-γ on GVHD target organs and GVL. After conditioning induced tissue damage, NK and NKT cells are activated through activating receptor ligands (eg, NKG2D) and inhibitory receptors (eg, killer inhibitory receptors [KIR]) and TCR ligation (in the case of NKT cells), resulting in rapid IFN-γ release and licensing of recipient APCs (ie, up-regulation of CD40 and CD80/86). Transplanted donor T cells are activated after interaction with these host APCs to undergo expansion and Th1/Tc1 differentiation. IFN-γ production by donor T cells augments gut damage via (1) promoting Th1 and Tc1 differentiation and subsequent cytolysis against target tissue (eg, via perforin/granzyme), (2) direct cytopathic effects of the cytokine itself on the GI tract, and (3) priming monocytes/macrophages such that they secrete quantities of TNF and lymphotoxin after signaling by TLR ligands that are themselves directly cytopathic to the GI tract. In contrast to these pathogenic effects, IFN-γ secretion by donor T cells is critical in the lung parenchyma to induce PDL1 expression, which subsequently inhibits donor T-cell survival and infiltration within this organ, preventing the development of idiopathic pneumonia syndrome (IPS). IFN-γ also promotes GVL effects via (1) the enhancement of CTL expansion and function (eg, perforin/granzyme-dependent killing) and (2) acting on leukemia cells directly (eg, to up-regulate MHC expression and death receptors), rendering them more susceptible to T cell–mediated cytolysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/23/10.1182_blood-2012-02-368076/4/m_zh89991291970002.jpeg?Expires=1769089466&Signature=ro5jA1dGEo2swG~zjah8-a-yT-yxbaRA16bCEP9~zWSCl~qKkRd8YSOYqnOPWgKEAm76CHnHzFqxVdj~YJpkTdI4NIUmsj71MXq7WzCmvKQKj9WBb93HoDTzSUScxvxzsZ~F6kAhRZp6DnOk7wUqvheQ1oYqRGHuRY2rVpX9~cOgsLM6Gns6RT1xuSGAyZd6nfQMvgzCJgAf0Nf-okUArk1tc-WN9KuvTENWjckK~n8x18tL8d-rUysIuM0dfkmbpA5aCQjBEPS7Wl3zR9BQCmN5zPXoQPPTxT8fr0tNNXbMhf9RiALa28w7q1BcUBUtOC~zmZljuZbneV3dNaYd7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal