Hematopoietic stem cell transplantation is the only curative treatment for many malignant hematologic diseases, with an often critical graft-versus-leukemia effect. Despite peritransplant prophylaxis, GVHD remains a significant cause of posthematopoietic stem cell transplantation morbidity and mortality. Traditional therapies have targeted T cells, yet immunostimulatory dendritic cells (DCs) are critical in the pathogenesis of GVHD. Furthermore, DCs also have tolerogenic properties. Monitoring of DC characteristics may be predictive of outcome, and therapies that target DCs are innovative and promising. DCs may be targeted in vivo or tolerogenic (tol) DCs may be generated in vitro and given in the peritransplant period. Other cellular therapies, notably regulatory T cells (Treg) and mesenchymal stem cells, mediate important effects through DCs and show promise for the prevention and treatment of GVHD in early human studies. Therapies are likely to be more effective if they have synergistic effects or target both DCs and T cells in vivo, such as tolDCs or Treg. Given the effectiveness of tolDCs in experimental models of GVHD and their safety in early human studies for type 1 diabetes, it is crucial that tolDCs be investigated in the prevention and treatment of human GVHD while ensuring conservation of graft-versus-leukemia effects.
Introduction
Hematopoietic stem cell transplantation (HSCT) remains the only curative therapy for many high-risk malignant hematologic diseases, as well as numerous life-threatening genetic and hematologic disorders. However, despite peritransplant prophylaxis, HSCT is frequently complicated by GVHD, which leads to significant morbidity and mortality. The risk of GVHD limits the broader application of HSCT where it has the potential to cure autoimmune diseases, facilitate transplant tolerance, and correct immunologic deficiencies, including HIV infection.1 Conventional immunosuppressants remain the mainstay of treatment for GVHD, yet they frequently fail and carry a significant risk for infection.2,3 It is therefore of significant interest to identify new, effective, and safe prophylactic and therapeutic approaches, particularly those that maintain the critical graft-versus-leukemia (GVL) effect of HSCT. In this review, we consider advances that have been made in understanding the role of dendritic cells (DCs) in GVHD and address the challenge of monitoring, targeting, and exploiting these cells to improve therapeutic outcomes.
Our understanding of the pathogenesis of GVHD has advanced significantly over the past 45 years, since Billingham proposed that GVHD is the result of immunocompetent donor cells recognizing recipient antigens (Ags) in an immunocompromised host unable to reject donor cells.4 The principal immunocompetent donor effector cells are T cells, and the vigor of the immune response is driven by differences in MHC and minor histocompatibility antigens (miHA). Furthermore, the crucial role of Ag-presenting cells (APCs), in particular uniquely well-equipped donor and recipient DCs, has begun to be elucidated, not only in GVHD, but also in the GVL effect of HSCT.
DC hematopoiesis and immunobiology
DCs are rare, heterogeneous bone marrow (BM)–derived professional APCs, first characterized in mouse spleen by Steinman and Cohn,5 that are distributed ubiquitously in blood, lymphoid, and peripheral tissues, especially at portals of entry. They arise from hematopoietic stem cells through specialized progenitor subsets6 and are important in innate and adaptive immune function and in determining the balance between immunity and tolerance. In the normal steady state, DCs reside in “immature” or “semimature” states in the periphery where they constantly take up and process self-Ags and maintain self-tolerance.7 Immunostimulatory DCs have undergone maturation after recognition of exogenous and endogenous alarmins/danger signals by Toll-like receptors (TLRs).8 These signals include pathogen-associated molecular patterns in the form of microbial products and danger-associated molecular patterns, such as products of damaged or dying cells (eg, high-mobility group protein B1 or DNA). DCs are also matured by CD40 ligation and by proinflammatory cytokines that can induce DC maturation ex vivo, independent of CD40 ligation. Maturation is associated with up-regulation of cell surface MHC gene products, costimulatory molecules (CD40, CD80, and CD86, in addition to CD83 in humans), and appropriate chemokine receptors (in particular CCR7) that enhance the ability of DCs to home to secondary lymphoid tissue. Therein they present Ag to Ag-specific T cells and induce T-cell activation/proliferation. In turn, activated T cells drive DCs toward terminal maturation. These aspects of DC immunobiology have been reviewed in detail.9,,–12
DCs develop from HSCs in the BM and are derived from both myeloid and lymphoid progenitors (Figure 1).13,,–16 This has been demonstrated in both mouse and human studies, in which all DC subsets can be generated from either a common lymphoid progenitor or common myeloid progenitor.6,17,–19 The hematopoietic growth factor fms-like tyrosine kinase 3 ligand (Flt3L) plays a central role in steady-state DC development; this is evidenced by the majority of DC precursors being Flt3+ (CD135+) and culture with Flt3L resulting in all major DC subsets.6,17,20,21 GM-CSF is also important in DC hematopoiesis, as it gives rise to DCs from monocytes and early progenitors in the absence of intact Flt3L signaling and produces DCs under inflammatory conditions.6,17 Monocyte/macrophage M-CSF is also a DC poietin and can drive DC generation in mice independently of Flt3L.22
DC hematopoiesis and subsets. (A) All identified DC subsets can be generated from either a common myeloid progenitor (CMP) or common lymphoid progenitor (CLP) depending on the cytokines and growth factors present. DCs can be broadly categorized as cDCs or precursor DCs. pDCs are understood to be a subset of precursor DCs that have plasma cell morphology, an immature phenotype, and secrete type I IFN after activation. Monocyte-derived DCs or “inflammatory DCs” are similar to cDCs in form and function and correlate with in vitro GM-CSF-generated DCs. cDCs can be categorized as lymphoid tissue resident and migratory DCs. DCs were categorized previously as lymphoid or myeloid (mDCs) based on the hypothesis that each had separate progenitors, a convention that has persisted in the experimental and clinical evaluation of DC subsets. Professional illustration by Alice Y. Chen. (B) In mice, pDCs are identified as CD11cloCD11b− Siglec-H+PDCA-1+, whereas in humans, they are lin−MHC II+CD11c−CD123(IL-3Rα)+BDCA2(CD303)+. Mouse mDCs are identified as CD11c+CD11b+B220− (CD45R−) NK1.1−, whereas human mDCs are lin− MHCII+CD11c+CD123−BDCA1(CD1b/c)+. Other phenotypic differences between mouse and human DC precursors are also listed in the table. HSC indicates hematopoietic stem cells; MPP, multipotent progenitor; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; pDC, plasmacytoid DC; mDC, myeloid DC; LN, lymph node; and LP, lamina propria.
DC hematopoiesis and subsets. (A) All identified DC subsets can be generated from either a common myeloid progenitor (CMP) or common lymphoid progenitor (CLP) depending on the cytokines and growth factors present. DCs can be broadly categorized as cDCs or precursor DCs. pDCs are understood to be a subset of precursor DCs that have plasma cell morphology, an immature phenotype, and secrete type I IFN after activation. Monocyte-derived DCs or “inflammatory DCs” are similar to cDCs in form and function and correlate with in vitro GM-CSF-generated DCs. cDCs can be categorized as lymphoid tissue resident and migratory DCs. DCs were categorized previously as lymphoid or myeloid (mDCs) based on the hypothesis that each had separate progenitors, a convention that has persisted in the experimental and clinical evaluation of DC subsets. Professional illustration by Alice Y. Chen. (B) In mice, pDCs are identified as CD11cloCD11b− Siglec-H+PDCA-1+, whereas in humans, they are lin−MHC II+CD11c−CD123(IL-3Rα)+BDCA2(CD303)+. Mouse mDCs are identified as CD11c+CD11b+B220− (CD45R−) NK1.1−, whereas human mDCs are lin− MHCII+CD11c+CD123−BDCA1(CD1b/c)+. Other phenotypic differences between mouse and human DC precursors are also listed in the table. HSC indicates hematopoietic stem cells; MPP, multipotent progenitor; CMP, common myeloid progenitor; CLP, common lymphoid progenitor; pDC, plasmacytoid DC; mDC, myeloid DC; LN, lymph node; and LP, lamina propria.
DC subsets
Overview of subsets
DCs can be broadly categorized as conventional DCs (cDCs) and precursor DCs (Figure 1).17 In the steady state, cDCs exhibit typical DC features (eg, cytoplasmic dendrites) and function (eg, Ag uptake, processing, and presentation).17 cDCs can be subdivided into migratory DCs, such as skin epidermal Langerhans cells (LCs) and dermal DCs, which present Ag in lymph nodes after its uptake in peripheral tissue, and resident DCs, which take up and present Ag within a lymphoid organ, such as splenic or thymic DCs (Table 1).6,17 Resident DCs can be further categorized in the mouse as CD8α−, which is the predominant splenic population, and CD8α+, which is the major thymic population.6,17 CD8α+ DCs are involved in Ag cross-presentation and show high IL-12 secretion.6,17 Thymic cDCs primarily present self-Ag and are important in self-tolerance through T cell–negative selection and the production of naturally occurring regulatory T cells (Treg).23,–25 Plasmacytoid DCs (pDCs) are a subset of precursor DCs which have an immature phenotype in the steady-state and plasma cell morphology (eg, lack dendrites).17 On activation, pDCs closely resemble cDCs in form and function.17 Monocyte-derived DCs, or “inflammatory DCs,” are similar to cDCs in form and function and correlate with in vitro GM-CSF-generated DCs.17
Phenotype and function of mouse conventional DC subsets
| Organ . | Phenotype . | Location . | Function and characteristics . |
|---|---|---|---|
| Lymphoid-tissue resident DCs | |||
| Spleen | CD8α+CD205+ | T-cell area and marginal zone | Uptake and cross-presentation of Ag from apoptotic cells on MHC class I |
| CD8α−33D1+ | Red pulp and bridging channels | Predominant population; uptake and presentation of Ag on MHC class II | |
| Thymus | CD8α+ | Medulla | Predominant population; cross-presentation of Ag; self-tolerance |
| CD8α− | Cortex, medulla, corticomedullary junction | Self-tolerance | |
| LN | CD8α+ | Cortex | Viral and self-Ag immunity; cross-presentation of Ag |
| CD8α− | Cortex | Unknown | |
| Migratory DCs | |||
| Skin | Langerin+ LC | Epidermis | Self-renew in situ, radiation resistant |
| CD103+CD11blo+ Langerin+ | Dermis | Viral and self-Ag immunity; cross-presentation of Ag | |
| CD103−CD11bhi− Langerin− | Dermis | Unknown | |
| Intestine | CD103+CD11blo | Peyer patches | Unknown |
| CD103+CD11b+ | Lamina propria | Transfer pathogenic bacteria from gut to mesenteric LN | |
| CD103−CD11bhi | Lamina propria | Transport Ag to mesenteric LN from gut lumen | |
| LN | CD11cintCD40hiMHCIIhiCCR7+ | Cortex | Transport Ag to LN from periphery |
| Organ . | Phenotype . | Location . | Function and characteristics . |
|---|---|---|---|
| Lymphoid-tissue resident DCs | |||
| Spleen | CD8α+CD205+ | T-cell area and marginal zone | Uptake and cross-presentation of Ag from apoptotic cells on MHC class I |
| CD8α−33D1+ | Red pulp and bridging channels | Predominant population; uptake and presentation of Ag on MHC class II | |
| Thymus | CD8α+ | Medulla | Predominant population; cross-presentation of Ag; self-tolerance |
| CD8α− | Cortex, medulla, corticomedullary junction | Self-tolerance | |
| LN | CD8α+ | Cortex | Viral and self-Ag immunity; cross-presentation of Ag |
| CD8α− | Cortex | Unknown | |
| Migratory DCs | |||
| Skin | Langerin+ LC | Epidermis | Self-renew in situ, radiation resistant |
| CD103+CD11blo+ Langerin+ | Dermis | Viral and self-Ag immunity; cross-presentation of Ag | |
| CD103−CD11bhi− Langerin− | Dermis | Unknown | |
| Intestine | CD103+CD11blo | Peyer patches | Unknown |
| CD103+CD11b+ | Lamina propria | Transfer pathogenic bacteria from gut to mesenteric LN | |
| CD103−CD11bhi | Lamina propria | Transport Ag to mesenteric LN from gut lumen | |
| LN | CD11cintCD40hiMHCIIhiCCR7+ | Cortex | Transport Ag to LN from periphery |
LN indicates lymph node.
Function of DC subsets
DC subsets differ in their immune functions, which has important implications for HSCT. Under steady-state conditions, human pDCs display lower levels of MHC and costimulatory molecules compared with conventional myeloid DCs (mDCs).26 In addition, because their Ag processing and loading ability are less efficient, pDCs stimulate T cells less effectively than mDCs.26 After their activation via TLR, pDCs secrete high levels of type 1 IFN and stimulate CD4+ and CD8+ T cells.11 This is in contrast to activated mDCs, which secrete IL-12 and promote T-helper type-1 (Th1) cell differentiation and CD8+ cytotoxic T lymphocyte (CTL) responses.26,27 pDCs have intrinsic tolerogenic properties; in the steady state, human thymic pDCs induce Treg, whereas liver and airway pDCs regulate oral and mucosal tolerance, respectively. pDCs have also been implicated in the regulation of disease activity in experimental models of autoimmunity and shown to exert disease-suppressing ability.28 This may be important after HSCT in terms of donor BM engraftment (tolerance), as well as for chronic GVHD (cGVHD), which has clinical features that overlap with autoimmune disease. Epidermal LCs may be immunostimulatory or tolerogenic, depending on their state of maturity, inciting immunogen, and the cytokine environment.29
Mouse versus human DC subsets
DC subsets have been well characterized, especially in mice, and also in humans and other species (Figure 1).13,14,30,–32 Most human work has been conducted in vitro; thus, knowledge of the in vivo function and development of human DC subsets is lacking. Direct comparisons between mouse and human DC subsets can be problematic because of phenotypic differences between the species (eg, multiple human DC subsets are capable of Ag cross-presentation and display high IL-12 secretion, and are thus comparable with the mouse CD8α+ DC subset).33 Likewise, flow cytometric characterization of DC subsets has been refined over time, and the evolution of “standard” DC markers makes comparisons between present and past studies difficult. Currently, in mice, pDCs are identified as CD11cloCD11b− sialic acid binding immunoglobulin-like lectin H (Siglec-H)+PDCA-1+, whereas in humans, they are lin−MHC II+CD11c−CD123(IL-3Rα)+BDCA2(CD303)+. Mouse mDCs are identified as CD11c+CD11b+B220− (CD45R−) NK1.1−, whereas human mDCs are lin−MHCII+CD11c+CD123−BDCA1 (CD1b/c)+.
Although characterization of human DC subsets has been more difficult because of both their rarity and difficulty of access, DC subsets in the skin have been well characterized, although recent studies have begun to elucidate subsets in the blood and other organs. The epidermis contains Langerin+ LCs, whereas the dermis contains CD1a+ and CD14+ DCs, the former of which has an unknown function.34 CD14+ DCs are involved in B-cell differentiation both by activation of naive B cells to plasma cells35,36 and induction of naive CD4+ T cells to T follicular helper cells.37 Compared with other human skin DC subsets, LCs are potent activators of Ag-specific CD8+ T cells, which may be explained in part by their production of IL-15.34,35 Conventional DC subsets appear to be comparable in human blood and spleen in the steady state, with 3 major populations described, all of which are lin−CD11c+HLA-DR+, and unlike mouse subsets, cannot be further distinguished by CD8α.33 BDCA3(CD141)+ DCs are thought to be the human equivalent of mouse CD8α+ DCs, based on cell adhesion molecule 1 expression and their ability to cross-present Ag and secrete high IL-12, although more recent studies indicate that other human cDC subsets share these abilities.33,38 BDCA1(CD1b/c)+ DCs may be comparable with mouse CD8α− DCs, whereas CD16+ DCs have been termed inflammatory monocytes, based on their high TNF-α and low IL-10 expression.33
DC tolerogenicity
In addition to their capacity to stimulate innate and adaptive immunity, DCs can induce and maintain tolerance.28,39,40 Tolerogenic (tol) DCs present Ag to T cells but lack adequate costimulatory ability, deliver inhibitory signals (eg, via the programmed death [PD] pathway), and produce tolerance-promoting cytokines (IL-10).39 TolDCs do not support Ag-specific T-cell activation and proliferation but instead facilitate T-cell anergy/apoptosis and/or the generation or expansion of Treg.39 Importantly, bidirectional feedback between tolDCs and Treg has been demonstrated in humans and mice, whereby tolDCs promote the generation of Treg from naive T cells and Treg generate tolDCs from DC progenitors.41 Regulation of immunosuppressive tryptophan catabolism in DCs via activation of indoleamine 2,3-dioxygenase (IDO) may be an important mechanism of action of Treg42 and may underlie transplant tolerance in vivo. The close relationship between Treg and DCs is illustrated by the observation that increases in DCs lead to increases in Treg, whereas constitutive absence of DCs leads to fatal autoimmunity.43,–45
Role of DCs in the pathogenesis of GVHD
Mouse studies have demonstrated that CD4+ T cell–dependent (MHC-mismatched) acute GVHD (aGHVD) can be induced by either host or donor APCs, whereas host APCs are required for the initiation of CD8+ T cell–dependent (MHC-matched, multiple miHA-mismatched) aGVHD and donor APC amplify the process.46,–48 Additional studies have tried to further characterize the contribution of different APC populations to the development of aGVHD. Whereas earlier mouse studies implicated host LCs in the pathogenesis of skin GVHD, more recent experiments using mice deficient in LCs question the relevance of LCs in the development of aGVHD.49,50
Less is known about the role of DCs in cGVHD because of variability in clinical presentation (de novo cGVHD vs cGVHD evolving from aGVHD) and the lack of relevant mouse models. Both host and donor APCs have been implicated, but with target organ specificity; skin cGVHD can be induced by either donor or host APCs, whereas donor APCs are dominant in intestinal cGVHD.51,52 Thymic independent and dependent pathways likely contribute to cGVHD. Autoreactive CD4+ T cells have been implicated in cGVHD, and cGVHD occurs in patients with little thymic function or in those with intact thymic negative selection.52,53 Mouse studies have implicated engrafted donor anti–host CD4+ T cells in the evolution of aGVHD to cGVHD, whereby donor CD4+ T cells are generated in the milieu of CD8+ T cell–mediated aGVHD thymic damage, likely due to failure of thymic DCs to delete autoreactive CD4+ T cells.54 This has important implications, both in the pathogenesis of cGVHD, but also in its prevention, as keratinocyte growth factor prevents cGVHD likely because of thymic protection.54
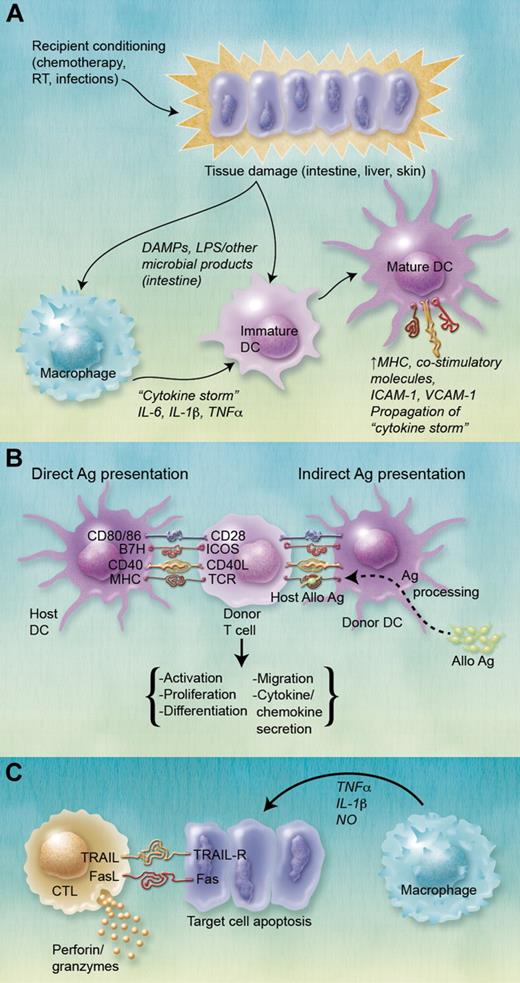
The development of GVHD, particularly aGVHD, has been divided traditionally into 3 phases (Figure 2). Phase 1 involves activation of APCs, particularly DCs, by cytokines released after recipient tissue damage. These DCs present acquired and processed Ag to T cells, which, in combination with simultaneous costimulation, leads to the second phase, donor T-cell activation. Mouse studies suggest that donor T-cell activation in GVHD requires costimulation via B7 family molecules (CD80/86)/CD28 and B7H/inducible costimulator and is inhibited by B7/CTL Ag (CTLA)–4 and PD-L1/PD interactions.55,56 After HSCT, DCs can present host Ag to donor T cells, either directly or indirectly. In the direct pathway, donor T cells are stimulated by allogeneic MHC or miHA molecules (in the more common MHC-matched setting) present on host APCs, whereas the indirect pathway involves presentation of acquired host Ags by engrafted donor APCs, particularly CD11c+ DCs.57 Mouse models have shown that the indirect pathway or “cross-presentation” of host Ag does not initiate aGVHD but that direct presentation by host DCs resistant to conditioning is required.46,48 Wang et al expanded knowledge of cross-presentation in an experimental model, demonstrating that donor APCs are activated by donor CD4+ T cells (initially activated by host APCs) dependent on CD40L and type I IFN, then cross-present acquired host hematopoietic and nonhematopoietic transmembrane proteins to donor CD8+ T cells.57 Using mAbs and/or transgenic/knockout donor mice, Markey et al examined the role of donor APC subsets and showed that donor cDCs are critical for cross-presentation of alloAg immediately after HSCT.58 Donor T-cell activation leads to the third phase, in which cytokines and cellular effectors, particularly CTLs, NK cells, and macrophages, mediate target cell injury and apoptosis.57
Role of DCs in the pathogenesis of GVHD. (A) Recipient pretransplant conditioning results in target organ tissue damage, leading to the so-called “cytokine storm,” a progressive amplification of proinflammatory cytokine production and immune activation as inflammatory cytokines feed forward unabated. IL-1β, IL-6, and TNF-α are particularly implicated in this process. In addition to proinflammatory cytokines, conditioning-released damage-associated molecular patterns (DAMPS) and translocation of lipopolysaccharide in the intestine also lead to the activation of host and subsequently donor DCs, including epidermal LCs and dermal DCs in the skin. Mature DCs up-regulate MHC, costimulatory, and intercellular adhesion molecule expression. (B) DCs present host allo-Ag to donor T cells. Host DCs resistant to conditioning present alloAg via the direct pathway, whereas transplanted donor DCs present processed alloAg peptides on MHC syngeneic with donor T cells via the indirect pathway. Donor T-cell activation requires Ag presentation via MHC molecules to the T-cell Ag receptor (TCR), as well as stimulation via various costimulatory molecules. This interaction results in T-cell activation, proliferation, differentiation (Th1, Th2), migration to GVHD target organs, and secretion of various chemokines and cytokines, importantly IFN-γ and IL-2. (C) Cellular and inflammatory effectors lead to target organ tissue damage. CTLs mediate target cell apoptosis via interactions between TNF and TNF receptors, TRAIL (TNF-related apoptosis-inducing ligand)/TRAIL-R and Fas (CD95)/FasL interactions and release of cytotoxic mediators (perforin and granzyme). Recruited macrophages release TNF-α, IL-1, and NO, which also damage target cells. RT indicates radiation therapy; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; ICOS, inducible costimulator; and NO, nitric oxide. (A-C) Professional illustrations by Alice Y. Chen.
Role of DCs in the pathogenesis of GVHD. (A) Recipient pretransplant conditioning results in target organ tissue damage, leading to the so-called “cytokine storm,” a progressive amplification of proinflammatory cytokine production and immune activation as inflammatory cytokines feed forward unabated. IL-1β, IL-6, and TNF-α are particularly implicated in this process. In addition to proinflammatory cytokines, conditioning-released damage-associated molecular patterns (DAMPS) and translocation of lipopolysaccharide in the intestine also lead to the activation of host and subsequently donor DCs, including epidermal LCs and dermal DCs in the skin. Mature DCs up-regulate MHC, costimulatory, and intercellular adhesion molecule expression. (B) DCs present host allo-Ag to donor T cells. Host DCs resistant to conditioning present alloAg via the direct pathway, whereas transplanted donor DCs present processed alloAg peptides on MHC syngeneic with donor T cells via the indirect pathway. Donor T-cell activation requires Ag presentation via MHC molecules to the T-cell Ag receptor (TCR), as well as stimulation via various costimulatory molecules. This interaction results in T-cell activation, proliferation, differentiation (Th1, Th2), migration to GVHD target organs, and secretion of various chemokines and cytokines, importantly IFN-γ and IL-2. (C) Cellular and inflammatory effectors lead to target organ tissue damage. CTLs mediate target cell apoptosis via interactions between TNF and TNF receptors, TRAIL (TNF-related apoptosis-inducing ligand)/TRAIL-R and Fas (CD95)/FasL interactions and release of cytotoxic mediators (perforin and granzyme). Recruited macrophages release TNF-α, IL-1, and NO, which also damage target cells. RT indicates radiation therapy; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; ICOS, inducible costimulator; and NO, nitric oxide. (A-C) Professional illustrations by Alice Y. Chen.
Role of DCs in GVL
Host DCs are required for full GVL effects, although donor APCs can initiate GVL activity when low levels of tumor are present.48,59,60 Li and Waller found that depletion of donor BM CD11b+ myeloid cells in an experimental model enhanced survival of recipients with tumor61 ; more recently, they reported that, conversely, addition of CD11b− cells, which were primarily precursor pDCs, augmented GVL without concomitant increase in GVHD.62 Clinical studies have demonstrated that increased graft pDC content is associated with relapse and decreased patient overall survival (OS).63 Low CD11c+ DCs, but not CD123+ DCs, in peripheral blood (PB) at the time of engraftment have also been associated with death and relapse.64 New insights concerning the role of both donor and recipient DC subsets in GVL, including graft precursor pDC content, and the impact of pretransplant manipulation of these subsets are clearly needed.
The role of DCs in GVL after donor leukocyte infusion has also been examined. In murine and clinical studies, GVL effects can be seen after donor leukocyte infusion without GVHD.65 The presence of host APCs and allo-MHC class I has been shown to be critical for GVL effects in mixed chimeras created in the fully MHC-mismatched setting, although results are not as clear in the MHC-matched minor Ag mismatched and clinical settings.66,–68 Furthermore, there is evidence that donor leukocyte infusion-induced GVL effects in mice are dependent on MHC alloAg, but not miHC or tumor-associated Ags, in a CD4+ and CD8+ T cell–dependent manner, and that MHC class II–expressing host APCs are required for maximal GVL activity.60 Unfortunately, although donor leukocyte infusion enhances the GVL effect, it is often complicated by GVHD.69,70
Influence of transplant factors on DCs in relation to GVHD
Given the role of host tissue damage in the pathogenesis of GVHD, it was thought that reduced intensity conditioning (RIC) would lead to less GVHD. However, whereas RIC has reduced transplant-related mortality, the incidence of aGVHD, although delayed, remains unchanged. In mouse HSCT after RIC, Turner et al demonstrated that, although the onset of GVHD was delayed, it was equally severe.71 The authors hypothesized that delays in GVHD were the result of maintenance of absolute numbers of host DCs and decreased TNF-α production, promoting Treg responses.71 As donor chimerism increased, donor-activated DCs increased and elevated TNF-α led to decreased Treg and onset of delayed, yet equally severe, GVHD.71
Conditioning regimens can differ in host irradiation; however, some human and mouse DCs are resistant to radiation, particularly dermal DCs and epidermal LCs.72,–74 In an experimental model, total body irradiation led to complete depletion of splenic and BM pDCs after 24 hours, whereas mDCs were maintained, but in decreased numbers.75 In addition, total body irradiation is important in DC activation; studies in mice have shown that inflammation from irradiation is critical for pDC but not mDC activation.26
HSCs may be obtained either directly from BM or from the PB after expansion with G-CSF. As reviewed by Korbling and Anderlini,76 despite a significant increase in donor T cells in PB stem cell grafts, there is surprisingly no increase in aGVHD, although there seems to be an increase in cGVHD.76 Numerous studies have documented increased graft pDCs with G-CSF mobilization, with potential implications for outcome of HSCT, including that these cells may favor Treg function.77,–79 G-CSF treatment has also been associated with decreased proinflammatory IL-12 production. In a mouse model of PB stem cell transplantation, G-CSF treatment of donors rather than recipients significantly reduced levels of TNF-α, probably via decreased donor DC TNF-α and IL-12 production.80 mDC IL-12 production was also significantly decreased in pediatric HSCT recipients who received G-CSF after transplantation.81 These differences must be taken into account when interpreting clinical studies.
DC analyses in relation to outcome of HSCT
DC engraftment
Clinical studies have revealed an association between low total DC numbers at the time of engraftment and decreased patient OS, increased relapse, and increased aGVHD (summarized in Table 2).64 Neither host DC count pretransplant nor graft DC count was associated with death or relapse.64 Although neither was independently significant, lower mDC count at engraftment was associated with decreased survival, increased relapse, and increased incidence of aGVHD.64 Lower circulating pDC count correlated only with increases in aGVHD.64 Skin GVHD has been associated with decreased human LC engraftment.49,82 However, this is thought to be a secondary effect related to steroid treatment and GVHD effector cells, as experimental studies have shown that donor T cells promote donor LC engraftment.49,82 Prospective studies are indicated to determine whether low DC count at the time of engraftment can be used as a predictive tool for GVHD.
HSCT outcome in relation to DC analyses
| Patient population . | DC measurement . | Outcome . | Reference . |
|---|---|---|---|
| Allo-HSCT (n = 49) | Total PB DC count at engraftment < 4.97 cells/μL | Survival ↓ | 64 |
| 92% PBSCT; 73% MAC | Relapse and aGVHD ↑ | ||
| Low PB mDCs (CD11c+) at engraftment | Survival ↓ | ||
| Relapse and aGVHD ↑ | |||
| Low PB pDCs (CD123+) at engraftment | aGVHD ↑ | ||
| Graft DC or DC count before transplant | No association with death/relapse | ||
| Allo-HSCT (n = 30) | Higher PB pDCs (BDCA-2+) > day 100 (donor) | cGVHD ↑ | 83 |
| 63% PBSCT; 83% MAC | |||
| Allo-HSCT (n = 24) | Higher total PB host DCs day 100 | Survival ↓ | 84 |
| 100% BM; 87% RIC | aGVHD and cGVHD (grade II-IV)↑ | ||
| Allo-HSCT (n = 40) | Low total PB DC, mDC (CD11c+), and pDC (CD123hi) counts | GVHD severity ↑ | 85 |
| 90% PBSCT; 52% RIC | > 7.9% CMRF-44+ CD11c+ DCs | aGVHD ↑ (sensitivity, 87.5%; specificity, 79.2%) | |
| CD83+/CD86+ CD11c+ DCs | No association with aGVHD | ||
| Allo-HSCT (n = 69) | Graft pDC (CD123+) > 2.3 × 106/kg | Relapse ↑ | 63 |
| 100% PBSCT; 54% MAC | OS and EFS ↓ | ||
| Allo-HSCT (n = 54) | Lower PB pDCs (lin−/CD11c−/ILT3+) 3 mo after HSCT (median 92 days) | aGVHD (grade II-IV) ↑ | 88 |
| 93% PBSCT; 100% RIC | OS and NRM ↓ | ||
| Late infections ↑ | |||
| Death ↑ | |||
| Patient population . | DC measurement . | Outcome . | Reference . |
|---|---|---|---|
| Allo-HSCT (n = 49) | Total PB DC count at engraftment < 4.97 cells/μL | Survival ↓ | 64 |
| 92% PBSCT; 73% MAC | Relapse and aGVHD ↑ | ||
| Low PB mDCs (CD11c+) at engraftment | Survival ↓ | ||
| Relapse and aGVHD ↑ | |||
| Low PB pDCs (CD123+) at engraftment | aGVHD ↑ | ||
| Graft DC or DC count before transplant | No association with death/relapse | ||
| Allo-HSCT (n = 30) | Higher PB pDCs (BDCA-2+) > day 100 (donor) | cGVHD ↑ | 83 |
| 63% PBSCT; 83% MAC | |||
| Allo-HSCT (n = 24) | Higher total PB host DCs day 100 | Survival ↓ | 84 |
| 100% BM; 87% RIC | aGVHD and cGVHD (grade II-IV)↑ | ||
| Allo-HSCT (n = 40) | Low total PB DC, mDC (CD11c+), and pDC (CD123hi) counts | GVHD severity ↑ | 85 |
| 90% PBSCT; 52% RIC | > 7.9% CMRF-44+ CD11c+ DCs | aGVHD ↑ (sensitivity, 87.5%; specificity, 79.2%) | |
| CD83+/CD86+ CD11c+ DCs | No association with aGVHD | ||
| Allo-HSCT (n = 69) | Graft pDC (CD123+) > 2.3 × 106/kg | Relapse ↑ | 63 |
| 100% PBSCT; 54% MAC | OS and EFS ↓ | ||
| Allo-HSCT (n = 54) | Lower PB pDCs (lin−/CD11c−/ILT3+) 3 mo after HSCT (median 92 days) | aGVHD (grade II-IV) ↑ | 88 |
| 93% PBSCT; 100% RIC | OS and NRM ↓ | ||
| Late infections ↑ | |||
| Death ↑ | |||
PBSCT indicates peripheral blood stem cell transplant; EFS, event-free survival; ILT3, immunoglobulin-like transcript 3; and NRM, nonrelapse mortality.
DC chimerism
There are conflicting data on DC chimerism after HSCT. Early human studies demonstrated an association between full donor chimerism and cGVHD compared with mixed chimerism in some control patients without cGVHD.83 Chan et al assessed DC chimerism 100 days after transplantation and found that host DC persistence correlated with severe aGVHD and cGVHD.84 There were significant differences between the 2 studies, however, with the latter involving primarily RIC regimens and samples differentiated in vitro and analyzed by DNA PCR banding, rather than by conventional flow cytometry.84 Given the differential effects of myeloablative conditioning (MAC) versus RIC, further studies on DC chimerism in both populations are warranted to resolve the impact of DC chimerism on development of aGVHD and cGVHD.
DC activation status
The activation status of DCs is likely important in and potentially predictive of GVHD. Lau et al85 examined expression of CMRF-44, a cell surface marker that is expressed early during the activation and maturation of human mDCs, but not on freshly isolated DCs from healthy controls. The incidence of circulating CMRF-44+CD11c+ DCs correlated with onset and severity of aGVHD and was found to be predictive when used as a screening test before the onset of GVHD.85 In the same study, cell surface expression of CD83 and CD86, both of which are increased on human DC activation and are important in T-cell costimulation, were not predictive of GVHD.85 Larger studies investigating the predictive role of these DC activation/maturation markers and their anti-inflammatory versus proinflammatory cytokine production, such as IL-10 or IL-12, respectively, should be performed. Analysis of donor versus host origin DC expression of these immunoregulatory molecules could enhance the insights from these further evaluations.
DC subsets
Before current immunophenotyping of DC subsets, Waller et al demonstrated an association between high BM graft presumptive pDC progenitors and decreased cGVHD,86 although the incidence of leukemic relapse was increased. A more recent clinical study did not find an association between G-CSF–mobilized PB graft pDC content and GVHD; however, it confirmed the increased incidence of relapse, as well as decreased OS and event-free survival.86 Because these studies used different stem cell sources, the difference in the incidence of GVHD may be accounted for, in part, by differences in cytokine release and/or DC activation status after G-CSF administration.86 The association between relapse and graft pDCs highlights the importance of preserving the GVL effect with any intervention to decrease GVHD.
Human DC subsets have also been examined in the posttransplant period. Reddy et al documented a dependent association between low CD11c+ DCs in PB at the time of engraftment and death, relapse and aGVHD64 ; low CD123+ DC count was associated with aGVHD only. More recent studies in patients after MAC and RIC found a significant correlation between pDC count and increased GVHD, as well as pDC and mDC count and increased GVHD severity.63,85,87 Low pDC count 3 months after RIC transplant was also associated with severe aGVHD, decreased OS, and increased nonrelapse mortality, notably from GVHD and late infections.88 As with other analyses, there have been conflicting reports, as an earlier study associated high pDC count with cGVHD, although this was at a median of 14.5 months after transplantation.83 Larger studies comparing DC subsets after various conditioning regimens may help elucidate the differences between studies.
DC subsets have been examined extensively in experimental GVHD. By adoptively transferring DCs into MHC class II–deficient recipient mice, both pDCs and cDCs were found to be sufficient to induce comparable donor CD4+ T cell–dependent GVHD, although pDCs required an inflammatory environment created by host irradiation for activation and donor T-cell priming.26 Thus, pDCs expressing alloAg were sufficient to prime alloreactive T cells and induce GVHD. Similar to human studies, low pDCs (depleted by 120G8 Ab to BM stromal cell Ag-2) in the BM graft led to increased aGVHD, whereas there was no association between GVHD and pDC count in G-CSF–mobilized grafts.75 The authors indicated that these latter pDCs were mature, which may account for the difference in incidence of GVHD. In addition, although cDC reconstitution did not differ between control and GVHD mice, pDC maturation was abrogated in GVHD.75 Interestingly, GVHD led to a suppressive precursor DC population that may contribute to immune paralysis after transplantation.75 These findings concerning the role of DC subsets provide important insight into potential strategies for tolerance induction in HSCT.
Influence of current GVHD therapies on DCs
Many current therapies significantly affect DC phenotype and function.89 More precisely, calcineurin inhibitors (CNI; cyclosporine or tacrolimus) suppress Ag presentation, whereas glucocorticosteroids inhibit DC maturation, activation, and production of TNF-α, IL-1β, and IL-12 after stimulation.90,–92 DCs generated in the presence of CNI or rapamycin (sirolimus; the serine-threonine kinase inhibitor of the mammalian target of rapamycin) have decreased costimulatory molecule expression and T-cell allostimulatory capacity.90,93 In addition, epidermal LCs exposed to glucocorticosteroids are phenotypically immature and expand Treg via TGF-β production.94
Ab therapy directed against immune cells is used both in the prevention and treatment of GVHD. Polyclonal anti-thymocyte globulin (ATG) Ab has been used before transplantation for T-cell depletion for decades.95 However, as reviewed by Mohty,96 ATG has diverse immunologic effects, including its impact on DCs. ATG inhibits experimental DC Ag uptake and maturation, induces complement-mediated lysis of DCs, and decreases the capacity of DCs to stimulate allogeneic T cells.96,97 In humans, ATG decreases DC Ag uptake, PB mDCs and pDCs, and mDC IL-12 production and allogeneic T-cell proliferation.66,67
Alemtuzumab (Campath-1H), a lymphocyte-depleting humanized anti-CD52 mAb, has been used for both GVHD prevention and treatment. As well as depleting donor T cells, alemtuzumab may also target host DCs.95 Although its effects on DCs are not well studied, alemtuzumab depletes human PB DCs in vivo but has few significant effects on LCs or dermal DCs, which only weakly express the epitope.98,99 Multiple mAbs against the IL-2 receptor (CD25) have shown efficacy in second-line treatment of GVHD.95 Although much of these effects have been attributed to direct binding to T cells, recent work using daclizumab (humanized anti-CD25 mAb) has shown that it potently inhibits Ag-specific T-cell activation by mature DCs.100,101
Prevention or treatment of GVHD by targeting DCs
Historically, T cells have been the primary target in GVHD, but given the important role of DCs in its pathogenesis, APCs also represent an important target. DCs may be manipulated using multiple approaches in vivo or in vitro, in the latter case for the production of tolDC vaccines with the ability to regulate immunity and suppress GVHD. Approaches being evaluated include the following.
Pharmacologic interventions
Histone deacetylase (HDAC) inhibitors, used clinically as anticancer drugs, reduce DC TLR-induced costimulatory molecule expression, proinflammatory cytokine release, and T-cell allostimulatory activity (summarized in Table 3). Further, they increase Treg number and function via increased IDO expression in a signal transducer and activator of T cells (STAT3)–dependent manner.102,103 HDAC inhibition decreases GVHD in several experimental models while preserving GVL.103,–105 Clinical testing of HDAC inhibition using agents, such as suberoylanilide hydroxamic acid (SAHA; vorinostat), in conjunction with CNI for the prophylaxis of GVHD after RIC allogeneic HSCT is in progress.103,104
Impact of interventional strategies on DCs and outcome in experimental HSCT
| Mechanism . | Therapy . | GVHD model; treatment . | Effect on DCs* . | Effect on GVHD . | Reference . |
|---|---|---|---|---|---|
| HDAC inhibition | SAHA or ITF2357 | MHC-mismatched BALB/c → B6; | TNF-α, IL-12, and IL-6 secretion ↓ | Survival ↑ | 104 |
| Host type DCs pretreated with HDAC inhibitor infused days −1, 0, and 2 | CD40/CD80/CD86 expression ↓ | Clinical score ↓ | |||
| Allogeneic T-cell proliferation ↓ (in vitro/in vivo) | Serum TNF-α ↓ | ||||
| Preserved GVL | |||||
| Proteasome inhibition | Bortezomib | MHC-mismatched BALB/c → B6; | Response to maturation signals ↓ | Survival ↑ with early treatment | 108 |
| Early (days 0-2) versus late (days 6-8) treatment | MHC class II, CD40/CD80/CD83/CD86 expression ↓ | Survival ↓ with late treatment | |||
| Apoptosis ↑ | Preserved GVL | ||||
| Allogeneic T-cell proliferation ↓ | |||||
| NF-κB inhibition | RelB−/− BM chimera recipients | MHC-mismatched BALB/c → B6 | CD11chi DCs (cDCs) ↓ | Survival ↑ | 109 |
| CD40/CD80/CD86 expression unchanged | Clinical score ↓ | ||||
| IL-12, IL-6, TNF-α secretion ↓ (after CD40L) | |||||
| CD4+ T-cell proliferation (in vitro/vivo); cytokine secretion ↓ (in vitro) | |||||
| No difference PDCA-1+ DCs (pDCs), Treg (in vivo) | |||||
| RelB−/− BM chimera donor | MHC-mismatched B6 → B6D2F1 | Late (> 3 wks) survival ↑ and clinical score ↓ | |||
| Anti-CD83 | Polyclonal Ab | Hu SCID; day 0 | Survival ↑ | 110 | |
| Preserved engraftment and GVL | |||||
| Mechanism . | Therapy . | GVHD model; treatment . | Effect on DCs* . | Effect on GVHD . | Reference . |
|---|---|---|---|---|---|
| HDAC inhibition | SAHA or ITF2357 | MHC-mismatched BALB/c → B6; | TNF-α, IL-12, and IL-6 secretion ↓ | Survival ↑ | 104 |
| Host type DCs pretreated with HDAC inhibitor infused days −1, 0, and 2 | CD40/CD80/CD86 expression ↓ | Clinical score ↓ | |||
| Allogeneic T-cell proliferation ↓ (in vitro/in vivo) | Serum TNF-α ↓ | ||||
| Preserved GVL | |||||
| Proteasome inhibition | Bortezomib | MHC-mismatched BALB/c → B6; | Response to maturation signals ↓ | Survival ↑ with early treatment | 108 |
| Early (days 0-2) versus late (days 6-8) treatment | MHC class II, CD40/CD80/CD83/CD86 expression ↓ | Survival ↓ with late treatment | |||
| Apoptosis ↑ | Preserved GVL | ||||
| Allogeneic T-cell proliferation ↓ | |||||
| NF-κB inhibition | RelB−/− BM chimera recipients | MHC-mismatched BALB/c → B6 | CD11chi DCs (cDCs) ↓ | Survival ↑ | 109 |
| CD40/CD80/CD86 expression unchanged | Clinical score ↓ | ||||
| IL-12, IL-6, TNF-α secretion ↓ (after CD40L) | |||||
| CD4+ T-cell proliferation (in vitro/vivo); cytokine secretion ↓ (in vitro) | |||||
| No difference PDCA-1+ DCs (pDCs), Treg (in vivo) | |||||
| RelB−/− BM chimera donor | MHC-mismatched B6 → B6D2F1 | Late (> 3 wks) survival ↑ and clinical score ↓ | |||
| Anti-CD83 | Polyclonal Ab | Hu SCID; day 0 | Survival ↑ | 110 | |
| Preserved engraftment and GVL | |||||
Hu SCID indicates human severe-combined immunodeficiency.
In vitro unless otherwise indicated.
Proteasome inhibitors have been studied in cancer and autoimmunity and are thought to induce apoptosis by blocking the degradation of proapoptotic proteins. Bortezomib, approved for the treatment of multiple myeloma, is thought to block the activation and nuclear translocation of NF-κB, a transcription factor central to DC maturation and inflammatory responses. Thus, inhibition of DC NF-κB activation with bortezomib or other inhibitors is an attractive strategy for GVHD prevention.106 Immature DCs treated with bortezomib fail to up-regulate MHC class II and costimulatory molecules in response to maturation signals, have decreased T-cell allostimulatory capacity, and are more susceptible to apoptosis.106,107 In experimental HSCT, bortezomib attenuates aGVHD yet preserves GVL.106,107 Whereas early treatment after HSCT prevents mild aGVHD in mice, later treatment increases mortality significantly,108 which may reflect loss of early effects on immature DCs. Notably, histopathologic observations in later bortezomib treatment have implicated severe colonic damage in increased GVHD-dependent mortality.106
RelB, an NF-κB family subunit, has been shown to be critical within both host and donor APCs for the induction and maintenance of experimental GVHD.109 RelB in APCs is required for differentiation of Th1 effectors, but not for expansion or function of donor Treg.109 Inhibition of nuclear RelB translocation, with RelB inhibitors targeted to DCs using Ab, thus appears to be an attractive strategy for therapy of GVHD.109 Although these studies confirm NF-κB in DCs as an important therapeutic target, they also urge caution when considering bortezomib for the treatment of established GVHD given that late (vs early) treatment in an experimental model significantly increased mortality.
Biologic interventions
Activated DCs may be targeted by mAbs against cell surface molecules, including CD83, which is up-regulated on DC maturation. There is recent evidence that anti-CD83 (polyclonal Ab) decreases T-cell proliferation induced by DCs while maintaining antiviral T cell memory.110 In an experimental model, anti-CD83 therapy prevented GVHD while preserving HSC engraftment and GVL.110 Costimulatory signal blockade also prevented experimental GVHD, with the most significant effect achieved by blocking inducible costimulator (using mAb) and CD28 (CD28−/− donor T cells) with intact CTLA-4 signaling.55 Further mechanistic and therapeutic studies of mAbs directed against activated DCs are clearly justified.
Generation of tolDCs for prevention or treatment of GVHD
DCs can be manipulated in vitro to produce tolDCs or “negative DC vaccines” for control of alloimmunity or allograft rejection (summarized in Table 4). TolDCs may be produced under specific culture conditions, by pharmacologic modification, or by cell sorting. Early studies showed that immature DCs generated from BM cells in GM-CSF, and with weak allostimulatory T-cell capacity, could prolong organ allograft survival.111,112 Subsequent reports have verified and extended these findings to show that immature or maturation-resistant tolDCs can promote tolerance in experimental organ and HSCT39,113,114 while still protecting against leukemia relapse.114
Use of tolDC therapy to inhibit GVHD in mice
| TolDC . | GVHD model; treatment . | Effect on DCs (in vitro) . | Effect on GVHD . | Reference . |
|---|---|---|---|---|
| DC conditioning | ||||
| Rapamycin | MHC-mismatched (B6 → BALB/c) | MHC class II, CD80/CD86 expression ↓ | Survival ↑ | 117 |
| Host-type DCs day 0 | Intact in vivo trafficking | Histopathology ↓ | ||
| Intact CCR5, CCR7, and CD62L | ||||
| VIP | MHC (B6 → BALB/c, BALB/c → B6) and miHA-mismatched (B6 → F1) Host-type DCs day 2 and/or day 5 | Donor CD4+ T-cell Ag-specific response ↓ Induce Treg | Survival ↑ (miHA > MHA; early > late for MHA) | 118 146 |
| SAHA (HDAC inhibitor) | MHC-mismatched (BALB/c → B6) | CD40/CD80 expression ↓ | Survival ↑ | 104 |
| Host-type DCs days −1, 0, and 2 | TNF-α ↓ | |||
| T-cell proliferation ↓ | ||||
| TGF-β, IL-10 + LPS (DCreg)* | MHC-matched, miHA-mismatched cGVHD (B10.D2 → BALB/c) | Induce anergy of Ag-specific T cells | Cutaneous GVHD ↓ | 129 |
| Host-type DCs days 2, 9, and 16 or days 18, 25, and 32 vs short-course rapamycin | TNF-α, IL-12p70, and IFN-γ ↓ | |||
| Induce Treg | ||||
| Subset (no DC conditioning) | ||||
| CD8α+ DCs | MHC (BALB/c → B6) and miHA (C3H.SW → B6) mismatched | T-cell proliferation ↓ | Survival ↑ | 124 |
| Host-type DCs days −8, −5 to −3, and −1 | IFN-γ and TNF-α ↓ | Histopathology ↓ | ||
| IL-10 ↑ | ||||
| CCR9+ pDCs | MHC-mismatched (BALB/c → B6) | CD40/CD80/CD86 expression ↓ | Survival ↑ | 128 |
| Host-type DCs day 0 (mobilized with Flt3L) | Intermediate expression of MHC class II | |||
| Induce Treg | ||||
| IL-12–producing effector T cells ↓ | ||||
| CD49+CD200R3+ DCs (naturally occurring DCreg) | MHC-matched, miHA-mismatched cGVHD (B10.D2 → BALB/c) | Anergy of Ag-specific T cells | Cutaneous GVHD ↓ | 130 |
| Host-type DCs days 2, 9, and 16 | TNF-α, IL-12p70, and IFN-γ ↓ | |||
| Induce Treg | ||||
| TolDC . | GVHD model; treatment . | Effect on DCs (in vitro) . | Effect on GVHD . | Reference . |
|---|---|---|---|---|
| DC conditioning | ||||
| Rapamycin | MHC-mismatched (B6 → BALB/c) | MHC class II, CD80/CD86 expression ↓ | Survival ↑ | 117 |
| Host-type DCs day 0 | Intact in vivo trafficking | Histopathology ↓ | ||
| Intact CCR5, CCR7, and CD62L | ||||
| VIP | MHC (B6 → BALB/c, BALB/c → B6) and miHA-mismatched (B6 → F1) Host-type DCs day 2 and/or day 5 | Donor CD4+ T-cell Ag-specific response ↓ Induce Treg | Survival ↑ (miHA > MHA; early > late for MHA) | 118 146 |
| SAHA (HDAC inhibitor) | MHC-mismatched (BALB/c → B6) | CD40/CD80 expression ↓ | Survival ↑ | 104 |
| Host-type DCs days −1, 0, and 2 | TNF-α ↓ | |||
| T-cell proliferation ↓ | ||||
| TGF-β, IL-10 + LPS (DCreg)* | MHC-matched, miHA-mismatched cGVHD (B10.D2 → BALB/c) | Induce anergy of Ag-specific T cells | Cutaneous GVHD ↓ | 129 |
| Host-type DCs days 2, 9, and 16 or days 18, 25, and 32 vs short-course rapamycin | TNF-α, IL-12p70, and IFN-γ ↓ | |||
| Induce Treg | ||||
| Subset (no DC conditioning) | ||||
| CD8α+ DCs | MHC (BALB/c → B6) and miHA (C3H.SW → B6) mismatched | T-cell proliferation ↓ | Survival ↑ | 124 |
| Host-type DCs days −8, −5 to −3, and −1 | IFN-γ and TNF-α ↓ | Histopathology ↓ | ||
| IL-10 ↑ | ||||
| CCR9+ pDCs | MHC-mismatched (BALB/c → B6) | CD40/CD80/CD86 expression ↓ | Survival ↑ | 128 |
| Host-type DCs day 0 (mobilized with Flt3L) | Intermediate expression of MHC class II | |||
| Induce Treg | ||||
| IL-12–producing effector T cells ↓ | ||||
| CD49+CD200R3+ DCs (naturally occurring DCreg) | MHC-matched, miHA-mismatched cGVHD (B10.D2 → BALB/c) | Anergy of Ag-specific T cells | Cutaneous GVHD ↓ | 130 |
| Host-type DCs days 2, 9, and 16 | TNF-α, IL-12p70, and IFN-γ ↓ | |||
| Induce Treg | ||||
VIP indicates vasoactive intestinal peptide; and LPS, lipopolysaccharide.
Depleted of CD40+CD80+CD86+ cells.
Pharmacologic manipulation of DCs (eg, using dexamethasone, rapamycin, or IL-10) renders DCs maturation-resistant and enhances their tolerogenic potential for inhibition of allograft rejection and GVHD. As an example, rapamycin-treated DCs (RAPA-DCs) resist maturation and have impaired capacity to stimulate allogeneic effector T cells yet promote Treg.93 When adoptively transferred to organ graft recipients, RAPA-DCs promote transplant survival and, in conjunction with a short course of host immunosuppression, can induce indefinite graft survival.93,115,116 When administered systemically in experimental GVHD, host-derived RAPA-DCs traffic to secondary lymphoid tissue and improve both survival and histopathologic grade of GVHD.117
Similarly, vasoactive intestinal peptide is an immunosuppressive neuropeptide that has been used to generate host-derived tolDCs that increase Treg and abrogate aGVHD while maintaining GVL.118 Interestingly, early administration (by day 5) of these tolDCs is critical in the MHC-mismatched model. They were more effective in the miHA-mismatched model regardless of timing.118
IDO is an important enzyme in tryptophan catabolism that is thought to be critical for control of Teff responses.119 After experimental GVHD, IDO expression in host APCs is increased via IFN-γ release by donor T cells. IDO−/− recipients have accelerated colonic GVHD and mortality, with enhanced T-cell proliferation and decreased apoptosis.120,121 Specific culture conditions (eg, low tryptophan or lipopolysaccharide and IFN-γ) can be used to generate tolDCs with increased IDO expression.122,123 Although these tolDCs have not been studied directly in experimental GVHD, DCs treated with the HDAC inhibitor SAHA display enhanced IDO expression and suppress experimental GVHD in an IDO-dependent manner.104 In addition, increasing colon IDO expression via the injection of kynurenine (tryptophan breakdown product) or a TLR7/TLR8 agonist (3M-011) abrogates experimental GVHD mortality.121
Cell sorting can be used to isolate/purify tolDCs. Murine CD8α+ DCs are the principal DC subset involved in cross-presentation (Table 1) and have tolerogenic properties.124 In both MHC- and miHA-mismatched models of aGVHD, immunization of recipients with ex vivo–generated and FACS-sorted autologous CD8α+ DC pretransplant reduces GVHD in an IL-10–dependent, Ag-specific manner.124 These results confirm the therapeutic ability of CD8α+ DCs to modify aGVHD, as shown in earlier studies in which Flt3L administration expanded CD8α+ DCs in vivo and reduced aGVHD.125 Ildstadt and colleagues have also described how CD8α+/TCR− “facilitating cells,” with a critical component of plasmacytoid precursor-like CD11c+/B220+/CD11b− cells, enhance HSC engraftment in mice without increased GVHD.126,127 This effect was attributed to the induction of Ag-specific chimeric Treg that suppress effector T cells. Murine CCR9+ pDCs, obtained via Flt3L-induced mobilization and cell sorting, display an immature phenotype and prevent experimental aGVHD via induction of Treg and suppression of IL-17–producing effector T cells while maintaining IFN-γ–producing effector T cells.128 Overall, distinct subsets of ex vivo–fashioned tolDCs or endogenous DCs have potential for therapy of GVHD, and an important question is which subset is best suited for therapeutic application.
So-called “regulatory DCs” (DCreg), generated by culturing BM in GM-CSF, IL-10, and TGF-β, are proposed to have greater therapeutic efficacy than conventional tolDCs.129 There is evidence that DCreg exclusively express CD200R3 and that naturally occurring mouse CD49+CD200R3+ DCs are identical phenotypically and functionally.130 Both BM-derived and naturally occurring recipient-type DCreg protect against cutaneous cGVHD in a multiple miHA- or MHA- mismatched model via the generation of donor inducible Treg and anergic, Ag-specific CD4+ T cells.130 Moreover, depletion of CD49+CD200R3+ cells before alloHSCT enhanced the progression of cGVHD.130
Cell therapies that target DCs in vivo
MSCs
Mesenchymal stem cells (MSCs) are rare, heterogeneous, pluripotent nonhematopoietic progenitors present in normal BM and adipose tissue that induce immune tolerance via effects on multiple immune cells, in particular DCs. Human MSCs impair DC maturation and induce T-cell hyporesponsiveness in a dose- and contact-dependent manner. The effect can be partly reversed by DC maturation and by blocking IL-10 or IL-6.131 TolDCs generated by coculture of DCs with human MSCs (MSC-DCs) induce Ag-specific Treg via activation of the Notch pathway, but they have not been studied in vivo.132,133
MSCs have shown promise in the prevention and treatment of GVHD. As reviewed by Baron and Storb,134 while various mouse models have generated conflicting results, they suggest the importance of MSC dose, timing, and activation status. Phase 1 and 2 human studies have demonstrated safety and possible efficacy, and multicenter randomized blinded trials are currently underway.134 Interestingly, the combination of rapamycin and MSCs after experimental cardiac transplantation led to long-term graft survival with significantly increased splenic Treg and tolDCs.135 This also highlights the capacity of synergistic therapies in the promotion of tolerance.135
MDSCs
Myeloid-derived suppressor cells (MDSCs) are heterogeneous hematopoietic precursor cells with immunosuppressive properties, first noted to aid tumor evasion in mice and humans.136 As reviewed by Lees et al,137 MDSCs modulate both innate and adaptive immunity. Although many of their functions are attributed to direct effects on T cells, MDSCs additionally inhibit the differentiation and maturation of DCs. In mice, MDSCs generated from BM cells in G-CSF, GM-CSF, and IL-13 (MDSC–IL-13) were more potent inhibitors of MHC-mismatched GVHD than conventional MDSCs.136 This inhibition was dependent on the L-arginine-depleting enzyme arginase.136 Importantly, MDSC–IL-13 do not impair the GVL effect in vivo.136
Treg
As a bidirectional tolerogenic feedback loop exists between Treg and tolDCs, Treg therapy supports tolerance through effects on DCs.41 DCs also control the number and function of Treg.44 Host APC alloAg expression is necessary and sufficient for Treg function in both miHA- and MHC-mismatched mouse models of GVHD, independent of APC IL-10 or IDO expression.138 In addition, human Treg (generated via CD127 [IL-7Rα] negative selection) and Treg-conditioned DCs can abrogate xenogeneic GVHD via induction of immunosuppressive PD-L1 expression on conditioned DCs and on effector T cells in vivo.56 Furthermore, Ag-specific Treg can be induced and expanded by DCs, as demonstrated by human monocyte-derived DCs in an IDO-dependent manner.139
Adoptive transfer of Treg is highly effective in the prevention of experimental GVHD; thus, phase 1 trials are underway with initial studies demonstrating safety and some efficacy.140,141 A major impediment to Treg therapy has been the generation of sufficient cell numbers, particularly for natural Treg.141 Interestingly, the addition of rapamycin (for restimulation of natural Treg or for the generation of iTreg with TGF-β) increases Treg yields, which may allow completion of dose escalation trials.141
Active clinical trials using interventions that target/impact DCs
There are numerous open clinical trials for the prevention or treatment of GVHD currently studying pharmacologic or biologic interventions and cellular therapies that target or impact DCs (Table 5). Although not listed in Table 5, there are also many ongoing trials assessing the impact of conventional GVHD therapies (eg, corticosteroids, CNI, rapamycin) used in new combinations and via different routes (eg, topical, intrahepatic). Cellular therapy remains particularly intriguing, with the majority of active studies using MSCs. A single trial has been underway testing autologous DCs in the setting of relapsed hematologic malignancy; although the DCs are not being used for the prevention or treatment of GVHD, GVHD is a primary outcome measure of the study and the trial will hopefully demonstrate safety and feasibility of DC therapy in the SCT setting.
Active clinical trials using interventions that target/impact DCs
| ID . | Condition . | Intervention . | Phase . | Study type . | Sponsor . |
|---|---|---|---|---|---|
| HDAC inhibition | |||||
| NCT00810602 | aGVHD | Vorinostat plus tacrolimus and mycophenolate after RIC related donor allogeneic transplant; prevention | II | Single-agent, open-label, non-randomized safety/efficacy | University of Michigan Cancer Center |
| NCT01111526 | aGVHD | Panobinostat (LBH589) plus corticosteroids; initial treatment | I/II | Non-randomized, open-label, safety/efficacy | H. Lee Moffitt Cancer Center and Research Institute |
| Proteasome inhibition | |||||
| NCT01158105 | Steroid-refractory cGVHD | Bortezomib; treatment | II | Single-agent, open-label, safety/efficacy | Baylor Research Institute |
| NCT00670423 | GVHD | Bortezomib plus tacrolimus and sirolimus after allogeneic PBSC transplant; prevention | I | Single-agent, open-label, safety | Indiana University School of Medicine |
| NCT01323920 | aGVHD | Bortezomib plus tacrolimus and methotrexate after myeloablative allogeneic SCT without HLA-matched related donor; prevention | II | Single-agent, open-label, safety/efficacy | Dana-Farber Cancer Institute |
| NCT01163786 | Bronchiolitis obliterans (cGVHD) | Bortezomib; treatment | II | Single-agent, open-label, safety/efficacy | Northwestern University |
| Antibody therapy | |||||
| NCT01012492 | aGVHD | Abatacept (CTLA4-Ig) plus cyclosporine and methotrexate after unrelated donor HSCT; prevention | II | Single group, open-label, safety | Emory University |
| Cellular therapy | |||||
| NCT00603330 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | II | Single-agent, open-label, efficacy | University Hospital of Liege |
| NCT00827398 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | I/II | Single-agent, open-label, safety/efficacy | UMC Utrecht |
| NCT00759018 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | NA | Expanded access (pediatrics) | Osiris Therapeutics |
| NCT00826046 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | NA | Expanded access (adults) | Osiris Therapeutics |
| NCT01522716 | Steroid-refractory cGVHD | MSCs; treatment | I | Single-agent, open-label, safety/efficacy | Karolinska Institute |
| NCT01045382 | GVHD | MSCs versus placebo in HLA-mismatched allogeneic transplant after non-myeloablative conditioning; prevention | II | Randomized, double-blind, safety/efficacy | University Hospital of Liege |
| NCT01222039 | cGVHD | Conventional therapy versus conventional therapy plus MSCs derived from adipose tissue; treatment | I/II | Multicenter, randomized, safety/efficacy | Fundacion Progreso y Salud, Spain |
| NCT00957931 | GVHD | Haploidentical MSCs in MUD HCT in patients with high-risk non-malignant RBC disorders after RIC; prevention | II | Non-randomized, open-label, efficacy | Stanford University |
| NCT01050764 | GVHD | Allogeneic Treg plus allogeneic conventional T cells after allogeneic MAC HCT with haploidentical related donor for patients with hematologic malignancies; prevention | II | Non-randomized, open-label, safety/efficacy | Stanford University |
| NCT00935597 | GVHD | Host DC infusion after allogeneic SCT for prevention or treatment of relapsed disease in patients with advanced hematologic malignancies | I | Non-randomized, open-label, safety/efficacy | Mt Sinai School of Medicine |
| ID . | Condition . | Intervention . | Phase . | Study type . | Sponsor . |
|---|---|---|---|---|---|
| HDAC inhibition | |||||
| NCT00810602 | aGVHD | Vorinostat plus tacrolimus and mycophenolate after RIC related donor allogeneic transplant; prevention | II | Single-agent, open-label, non-randomized safety/efficacy | University of Michigan Cancer Center |
| NCT01111526 | aGVHD | Panobinostat (LBH589) plus corticosteroids; initial treatment | I/II | Non-randomized, open-label, safety/efficacy | H. Lee Moffitt Cancer Center and Research Institute |
| Proteasome inhibition | |||||
| NCT01158105 | Steroid-refractory cGVHD | Bortezomib; treatment | II | Single-agent, open-label, safety/efficacy | Baylor Research Institute |
| NCT00670423 | GVHD | Bortezomib plus tacrolimus and sirolimus after allogeneic PBSC transplant; prevention | I | Single-agent, open-label, safety | Indiana University School of Medicine |
| NCT01323920 | aGVHD | Bortezomib plus tacrolimus and methotrexate after myeloablative allogeneic SCT without HLA-matched related donor; prevention | II | Single-agent, open-label, safety/efficacy | Dana-Farber Cancer Institute |
| NCT01163786 | Bronchiolitis obliterans (cGVHD) | Bortezomib; treatment | II | Single-agent, open-label, safety/efficacy | Northwestern University |
| Antibody therapy | |||||
| NCT01012492 | aGVHD | Abatacept (CTLA4-Ig) plus cyclosporine and methotrexate after unrelated donor HSCT; prevention | II | Single group, open-label, safety | Emory University |
| Cellular therapy | |||||
| NCT00603330 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | II | Single-agent, open-label, efficacy | University Hospital of Liege |
| NCT00827398 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | I/II | Single-agent, open-label, safety/efficacy | UMC Utrecht |
| NCT00759018 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | NA | Expanded access (pediatrics) | Osiris Therapeutics |
| NCT00826046 | Grade II to IV steroid-refractory aGVHD | MSCs; treatment | NA | Expanded access (adults) | Osiris Therapeutics |
| NCT01522716 | Steroid-refractory cGVHD | MSCs; treatment | I | Single-agent, open-label, safety/efficacy | Karolinska Institute |
| NCT01045382 | GVHD | MSCs versus placebo in HLA-mismatched allogeneic transplant after non-myeloablative conditioning; prevention | II | Randomized, double-blind, safety/efficacy | University Hospital of Liege |
| NCT01222039 | cGVHD | Conventional therapy versus conventional therapy plus MSCs derived from adipose tissue; treatment | I/II | Multicenter, randomized, safety/efficacy | Fundacion Progreso y Salud, Spain |
| NCT00957931 | GVHD | Haploidentical MSCs in MUD HCT in patients with high-risk non-malignant RBC disorders after RIC; prevention | II | Non-randomized, open-label, efficacy | Stanford University |
| NCT01050764 | GVHD | Allogeneic Treg plus allogeneic conventional T cells after allogeneic MAC HCT with haploidentical related donor for patients with hematologic malignancies; prevention | II | Non-randomized, open-label, safety/efficacy | Stanford University |
| NCT00935597 | GVHD | Host DC infusion after allogeneic SCT for prevention or treatment of relapsed disease in patients with advanced hematologic malignancies | I | Non-randomized, open-label, safety/efficacy | Mt Sinai School of Medicine |
PBSC indicates peripheral blood stem cell; MUD, matched-unrelated donor; RBC, red blood cells; and NA, not applicable.
Conclusions
Despite therapies that broadly target effector T cells or globally suppress immunity, GVHD remains a significant cause of post-HSCT morbidity and mortality. Given the tolerogenic potential of some DC subsets and the critical role of others in the pathogenesis of GVHD, differences in DC characteristics may be used to predict outcome, whereas targeting DCs is an innovative treatment approach. Likewise, DCs may be targeted directly in vivo through molecular pathways or cell surface expression of maturation markers or costimulatory molecules, or tolDCs may be generated in vitro and given in the peritransplant period (summarized in Figure 3). Other cellular therapies, including Treg, mediate dominant immunosuppressive effects by restraining DC stimulatory functions. Given the importance of the GVL effect, any therapy targeting or using DCs must conserve this process.
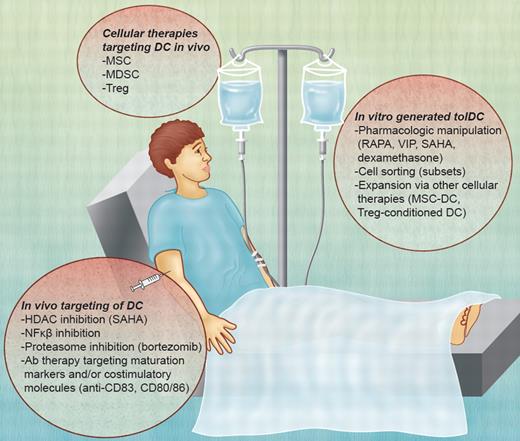
Potential DC-based therapies for GVHD. TolDCs can be used as a negative cellular vaccine after in vitro generation via their pharmacologic manipulation, cell sorting (subsets), or expansion after interaction with other immune regulatory cell populations. In vivo, DCs can be targeted by the inhibition of molecular pathways (HDAC; NF-κB) or the expression of maturation markers or costimulatory molecules (eg, CD80/CD86; CD83). Other cellular therapies, such as MSCs, MDSCs, and Treg, mediate immunosuppressive effects through DCs. VIP indicates vasoactive intestinal peptide. Professional illustration by Alice Y. Chen.
Potential DC-based therapies for GVHD. TolDCs can be used as a negative cellular vaccine after in vitro generation via their pharmacologic manipulation, cell sorting (subsets), or expansion after interaction with other immune regulatory cell populations. In vivo, DCs can be targeted by the inhibition of molecular pathways (HDAC; NF-κB) or the expression of maturation markers or costimulatory molecules (eg, CD80/CD86; CD83). Other cellular therapies, such as MSCs, MDSCs, and Treg, mediate immunosuppressive effects through DCs. VIP indicates vasoactive intestinal peptide. Professional illustration by Alice Y. Chen.
Further understanding of the precise immunoregulatory properties of DCs and the development of DC-based therapies for GVHD will expand HSCT use beyond treatment of malignant disease and allow its use in patients lacking MHC-matched donors. Early work by Shlomchik et al elucidated the critical role of miHA expression by host hematopoietic APCs for CD8+ T cell–driven GVHD2,46 ; thus, therapies that orchestrate the successful and timely suppression and/or ablation of host DCs are expected to be particularly beneficial to patients after MHC-matched HSCT. Very recent findings suggest that recipient nonhematopoietic APCs in target organs may be central to promoting indirect CD4+ T cell-mediated aGVHD.142 In these studies, host CD11c+ DCs suppressed GVHD development. These and other data discussed herein underscore the importance of developing a clear understanding of DC involvement in the complex immunopathology of GVHD. Likewise, infused recipient Ag-pulsed donor tolDCs or recipient tolDCs presenting alloAg and miHA have the potential to prevent the pathologic alloresponses of donor T cells and benefit HSCT patients given either MHC-matched or mismatched transplants.
Finally, given the role of both DCs and T cells in the pathogenesis of GVHD, synergistic therapies or those that target both cell types in vivo may be more effective. Cellular therapies, specifically tolDCs and Treg, are intriguing in their ability to modulate one another in vivo. Importantly, cellular therapies have begun in humans. Human tolDCs have been generated and characterized in vitro using clinical-grade reagents.143 Recently, a DC-based vaccine for the treatment of prostate cancer was approved by the FDA,144 and the first report has appeared of a phase 1 safety study of tolDCs in patients with type 1 (autoimmune) diabetes.145 As other forms of innovative cell therapy, including testing of Treg, are underway for the prevention of GVHD,140,141 there would appear to be adequate justification for phase 1 studies of tolDCs alone and in combination with Treg in HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Brian Rosborough for critical reading of the manuscript.
This work was supported by the National Institutes of Health (grants R01AI67541 and R01AI60994, A.W.T.; grants R01HL93716 and RO1GM63569, M.Y.M.; and grant K99/R00 HL97155, H.R.T.) and the Pittsburgh Foundation (M.Y.M.).
National Institutes of Health
Authorship
Contribution: E.O.S. and A.W.T. wrote the manuscript; H.R.T. assisted in writing the manuscript and generation of the figures; and M.Y.M. assisted in editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angus W. Thomson, Starzl Transplantation Institute, University of Pittsburgh School of Medicine, 200 Lothrop St, BST W1540, Pittsburgh, PA 15261; e-mail: thomsonaw@upmc.edu.