Cancer is a leading cause of death and disability in sub-Saharan Africa and will eclipse infectious diseases within the next several decades if current trends continue. Hematologic malignancies, including non-Hodgkin lymphoma, leukemia, Hodgkin lymphoma, and multiple myeloma, account for nearly 10% of the overall cancer burden in the region, and the incidence of non-Hodgkin lymphoma and Hodgkin lymphoma is rapidly increasing as a result of HIV. Despite an increasing burden, mechanisms for diagnosing, treating, and palliating malignant hematologic disorders are inadequate. In this review, we describe the scope of the problem, including the impact of endemic infections, such as HIV, Epstein-Barr virus, malaria, and Kaposi sarcoma–associated herpesvirus. We additionally describe current limitations in hematopathology, chemotherapy, radiotherapy, hematopoietic stem cell transplantation, and supportive care and palliation. We review contemporary treatment and outcomes of hematologic malignancies in the region and outline a clinical service and research agenda, which builds on recent global health successes combating HIV and other infectious diseases. Achieving similar progress against hematologic cancers in sub-Saharan Africa will require the sustained collaboration and advocacy of the entire global cancer community.
Introduction
Mutu umodzi susenza denga — “A single head cannot support a roof alone” (Malawian Chichewa proverb)
Cancer is a major cause of death and disability in developing countries, where health systems are poorly equipped to deal with this challenge. Sub-Saharan Africa, in particular, is experiencing a marked increase in burden, with more than 1 million incident cancers and nearly 800 000 cancer-related deaths projected in the year 2030, representing an approximately 85% increase from 2008.1 By comparison, HIV is responsible for 1.2 million deaths, tuberculosis for 250 000 deaths, and malaria 709 000 deaths annually across sub-Saharan Africa, with mortality declining for each of these infectious diseases in recent years.2,–4
As a result, cancer figured prominently in the recent United Nations General Assembly meeting on noncommunicable diseases. The September 2011 meeting marked the second time in history that a General Assembly meeting was devoted to a health-related issue, the first being the 2001 meeting that catalyzed the global response to HIV. Of participating civil society organizations, 23 were dedicated to cancer, including the American Cancer Society, American Society of Clinical Oncology (ASCO), European Society for Medical Oncology, LIVESTRONG, and Union for International Cancer Control.
Of all cancers occurring in sub-Saharan Africa, hematologic malignancies have emerged as a major cause of morbidity and mortality. Non-Hodgkin lymphoma (NHL), leukemia, Hodgkin lymphoma (HL), and multiple myeloma together accounted for 8.7% of incident cancer diagnoses and 9.9% of cancer deaths in 2008, with NHL being the sixth most common cancer in the region.1 Treatment of hematologic malignancies in resource-rich settings is increasingly associated with unprecedented rates of long-term cure and control. Conversely, and despite their increasing impact, mechanisms for diagnosing, treating, and palliating these conditions in sub-Saharan Africa are inadequate. Despite numerous obstacles, hematologic malignancies can be effectively treated and cured in resource-limited settings. In this article, we describe the problem and survey resources and obstacles toward meeting this urgent global health challenge, emphasizing biomedical and public health considerations. Economic and political issues, although critically important, are beyond the scope of our review. Finally, we outline a clinical service and research agenda to address malignant hematologic disorders in the region, a problem that demands the sustained collaboration and advocacy of the global cancer community.
Epidemiology
GLOBOCAN 2008 estimates and 2030 projections summarizing the burden of hematologic malignancies are shown in Table 1.1 Despite serving as the reference standard for cancer burden worldwide, GLOBOCAN estimates suffer from several limitations with respect to sub-Saharan Africa. First, projections are derived from population growth and aging assuming stable cancer-specific incidence rates. Second, cancer registration is sparse because of difficulties defining and enumerating populations residing in a particular area, as well as challenges obtaining accurate cancer diagnoses. Cancer registries covered only 11% of Africa in 2006,5 and data from small regions of only 2 countries in sub-Saharan Africa (Uganda and Zimbabwe) are included in the most recent Cancer Incidence in 5 Continents World Health Organization (WHO) monograph.6 Third, mortality statistics are similarly limited with only 3 countries (Mauritius, Seychelles, and South Africa) contributing to the WHO mortality registry.7
Burden of hematologic malignancies in sub-Saharan Africa: GLOBOCAN 2008 estimates and 2030 projections
| . | Incidence (2008) . | Mortality (2008) . | Incidence (2030) . | Mortality (2030) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | Rate* . | No. . | Rate* . | No. . | % increase . | No. . | % increase . | |
| NHL | 26 224 | 4.6 | 21 822 | 3.8 | 45 300 | 73 | 37 396 | 71 |
| Leukemia | 13 091 | 2.4 | 12 404 | 2.2 | 23 120 | 77 | 21 804 | 76 |
| HL | 5292 | 0.9 | 4425 | 0.8 | 9196 | 74 | 7825 | 77 |
| Multiple myeloma | 3291 | 0.8 | 3010 | 0.8 | 6277 | 91 | 5755 | 91 |
| Total | 47 898 | 8.7 | 41 661 | 7.6 | 83 893 | 75 | 72 778 | 75 |
| . | Incidence (2008) . | Mortality (2008) . | Incidence (2030) . | Mortality (2030) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | Rate* . | No. . | Rate* . | No. . | % increase . | No. . | % increase . | |
| NHL | 26 224 | 4.6 | 21 822 | 3.8 | 45 300 | 73 | 37 396 | 71 |
| Leukemia | 13 091 | 2.4 | 12 404 | 2.2 | 23 120 | 77 | 21 804 | 76 |
| HL | 5292 | 0.9 | 4425 | 0.8 | 9196 | 74 | 7825 | 77 |
| Multiple myeloma | 3291 | 0.8 | 3010 | 0.8 | 6277 | 91 | 5755 | 91 |
| Total | 47 898 | 8.7 | 41 661 | 7.6 | 83 893 | 75 | 72 778 | 75 |
Data are from Ferlay et al.1
Age-standardized rate per 100 000 person-years.
In Uganda, despite periods of interruption, the Kampala Cancer Registry provides important data regarding temporal trends in cancer incidence. It recently reported a 2- to 3-fold increase in NHL incidence rates from 1991 to 2006, ranking it among the most rapidly increasing incident cancers.8 Similarly, reports from centers in Kenya, South Africa, and Tanzania all suggest increasing NHL burden since the onset of the HIV epidemic,9,–11 with some suggestion that HL is also increasing.12 Incidence trends for leukemia and multiple myeloma are less clear. However, increasing NHL and HL incidence suggest that GLOBOCAN 2030 projections are significantly underestimated and that lymphoma will increase as a proportional contributor to overall cancer burden in the coming decades.
Impact of endemic infections
Of cancers occurring in Africa, 36% result from infection, which is double the world average.13 In this context, the impact of several endemic infections on hematologic malignancies deserves examination.
HIV
In resource-rich countries, HIV confers a higher risk of NHL, HL, and leukemia.14,–16 A similarly increased risk of NHL and HL has been reported in sub-Saharan Africa.17,18 In the antiretroviral therapy (ART) era in developed countries, NHL incidence declined but has since stabilized, whereas HL incidence has been stable and possibly even increased.14,15,19 Cancer has increased as a proportional contributor to HIV mortality and now accounts for 25% to 35% of deaths, with NHL being the most frequent cancer-related cause.20,–22 Risk factors for NHL include lower CD4 count and cumulative HIV viremia.23,–25 Interestingly, risk for HL may be highest during the first months after ART initiation and with moderate rather than severe CD4 lymphopenia.26,27
Despite having 12% of the world's population, sub-Saharan Africa is home to 22.9 million (68%) of the 34.0 million people worldwide living with HIV.2 Although there have been significant achievements scaling up access to ART, there remain many obstacles. HIV-infected persons in sub-Saharan Africa initiate ART at lower CD4 counts than developed countries. Delayed ART initiation leads to greater lifetime CD4 lymphopenia and HIV viremia, placing patients at higher risk for hematologic malignancies.24,25 There remain marked country-to-country variations in ART coverage, with coverage estimates for eligible patients ranging from 3% to 5% in Somalia and Sudan, to 88% to 93% in Rwanda and Botswana in 2010.2 ART coverage in the region as a whole is estimated to be 49%, compared with 75% in the United States.2,28 Revised 2010 WHO guidelines recommend ART at CD4 counts less than 350 cells/mm3 in resource-limited settings, replacing the prior threshold of less than 200 cells/mm3.29 However, even if updated WHO guidelines are universally implemented, there will remain a significant “treatment gap” between developing and developed countries, where many guidelines now recommend ART at CD4 counts more than 500 cells/mm3.30,31 The risk of malignant hematologic disorders may also be enhanced by higher frequency of endemic oncogenic coinfections in sub-Saharan Africa. As the global epidemic evolves, hematologic cancers may therefore become major contributors to HIV morbidity and mortality in the region, to an equal or even greater degree than resource-rich settings. Consistent increases in NHL, and to a lesser extent HL, have been noted across the region since the onset of the HIV epidemic, and HIV prevalence among NHL and HL patients in many settings ranges from 30% to 70%.10,12,32,–34
In resource-rich countries, clinical trials and observational cohorts have demonstrated comparable outcomes for HIV-associated NHL and HL to patients without HIV,35,–37 whereas others have found HIV to be independently associated with mortality.38 There are limited data examining the impact of HIV on NHL survival in sub-Saharan Africa, although studies suggest that concurrent ART may render survival similar to patients without HIV.33,39,40
EBV
The discovery of EBV as the first virus to be pathogenically implicated in human cancer originated in sub-Saharan Africa nearly 50 years ago, when specimens collected by Denis Burkitt from Ugandan children with a newly described jaw tumor were cultured and examined via electron microscopy by colleagues in London. EBV has since been recognized for its ability to immortalize B lymphocytes, as well as its association with Burkitt lymphoma (BL), HL, nasal NK-T cell lymphoma, and B-cell NHL in patients with immunosuppression, including HIV.41 The kinetics of EBV acquisition differ in sub-Saharan Africa from developed countries. Infection occurs via contact with oral secretions, and seroprevalence is nearly universal throughout sub-Saharan Africa with acquisition in early childhood, whereas seroprevalence is approximately 75% in developed countries with acquisition later in life coincident with sexual activity.42,43 Although the majority of the world's population is infected with EBV, there are marked global variations in the incidence of EBV-associated cancers, perhaps resulting from host genetics, environmental factors, or viral genetic variation, with EBV having a striking and geographically unique association with BL and HL in sub-Saharan Africa.44 EBV-encoded RNA has been demonstrated in more than 50% of unselected lymphomas from sub-Saharan Africa, with BL, HL, and plasmablastic lymphoma having the highest rates of association.32,34 In addition, HIV increases EBV-infected B-lymphocytes while decreasing cytotoxic T-cell activity against infected B cells,42 creating potential oncogenic synergy among coinfected persons.
Malaria
Sub-Saharan Africa's share of global malaria (∼ 90%) is even more disproportionate than its share of HIV.4 Endemic childhood BL has been ecologically linked to malaria since its earliest description.45 In addition, malaria suppression programs in Tanzania have resulted in decreased BL incidence.46 Elevated levels of nonprotective whole schizont antibodies, reflecting malaria-related immune activation, have been demonstrated among children with BL in Malawi and Uganda,47,48 whereas protective antibodies against Plasmodium falciparum serine repeat antigen have been associated with lower BL risk in Ghana.49 A clear etiologic link has not been established, yet P falciparum induces polyclonal expansion of B cells, impairs EBV-specific T-cell immune responses,50 and may preferentially stimulate EBV replication and expansion of EBV-positive B-cell expression by its cysteine-rich interdomain region 1α,51 all of which suggest a possible etiologic role. Alternatively, malaria transmission is influenced by pesticides, bed-nets, sociocultural practices, and other ecologic factors, which may modulate BL risk by affecting immune responses to EBV or malaria, or by influencing additional cofactors, such as intestinal parasites.52
KSHV
Since identification as the etiologic agent of Kaposi sarcoma (KS), Kaposi sarcoma–associated herpesvirus (KSHV) has been associated with 2 lymphoproliferative diseases occurring primarily in HIV-infected persons, primary effusion lymphoma and multicentric Castleman disease. KSHV seroprevalence is uncommon (< 5%) in developed countries, although higher among men who have sex with men (up to 25% if HIV-negative and 75% if HIV-positive) with infection typically acquired via sexual intercourse.53,–55 Conversely, KSHV infection is endemic in sub-Saharan Africa, occurring frequently in childhood and reaching a prevalence of 30% to 60% by 15 years of age.53,56 In part because of geographic overlap of HIV and KSHV, the region has an enormous KS burden, which ranks as the fourth most common cancer in the general population.1 Unlike developed countries, KS incidence has not declined significantly despite ART.8 For unclear reasons, however, there does not seem to be a corresponding burden of KSHV-associated hematologic malignancies, although primary effusion lymphoma and multicentric Castleman disease are probably underdiagnosed given current hematopathology limitations. Only 7 (0.3%) primary effusion lymphoma cases were identified in a retrospective series of 2225 lymphoproliferative disorders in South Africa from 2007 until 2009.10 Similarly, among 144 cases of NHL and reactive lymphadenopathy in Uganda assessed for KSHV, only 3 (1 NHL, 2 multicentric Castleman disease) were positive.57
Available resources
Despite increasing burden, resources to diagnose, treat, and palliate hematologic malignancies in sub-Saharan Africa are scarce. Moreover, services are concentrated within major population centers in more economically advanced countries, despite the fact that 63% of the population lived in rural areas in 2010.58 For illustration, the distribution of radiotherapy and hematopoietic stem cell transplantation (HSCT) facilities is shown in Figure 1.59,60 Although distribution of all pathology and cancer treatment facilities is less well registered, it probably follows a similar pattern.
Distribution of radiotherapy and hematopoietic stem cell transplant services in sub-Saharan Africa.
Distribution of radiotherapy and hematopoietic stem cell transplant services in sub-Saharan Africa.
Hematopathology
Diagnostic hematopathology remains limited, and pathologist scarcity is symptomatic of critical health worker shortages in the region. Pathologist availability in Malawi, Sudan, Tanzania, and Uganda ranges from 0.1 to 1.3 pathologists per million population, with concentration in major cities.61,62 By comparison, there were 62.3 pathologists per million population in the United States in 2009.63 The International Network for Cancer Treatment and Research (INCTR) convened a panel, which visited national referral hospitals in Kenya, Nigeria, Tanzania, and Uganda, to evaluate infrastructure for diagnosing lymphoma.34 Key findings included highly variable equipment and personnel, overuse of suboptimal fine needle aspiration specimens, variable turnaround times, variable histology and cytology preparations with lower standards than developed countries, a tendency for BL overdiagnosis, and absence of immunohistochemistry. Cytogenetic, molecular, and FISH techniques, which are core elements of hematopathology in the developed world, are nonexistent. Pathologists were well trained and adequate in number for institutional workloads, but too few at the national level with inadequate numbers of trainees, and suffered from limited reference materials and training opportunities. Of 467 histologic samples interpreted as lymphoma by participating centers, 393 (84%) were assessable by the panel, with 364 (93%) assessed specimens confirmed as lymphoma on independent review, suggesting a high level of diagnostic accuracy when specimen quality was sufficient. The INCTR has been working to improve diagnostic pathology infrastructure, including improvements in specimen collection and data management, as well as translational research partnerships between developed countries and centers in sub-Saharan Africa. Similarly, the National Cancer Institute (NCI) supports the Sub-Saharan Africa Lymphoma Consortium, an effort to characterize lymphomas from 10 regional referral centers, in consultation with United States hematopathologists using a tissue microarray platform and digital microscopy.
Chemotherapeutic agents
Twenty-two chemotherapeutic agents are included in March 2011 WHO Model Lists of Essential Medicines for adults and children, 20 of which are active in hematologic malignancies and shown in Table 2.64 All agents appear in the complementary rather than core list, as a result of need for specialized facilities, higher cost, and lower cost-effectiveness compared with core list medicines. The median year of US Food and Drug Administration approval for included medicines is 1969, and it has been proposed that cisplatin, imatinib, melphalan, and rituximab be added. Imatinib is available in some settings under a donation program and has been used with good results in chronic myelogenous leukemia in Kenya.65 Developed countries have recently suffered from critical shortages of many generic chemotherapeutic agents designated as WHO essential medicines. Although the impact of global shortages on cancer programs in sub-Saharan Africa is difficult to quantify, governments may be less able to secure adequate quantities of cancer medicines when competing with developed countries under conditions of limited international supply. Pharmacy stock-outs have long hampered HIV and tuberculosis treatment programs in sub-Saharan Africa. Drug shortages have similarly interfered with lymphoma treatment even in the context of an internationally funded clinical trial.33,40 A global commitment to providing essential drugs to developing countries is clearly articulated in Millennium Development Goal 8.E. Nevertheless, availability of essential medicines in sub-Saharan Africa was only 44.3% in public and 55.3% in private health facilities during the period 2001 to 2009, with prices globally on average 2.7 times higher in the public sector and 6.1 times higher in the private sector than international reference prices.66 Counterfeit and substandard medicines also pose a threat, and the extent to which cancer medicines are affected is unknown. Furthermore, uncertainty regarding optimal dosing schedules for the setting as well as lack of trained health workers and equipment (eg, infusion pumps), are additional obstacles to successful chemotherapy administration. However, with adequate support and training, local physicians and nurses working even in rural areas without oncologists can deliver chemotherapy safely and effectively.67
Chemotherapeutic agents active in hematologic malignancies included in the 2011 World Health Organization Model Lists of Essential Medicines
| Medicine . | Year of FDA licensure . | FDA hematologic malignancy indications . | Other clinical uses . | Dose . | AWP (US dollars)* . | AWP per dose (US dollars)† . |
|---|---|---|---|---|---|---|
| Asparaginase | 1978 | ALL | AML | 6000 units/m2 IV | 0.007/unit | 84 |
| Bleomycin | 1973 | HL, NHL | 10 units/m2 IV | 3.406/unit | 68 | |
| Carboplatin | 1989 | HL, NHL | AUC 5 IV | 0.407/mg | 325 | |
| Chlorambucil | 1957 | CLL, HL, NHL | 0.4 mg/kg PO | 1.141/mg | 37 | |
| Cyclophosphamide | 1959 | ALL, AML, CLL, CML, HL, NHL, MM | 750 mg/m2 IV | 0.050/mg | 75 | |
| Cytarabine | 1969 | ALL, AML, CML | HL, NHL | 2000 mg/m2 IV | 0.029/mg | 114 |
| Dacarbazine | 1975 | HL | 375 mg/m2 IV | 0.106/mg | 80 | |
| Daunorubicin | 1987 | ALL, AML | CML, NHL | 45 mg/m2 IV | 4.515/mg | 406 |
| Doxorubicin | 1974 | ALL, AML, CLL, HL, NHL, MM | 60 mg/m2 IV | 1.108/mg | 133 | |
| Etoposide | 1983 | ALL, AML, HL, NHL, MM | 50 mg/m2 IV | 0.585/mg | 58 | |
| Fluorouracil | 1962 | ALL | 15 mg/kg IV | 0.010/mg | 12 | |
| Hydroxyurea | 1967 | CML | AML, ALL, NHL | 30 mg/kg PO | 0.003/mg | 8 |
| Ifosfamide | 1988 | ALL, HL, NHL, MM | 5000 mg/m2 IV | 0.056/mg | 563 | |
| Mercaptopurine | 1953 | ALL | AML, CML, NHL | 2.5 mg/kg PO | 0.072/mg | 14 |
| Methotrexate | 1959 | ALL, NHL | AML, HL | 1000 mg/m2 IV | 0.154/mg | 308 |
| Paclitaxel | 1992 | NHL, MM | 175 mg/m2 IV | 0.964/mg | 337 | |
| Procarbazine | 1969 | HL | NHL, MM | 100 mg/m2 PO | 1.114/mg | 223 |
| Thioguanine | 1966 | AML | 2 mg/kg PO | 0.262/mg | 42 | |
| Vinblastine | 1965 | HL, NHL | 6 mg/m2 IV | 2.738/mg | 33 | |
| Vincristine | 1963 | ALL, HL, NHL | CLL, MM | 1.4 mg/m2 IV | 13.090/mg | 26 |
| Medicine . | Year of FDA licensure . | FDA hematologic malignancy indications . | Other clinical uses . | Dose . | AWP (US dollars)* . | AWP per dose (US dollars)† . |
|---|---|---|---|---|---|---|
| Asparaginase | 1978 | ALL | AML | 6000 units/m2 IV | 0.007/unit | 84 |
| Bleomycin | 1973 | HL, NHL | 10 units/m2 IV | 3.406/unit | 68 | |
| Carboplatin | 1989 | HL, NHL | AUC 5 IV | 0.407/mg | 325 | |
| Chlorambucil | 1957 | CLL, HL, NHL | 0.4 mg/kg PO | 1.141/mg | 37 | |
| Cyclophosphamide | 1959 | ALL, AML, CLL, CML, HL, NHL, MM | 750 mg/m2 IV | 0.050/mg | 75 | |
| Cytarabine | 1969 | ALL, AML, CML | HL, NHL | 2000 mg/m2 IV | 0.029/mg | 114 |
| Dacarbazine | 1975 | HL | 375 mg/m2 IV | 0.106/mg | 80 | |
| Daunorubicin | 1987 | ALL, AML | CML, NHL | 45 mg/m2 IV | 4.515/mg | 406 |
| Doxorubicin | 1974 | ALL, AML, CLL, HL, NHL, MM | 60 mg/m2 IV | 1.108/mg | 133 | |
| Etoposide | 1983 | ALL, AML, HL, NHL, MM | 50 mg/m2 IV | 0.585/mg | 58 | |
| Fluorouracil | 1962 | ALL | 15 mg/kg IV | 0.010/mg | 12 | |
| Hydroxyurea | 1967 | CML | AML, ALL, NHL | 30 mg/kg PO | 0.003/mg | 8 |
| Ifosfamide | 1988 | ALL, HL, NHL, MM | 5000 mg/m2 IV | 0.056/mg | 563 | |
| Mercaptopurine | 1953 | ALL | AML, CML, NHL | 2.5 mg/kg PO | 0.072/mg | 14 |
| Methotrexate | 1959 | ALL, NHL | AML, HL | 1000 mg/m2 IV | 0.154/mg | 308 |
| Paclitaxel | 1992 | NHL, MM | 175 mg/m2 IV | 0.964/mg | 337 | |
| Procarbazine | 1969 | HL | NHL, MM | 100 mg/m2 PO | 1.114/mg | 223 |
| Thioguanine | 1966 | AML | 2 mg/kg PO | 0.262/mg | 42 | |
| Vinblastine | 1965 | HL, NHL | 6 mg/m2 IV | 2.738/mg | 33 | |
| Vincristine | 1963 | ALL, HL, NHL | CLL, MM | 1.4 mg/m2 IV | 13.090/mg | 26 |
ALL indicates acute lymphocytic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; MM, multiple myeloma; IV, intravenous; PO, per os (oral); and AUC, area under the curve.
AWP indicates average wholesale price in US dollars based on data from the Red Book 2010. When multiple package sizes and manufacturers were listed, an average of all AWPs was used.
AWP per dose assumes a body surface area of 2 m2 and weight of 80 kg. Calculation for carboplatin is based on a maximum dose of 800 mg corresponding to a creatinine clearance of 135 mL/min. Calculation for vincristine is based on a maximum dose of 2 mg.
Radiotherapy
In sub-Saharan Africa, 64% of patients with NHL, 75% with HL, and 38% with myeloma have been estimated to need radiotherapy at diagnosis.68 The International Atomic Energy Agency has set a global minimum standard of one radiotherapy unit per million persons, whereas developed countries may have up to 6 units per million persons.69 Currently, 18% of radiotherapy need in Africa is fulfilled, and 21 countries in sub-Saharan Africa have no operational units.60,69 Among radiotherapy centers, there are wide variations in staff and equipment, as well as occasional absence of simulators and treatment-planning systems.60 In addition to increasing capacity, International Atomic Energy Agency is facilitating the gradual transition from cobalt-60 to linear accelerator units, as well as implementation of newer techniques such as three-dimensional conformal and intensity modulated therapy, which allow greater radiotherapy safety and efficacy.69
HSCT
HSCT is available in 6 centers in sub-Saharan Africa, all in South Africa.59 Between 1995 and 2011, 902 HSCTs (44% autologous, 43% matched sibling, 9% unrelated) were performed in South Africa and reported to the Center for International Bone Marrow Transplant Research, with leading indications being NHL, multiple myeloma, acute myeloid leukemia, and chronic myelogenous leukemia (Sandra Korman, Center for International Bone Marrow Transplant Research, email communication, December 29, 2011). Outcomes were comparable to developed countries, with 5-year survival rates of 52% for autologous HSCT and 50% for allogeneic HSCT. Even within South Africa, availability is less than 50 HSCTs per 10 million population, compared with more than 300 HSCTs per 10 million population in developed countries.70 Building an infrastructure to support allogeneic HSCT may not be feasible in the near term throughout much of sub-Saharan Africa. However, high-dose therapy with autologous HSCT has the potential for long-term cure in relapsed or refractory HL and NHL, particularly for patients with response to second-line chemotherapy for whom HSCT mortality is less than 5%.59,71,72 In addition, autologous HSCT in developed countries is increasingly performed on an outpatient basis with good results73 and is safe and effective in patients with HIV.74 Developing strategies to extend autologous HSCT to selected patients at national referral hospitals, or at regional centers serving multinational blocs, including adaptation of the HSCT procedure to local conditions, is a reasonable priority in coming decades.
Supportive care and palliation
In addition to better cancer treatments, many of the recent survival gains among patients with hematologic malignancies in developed countries have resulted from improved supportive care. In sub-Saharan Africa, where endemic infectious burden is high, and where patients frequently have HIV and other immunosuppressive conditions, such as malnutrition, infectious complications of cancer therapy present a significant obstacle. Cancer treatment increases risk of tuberculosis in endemic areas.75,76 Bacteremia studies from sub-Saharan Africa are scarce and have focused on community-acquired bacteremia. Few existing nosocomial bacteremia studies suggest a distinct microbiologic spectrum from community-acquired bacteremia, similar to developed countries.77,78 No studies systematically investigate causes of febrile neutropenia in sub-Saharan Africa, although febrile neutropenia occurs in more than 80% of patients with hematologic malignancies in developed countries,79 and frequently complicates treatment of children in Malawi and South Africa.80,81 Available intravenous antibacterial agents include penicillin, ampicillin, cloxacillin, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, metronidazole, and sulfamethoxazole-trimethoprim.64 Vancomycin, ceftazidime, and imipenem, agents that are more typically used for empiric treatment of febrile neutropenia in developed countries, are WHO complementary rather than core medicines and not typically available. Given the high prevalence of HIV and malnutrition, gram-negative enteric pathogens are likely to predominate as bacterial causes of febrile neutropenia,81 organisms for which there is increasing resistance in sub-Saharan Africa.82,83 In addition, antifungal agents active against invasive molds are lacking. Amphotericin and flucytosine are the only systemic antifungal agents other than fluconazole appearing in the WHO essential medicines list, both of which are complementary medicines and typically unavailable.64
Apart from a limited anti-infective formulary, lack of transfusion support presents another major obstacle. Only 41.5% of the transfusion demand for Africa as a whole was met in 2006, with most countries lacking centralized national systems for blood collection and banking, often relying on hospital-based systems which require “replacement” donations from family members or paid donors when dispensing units.84,85 Compared with red cell supply, there is even less capacity for platelet transfusion,84 given technical barriers to preparation of pooled products from multiple donors or apheresis products from single donors, as well as short shelf life and storage requirements of platelet products themselves. In addition, there remains a persistent risk of acquiring infectious agents via transfusion.84,85
Finally, palliation is a core function of oncologists in the developed world, and patients often die of cancer even when optimally treated. Palliative services in sub-Saharan Africa are scarce. They are constrained by opioid availability, as well as regulatory barriers that interfere with opioid administration even when available, despite the fact that morphine has been a WHO essential medicine since 1977.86 Scaling up palliative care has recently become an international priority, including WHO-sponsored initiatives to develop comprehensive palliative care programs with a community health approach in Botswana, Ethiopia, Tanzania, Uganda, and Zimbabwe.87
Current treatment and outcomes
Contemporary outcomes among patients treated for NHL in sub-Saharan Africa are summarized in Table 3. Studies are consistent in demonstrating modest survival rates among adults and children treated with generic single-agent and multiagent chemotherapy regimens. Studies are also consistent in demonstrating a marked survival benefit with concurrent ART among patients with HIV (overall survival 50%-60% with ART vs < 20% without ART).33,40 The pediatric oncology community has long been engaged in sub-Saharan Africa and has shown that prospective, multicenter studies testing specific protocols in childhood endemic BL, as well as nephroblastoma and neuroblastoma, are feasible even in the poorest countries. These studies led to the gradual adaptation of regimens from developed countries to local realities. For instance, the French-African Pediatric Oncology Group implemented intensive BL regimens modeled after those in Europe in 6 African units.88 Acceptable results were achieved in North Africa, whereas in sub-Saharan Africa, the balance of efficacy and toxicity led to eventual replacement of more intensive protocols with single-agent cyclophosphamide and intrathecal methotrexate, a regimen that results in a 50% cure rate at a cost of less than $50 (US) per child, with more intensive regimens reserved for advanced, relapsed, and refractory cases.89,90 Efforts are ongoing to develop similar locally adapted pediatric protocols for acute lymphocytic leukemia, HL, retinoblastoma, and brain tumors.91,92 The adult oncology community has been slower to develop such collaborations. Although South African centers routinely participate in trials of modern cancer therapies, we are aware of only one registered clinical trial to date assessing a specific treatment regimen for adult patients with hematologic malignancy in a resource-limited setting in sub-Saharan Africa.40 The literature regarding treatment and outcomes of adult patients is therefore sparse, with sporadic existing studies reflecting overwhelmingly single-center, retrospective experiences with often limited patient numbers, incomplete clinical and histopathologic characterization, absent cytogenetic and molecular characterization, nonstandardized treatment schemes, incomplete longitudinal assessment with frequent loss to follow-up, and absent health-related quality of life and survivorship measures. It is therefore difficult to collate a cumulative experience from the region for most hematologic cancers, and data for NHL are presented solely for illustration as the most robustly characterized malignancy to date.
Representative contemporary outcomes among patients treated for NHL in sub-Saharan Africa
| . | Study type . | Population . | Median age, y . | Countries . | Years . | Stage . | Treatment . | Comments . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| Bateganya et al33 | Retrospective | 160 NHL (51 HIV-NHL; median CD4 142; 75.6% ART) | 37 | Uganda | 2004-2008 | I/II, 9%; III/IV, 91%; B, 76% | CHOP | HIV without ART hazard ratio for death, 8.99; HIV with ART OS equivalent to non-HIV | Median OS 61 d |
| Mwanda et al40 | Prospective | 49 HIV-NHL; median CD4 198; 37% ART | 39 | Kenya; Uganda | 2001-2005 | I/II, 31%; III/IV, 69%; B, 88% | First line DMOC; second line CHOP | Second line in 22%; ART strongly influenced OS (P = .0007) | Median OS 12.3 mo; 5-y OS 33% |
| Traoré et al89 | Prospective | 178 BL | 7 | Burkina-Faso; Cameroon; Côte d'Ivoire; Madagascar; Mali; Senegal | 2001-2004 | I/II, 24%; III/IV, 76% | First line CPM, IT MTX, IT HC; second line COPM, CYM | Second line in 43%; Stage strongly influenced OS (P = .02) | 2-y OS 50.5% |
| Hesseling et al90 | Prospective | 127 BL | 7.9 | Cameroon | 2008-2009 | I/II, 16%; III/IV, 84% | Stage I-III CPM, IT MTX, IT HC; stage IV or no CR, CPM, MTX,VIN, IT MTX, IT HC | Stage strongly influenced EFS | 1-y EFS 61% |
| . | Study type . | Population . | Median age, y . | Countries . | Years . | Stage . | Treatment . | Comments . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| Bateganya et al33 | Retrospective | 160 NHL (51 HIV-NHL; median CD4 142; 75.6% ART) | 37 | Uganda | 2004-2008 | I/II, 9%; III/IV, 91%; B, 76% | CHOP | HIV without ART hazard ratio for death, 8.99; HIV with ART OS equivalent to non-HIV | Median OS 61 d |
| Mwanda et al40 | Prospective | 49 HIV-NHL; median CD4 198; 37% ART | 39 | Kenya; Uganda | 2001-2005 | I/II, 31%; III/IV, 69%; B, 88% | First line DMOC; second line CHOP | Second line in 22%; ART strongly influenced OS (P = .0007) | Median OS 12.3 mo; 5-y OS 33% |
| Traoré et al89 | Prospective | 178 BL | 7 | Burkina-Faso; Cameroon; Côte d'Ivoire; Madagascar; Mali; Senegal | 2001-2004 | I/II, 24%; III/IV, 76% | First line CPM, IT MTX, IT HC; second line COPM, CYM | Second line in 43%; Stage strongly influenced OS (P = .02) | 2-y OS 50.5% |
| Hesseling et al90 | Prospective | 127 BL | 7.9 | Cameroon | 2008-2009 | I/II, 16%; III/IV, 84% | Stage I-III CPM, IT MTX, IT HC; stage IV or no CR, CPM, MTX,VIN, IT MTX, IT HC | Stage strongly influenced EFS | 1-y EFS 61% |
B indicates B symptoms; OS, overall survival; EFS, event-free survival; CR, complete remission; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; DMOC, dose-modified oral chemotherapy (lomustine, etoposide, cyclophosphamide, procarbazine); CPM, cyclophosphamide; IT, intrathecal; MTX, methotrexate; HC, hydrocortisone; COPM, vincristine, prednisone, cyclophosphamide, methotrexate, intrathecal methotrexate, intrathecal hydrocortisone; CYM, intrathecal methotrexate, intrathecal cytarabine, intrathecal hydrocortisone; and VIN, vincristine.
Future directions
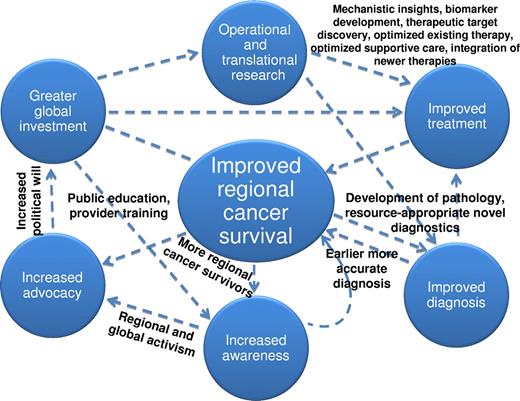
Improving survival for hematologic malignancies in sub-Saharan Africa is a formidable task. We propose a conceptual framework to achieve this goal (Figure 2), a model that will pay collateral dividends toward reducing morbidity and mortality from nonhematologic malignancies as well. Progress against HIV and other infectious diseases has resulted from collaborative solutions developed by international partners working with local scientific, political, and civil society leaders. Successful cancer programs must also be “locally grown.” There will be legitimate debate about what is appropriate, implementable, and achievable throughout the region. However, clearly articulated, aspirational goals have been vitally important in enabling current progress against infectious diseases, including calls for HIV and malaria elimination despite the fact that these 2 diseases continue to claim almost 2 million lives per year in sub-Saharan Africa. We think that a long-term, aspirational strategy for cancer is no less important, and specific recommendations in this regard are as follows.
A conceptual framework to improve survival among patients with hematologic malignancies in sub-Saharan Africa.
A conceptual framework to improve survival among patients with hematologic malignancies in sub-Saharan Africa.
Develop conventional and novel diagnostics adapted to the setting
There is a critical need to expand histopathology, improve tissue sampling and processing, and implement basic immunohistochemistry. However, grafting pathology systems from developed countries into dissimilar settings without modification is unlikely to be successful. Conventional procedures may need to be supplemented, and even supplanted in some instances, by diagnostic algorithms, tissue microarrays, and digital microscopy approaches suited to local conditions, as promoted by the INCTR and Sub-Saharan Africa Lymphoma Consortium.34,93 In addition, cancers are increasingly distinguished by signature genetic aberrations and downstream molecular events. Presently, genotypic and nucleic acid amplification assays are not widely implementable in the region given inadequate existing laboratory facilities. However, PCR assays using dried blood spots have been specifically developed for use in resource-limited environments to allow HIV diagnosis in infancy, HIV RNA monitoring, and detection of HIV resistance.94 Similarly, a fully automated, cartridge-based molecular diagnostic system has been developed to diagnose tuberculosis and detect drug resistance, allowing earlier initiation of optimal therapy.95 These technologies have been designed for use under conditions of decentralized specimen collection, delayed specimen transport, and limited laboratory technical capacity. Their cost is acceptable for sub-Saharan Africa, and they have been successfully implemented even in lower-level health centers.94,95 It is conceivable that similar technologies may one day facilitate cancer diagnosis, treatment, and response assessment in settings without traditional pathology services or advanced diagnostic imaging, similar to current trends in developed countries.
Make cancer medicines affordable and explore the integration of newer treatments
The molecular age has catalyzed a shift away from cytotoxic treatment toward “targeted” therapies directed at immunophenotypes, mutations, and gene products. Noncytotoxic agents, which have augmented the armamentarium for hematologic malignancies in recent years, include monoclonal antibodies, antibody-drug conjugates, small molecule tyrosine kinase inhibitors, immunomodulatory agents, proteasome inhibitors, and histone deacetylase inhibitors. The optimal application for many of these agents remains under investigation even in resource-rich settings, and they are prohibitively expensive for use in sub-Saharan Africa at present. However, leaving aside cost considerations, they are also in many respects ideal treatments for the region. Some are orally administered, obviating need for infusion, and many have more manageable toxicities, including less myelosuppression and infectious risk compared with conventional cytotoxic agents. As noted previously, the “newest” WHO essential cancer medicine is 20 years old, and we believe withholding newer treatments from patients in sub-Saharan Africa for several more decades opposes the WHO and United Nations foundational principle of health as a human right. Developing platforms to study integration of newer agents is important in settings where genetic differences, prevalent comorbid illnesses, nutritional status, interactions with coadministered medications, adherence, and other factors may result in efficacy, safety, and cost-effectiveness profiles very different from previously studied populations. Negotiated agreements with manufacturers to provide HIV medicines at low cost (often ∼ 10% of the US retail price), as led by the Clinton Health Access Initiative, have been a cornerstone of successful ART in sub-Saharan Africa.96 Similar tiered pricing arrangements may be feasible for selected cancer treatments proven to be safe and effective in the region, especially if associated with increased rates of long-term cure. Patents for rituximab and imatinib are set to expire soon, and generic formulations and biosimilars are already in development and even clinical use.
Use existing care and research infrastructure to study and control cancer
International efforts to control HIV and other infectious diseases have led to health care investment in settings where there was little preexisting capacity, as well as robust clinical trial networks capable of enrolling patients and following them in settings where this has been historically challenging. By adapting this infrastructure to study cancer, there is an opportunity to build on recent global health successes. For instance, the AIDS Malignancy Consortium (AMC) and AIDS Clinical Trials Group (ACTG) currently cosponsor 2 trials of HIV-associated KS (AMC066-ACTG 5263 and AMC067-ACTG 5264), which are enrolling patients throughout sub-Saharan Africa, and these networks can be harnessed to study hematologic cancers in patients with and without HIV. Even long-settled questions in resource-rich settings, such as the tolerability, benefit-risk profile, and optimal dosing of CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone) for NHL, have never been prospectively assessed in sub-Saharan Africa in patients with or without HIV, despite the fact that CHOP serves as the de facto regional standard. Addressing such pressing clinical questions through existing networks would provide immediately translatable results to guide treatment for tens of thousands of patients in the region.
Increase global funding for cancer and rigorously assess cost-effectiveness of cancer control measures
Sub-Saharan Africa accounted for 5.4% of new global cancer cases in 2009 but only 0.2% of global cancer spending, with South Africa by itself accounting for 51% of the total $581 million (US) in regional cancer expenditures.97 By comparison, $2.8 billion (US) was spent on HIV in sub-Saharan Africa in 2009.98 The ability of governments and health systems to sustainably finance modern cancer care has been challenged even in developed countries. In sub-Saharan Africa, resource constraints demand a reasoned debate as to the optimal application and expansion of existing resources, a debate that will require contributions from patients, clinicians, policy makers, economists, and ethicists. Compared with chronic diseases, such as HIV, cancer care will be predictably “cost-dense.” For example, 6 cycles of CHOP costs approximately $1500 (US). However, if treatment is followed by decades of event-free survival, cost-effectiveness may compare favorably with other accepted interventions. Current annual costs for ART are approximately $200 (US) for first-line treatment and approximately $500 to $600 (US) for second-line treatment in resource-limited settings.96,98 Submitting cancer control measures to formal cost-effectiveness analysis, as well as developing ethical regional standards for cost-effective cancer care will be important future priorities.
Increase human capital investments for cancer
Although nononcologist providers can deliver cancer treatment safely and effectively,67 critical health worker shortages must be addressed. There is an urgent need to invest in a laboratory, pharmacy, and clinical workforce that is comfortable treating cancer patients. Training efforts have become a major focus for international organizations (American Society of Hematology, ASCO, INCTR, NCI), and African organizations (West African College of Physicians, African Organization for Research and Training in Cancer), among others. As one example, the NCI currently supports pathology training workshops over a 3-year period in Kenya to improve regional capacity. Specific funding mechanisms to support training activities are available through such programs as the Medical Education Partnership Initiative. However, building a dedicated oncology workforce and reversing decades of “brain drain” will similarly require decades of sustained commitment, and developing interim strategies is essential. Progress against infectious diseases has often relied on innovative human resource solutions, such as task shifting, to compensate for physician shortages.99 Videoconferencing and telepathology may also be important approaches, and the suitability of such strategies for cancer care in the region should be further explored.
In conclusion, given the enormous challenges and rapidly increasing burden, some may feel nihilistic about prospects for controlling hematologic malignancies in sub-Saharan Africa. However, the global response to HIV provides an important lesson, although there remain significant obstacles and much work still to be done. Where there was existential anxiety about the fate of entire societies, there is now increasing optimism and open calls to end the epidemic in our lifetimes, all as a result of sustained international advocacy, collaboration, and investment. There is now a similar opportunity to substantially influence cancer control in sub-Saharan Africa in the years to come. By meeting this challenge, we will mitigate the suffering of millions afflicted by cancer and transform many patients into long-term survivors. We will also live up to the mutualistic ideal exemplified by the earliest BL collaborations in Uganda, and potentially gain insights that can drive the development of new therapies applicable around the world.
Authorship
Contribution: S.G. conceived, wrote, and revised the manuscript; and W.A.W., S.J.L., T.C.S., K.N.N., P.N.K., C.C., P.B.H., and R.T.M. contributed comments and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satish Gopal, Program in Global Oncology, Lineberger Comprehensive Cancer Center, UNC-Chapel Hill, Physicians' Office Bldg, 170 Manning Dr, CB 7305, Chapel Hill, NC 27599-7305; e-mail: gopal@med.unc.edu.