Abstract
An explorative study evaluates the efficacy of Helicobacter pylori (HP) eradication (HPE) therapy on early-stage gastric diffuse large B-cell lymphomas (DLBCLs) without features of mucosa-associated lymphoid tissue (MALT), the pure (de novo) DLBCLs, in comparison with its efficacy on high-grade transformed gastric MALT lymphomas, the DLBCL(MALT). In total, 50 patients of stage IE/IIE1 HP-positive gastric DLBCLs with frontline HPE treatment were included. HP infection was successfully eradicated in 100% (16/16) of the pure (de novo) DLBCL patients and 94.1% (32/34) of the DLBCL(MALT) patients. In total, 68.8% (11/16) of pure (de novo) DLBCL patients and 56.3% (18/32) of DLBCL(MALT) patients achieved complete pathologic remission (pCR) after HPE therapy. The median time to pCR was 2.1 months (95% confidence interval, 0.6%-3.7%) for pure (de novo) DLBCLs and 5.0 months (95% confidence interval, 2.8%-7.5%; P = .024) for DLBCL(MALT). At a median follow-up of 7.7 years, all patients with pCR after HPE therapy were alive and free of lymphomas, except for one patient with pure (de novo) DLBCL who died of lung cancer. Similar to DLBCL(MALT), a substantial portion of early-stage HP-positive gastric pure (de novo) DLBCLs remains HP-dependent and responds to antibiotic treatment. Prospective studies to validate the findings are warranted.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 5057.
Disclosures
The authors, Associate Editor Jacob M. Rowe, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe short-term outcomes of Helicobacter pylori (HP) eradication (HPE) therapy in patients with early-stage HP-positive gastric pure (de novo without features of mucosa-associated lymphoid tissue [MALT]) diffuse large B-cell lymphoma (DLBCL).
Describe short-term outcomes of HPE in patients with early-stage HP-positive gastric DLBCL(MALT).
Describe long-term outcomes of HPE in patients with early-stage HP-positive gastric DLBCL.
Release date: May 24, 2012; Expiration date: May 24, 2013
Introduction
Diffuse large B-cell lymphoma (DLBCL) of the stomach is a heterogeneous disease entity that includes lymphomas with and without features of mucosa-associated lymphoid tissue (MALT) lymphoma in the classification of World Health Organization (WHO).1-3 The main reason to include high-grade transformed MALT lymphoma into the category of DLBCL and renamed as DLBCL with features of MALT lymphoma, DLBCL(MALT), in WHO classification was based on earlier preclinical and clinical observations suggesting high-grade transformed MALT lymphomas were usually Helicobacter pylori (HP)–independent and no longer responded to antibiotics as their low-grade counterpart did.1,4-6 However, we and other investigators have recently shown that certain proportion of early-stage HP-positive gastric DLBCL(MALT) could achieve long-term complete pathologic remission (pCR) after frontline HP eradication (HPE) therapy.7-10
In 1988, Issacson et al11 proposed a new classification for gut lymphoma in which DLBCLs of the gastrointestinal tract would include high-grade transformed MALT lymphoma without a low-grade component or de novo DLBCL of peripheral lymph nodal equivalent. Maybe because of the recommendation of WHO advisory committee that suggested all gastric DLBCL, no matter originating from high-grade transformed MALT lymphoma or de novo, should be treated with aggressive chemotherapy and there is no marker to differentiate the origin of pure (de novo) DLBCL in current clinical practice, larger series investigating the therapeutic efficacy of frontline antibiotics in gastric pure (de novo) DLBCL are seldom reported.
With laser capture microdissection followed by IgH rearrangement analysis, we further demonstrated that large cell and MALT lymphoma components in HP-independent gastric DLBCL(MALT) might be different in clonality and respond differently to antibiotic treatment.12 Of the 2 different clones in one particular patient, his DLBCL remitted completely, but the MALT component remained unresolved after HPE therapy.12 The finding suggests some HP-positive pure (de novo) DLBCL (without features of MALT lymphoma or of same clonality) may remain HP-dependent and respond to antibiotics, in accordance with several recent anecdotal case reports showing HPE treatment could result in pCR of early-stage gastric pure (de novo) DLBCLs.13-15
In 2001, we reported the efficacy of antibiotics in treating 16 patients with newly diagnosed stage IE gastric high-grade MALT lymphoma, DLBCL(MALT), associated with HP infection,7 and antibiotics have became a frontline treatment for localized (stage IE/IIE1) HP-positive gastric DLBCL(MALT) in our institutions.8 Antibiotics also have been prescribed for patients with localized HP-positive gastric pure (de novo) DLBCL who decided to take a brief trial of antibiotics after fully informed about the pros and cons of antibiotic therapy and chemotherapy. Patients have been protected from nonsupervised lymphoma progression by a relatively intensive endoscopy follow-up schedule.7,8,10
Here, we report the results of a retrospective study evaluating the efficacies of HPE therapy on stage IE/IIE1 HP-positive, pure (de novo) DLBCLs of the stomach in comparison with that on DLBCL(MALT) in a multicenter, prospective study.
Methods
Patients, histologic diagnosis, and study design
In 2001, we reported the results of a multicenter, prospective study on 16 patients with newly diagnosed stage IE gastric HP-positive high-grade transformed MALT lymphoma, DLBCL(MALT), treated with antibiotics alone.7 Since then, the prospective trial for gastric DLBCL(MALT) has remained open for patient enrollment.8 We also prescribed antibiotics in National Taiwan University Hospital for patients with localized HP-positive gastric pure (de novo) DLBCL who decided to take a brief trial of HPE therapy after fully informed about the pros and cons of antibiotic therapy and chemotherapy.
We retrospectively reviewed the medical and pathologic records of National Taiwan University Hospital. Between June 2002 and June 2009, patients with newly diagnosed primary DLBCL of the stomach were identified, and their histologic sections of initial gastric biopsy specimens were carefully reviewed by 2 independent, experienced hematopathologists to ensure the diagnosis of DLBCL and to reassess the presence or absence of MALT lymphoma component. Of each patient, histologic sections of a minimum of 6 endoscopic biopsies from the tumor lesions should be available, in accordance with the literature or the European Gastrointestinal Lymphoma Study Group consensus.16-19
Diagnosis of DLBCL was based on the criteria of WHO classification as hematologic malignancies that manifest as compact clusters, confluent aggregates, or sheets of large lymphoma cells with distinctive nucleoli.1-3 Tumors without histologic evidence of MALT lymphoma, meaning dense infiltration of centrocyte-like cells in the lamina propria and typical lymphoepithelial lesions, were classified as pure (de novo) DLBCL.1,3,20,21 Those with histologic evidence of MALT were classified as DLBCL(MALT).20,21 All specimens also had immunohistochemical staining of CD20, CD5, CD3, and CD43 and additional study with anti-cytokeratin (1:50, clone AE1/AE3; Ylem) to identify lymphoepithelial lesions in the context of the minimal MALT lymphoma components in DLBCL.22 This explorative study has been approved by the Institutional Review Board of National Taiwan University Hospital.
HPE therapy
The presence of an HP infection was confirmed at baseline for each case by positive results of at least one of these tests: histologic examination, rapid urease test, bacterial culture, urea breath test, and serology.19,23,24 The HPE regimen was 500 mg of amoxicillin and 250 mg metronidazole 4 times daily, with either 120 mg of bismuth subcitrate 4 times daily or 20 mg omeprazole twice daily for 4 weeks.7,8 After March 1996, the HPE regimen was changed to 500 mg amoxicillin 4 times daily, 500 mg clarithromycin twice daily plus 20 mg of omeprazole twice daily for 2 weeks.7,8,25
Clinical and histologic evaluations
Staging workups included a detailed physical examination, inspection of Waldeyer ring, computed tomography (CT) of the chest and abdomen, and bone marrow aspiration and biopsy.19 Tumors were staged based on CT findings and a complimentary endoscopic ultrasonography examination that evaluated the depth of tumor infiltration and the status of perigastric lymph nodes according to Musshoff modification of the Ann Arbor staging system in which stage IE tumors are confined to the wall of the stomach and stage IIE1 tumors have perigastric lymph node involvement. Gastric wall involvement was mainly evaluated by endoscopic ultrasonography.
Patients were scheduled for the first follow-up upper gastrointestinal endoscopic examination 4 to 6 weeks after completing antibiotic therapy, which was repeated every 6 to 8 weeks until pCR was achieved or until treatment failed. At each endoscopic examination, 4 to 6 biopsy specimens from the antrum and body of the stomach were evaluated for HP infection, and a minimum of 6 biopsy samples from each of the tumors and suspicious areas were histologically evaluated.19 HP infection status was determined by histologic examination, biopsy urease test, bacterial culture, urease breath test, or a combination. Histologic features were evaluated using the scoring system for MALT lymphoma of Wotherspoon et al.26 The proportion of large cells was evaluated. The status of pCR was defined as a Wotherspoon score of 2 or less on every histologic section of the biopsy specimens, and partial pathologic remission was defined as the presence of lesions with Wotherspoon score of 3 and no lesions with scores of 4 or 5 on any histologic section of biopsy specimens. Pathologic remission was confirmed by a second biopsy within 3 months. Patients with pCR had an endoscopic examination of the stomach and CT of the abdomen every 3 to 6 months for the first 12 months and every 6 to 12 months thereafter. Treatment failure, or progressive disease, was defined as grossly progressive disease on follow-up endoscopic examination or grossly stable but with the presence of a persistent or increasing proportion of large cells on microscopic examination and was referred immediately for systemic chemotherapy.
Statistical analysis
Discrete variables were compared by the χ2 test, Fisher exact test, Student t test, or 1-way ANOVA. Analysis was conducted using the follow-up data available on December 31, 2010. Overall survival was calculated from the date of initial treatment to the date of death because of any cause. Survival was estimated by the Kaplan-Meier method and compared by log-rank test. Differences between the results of the comparative tests were considered statistically significant if the 2-sided P value was less than .05.
Results
Clinicopathologic features
Between June 2002 and June 2009, 104 patients with newly diagnosed primary gastric DLBCL were identified. The final histologic diagnosis was pure (de novo) DLBCL in 77 patients and DLBCL(MALT) in 27 patients. Of those with pure (de novo) DLBCL patients, 46 (59.7%) were of stage IE or IIE1 (localized diseases), and 31 (40.3%) were of stage IIE2 or beyond (disseminated disease). Sixteen of the 46 (34.8%) patients with stage IE or IIE1 gastric pure (de novo) DLBCL and having received frontline antibiotics therapy were included into the study (Table 1). The remaining 30 (65.2%) patients with stage IE or IIE1 gastric pure (de novo) DLBCL who received systemic frontline chemotherapy as well as the 34 accruals who participated in our multicenter, prospective trial for DLBCL(MALT) between June 1995 and June 2009 were included to serve as control (Table 1). Twenty-four patients of the latter group had been presented in our previous extension report.8 The demography of the 3 groups of patients was listed against their histologic diagnosis and frontline treatment in Table 1, showing no statistically significant differences in age, sex, endoscopic appearance, lesion site, and depth of tumor infiltration among these 3 groups.
Clinicopathologic characteristics of patients with early-stage, gastric DLBCL with and without histologic evidence of MALT origin
| Frontline treatment . | Pure (de novo) DLBCL . | DLBCL(MALT) . | P* . | |
|---|---|---|---|---|
| Chemotherapy, n = 30 . | Antibiotics, n = 16 . | Antibiotics, n = 34 . | ||
| Clinicopathologic characteristics | ||||
| Median age, y (range) | 65 (20-87) | 63 (34-88) | 55 (35-83) | .283† |
| Sex, male/female | 16/14 | 6/10 | 12/22 | .312‡ |
| Stage | .022‡ | |||
| IE, n (%) | 19 (63.3) | 9 (56.3) | 30 (88.3) | |
| IIE1, n (%) | 11 (36.7) | 7 (43.8) | 4 (11.7) | |
| Endoscopic features, n (%) | .537§ | |||
| Gastritis-like or multiple erosion on infiltrative mucosa | 7 (23.3) | 3 (18.8) | 11 (32.4) | |
| Ulceration or ulcerated mass | 18 (60.0) | 11 (68.8) | 18 (52.9) | |
| Erosions on giant nodular folds | 3 (10.0) | 1 (6.3) | 3 (8.8) | |
| Mixed | 2 (6.7) | 1 (6.3) | 2 (5.9) | |
| Location of tumor(s), n (%) | .790§ | |||
| Antrum | 12 (40.6) | 6 (37.5) | 10 (29.4) | |
| Angularis | 1 (3.3) | 0 (0) | 3 (8.8) | |
| Middle body, lower body, or both | 7 (23.3) | 5 (31.3) | 8 (23.5) | |
| Upper body, fundus, or both | 3 (10.0) | 1 (6.3) | 3 (8.8) | |
| ≥ 2 components | 7 (23.3) | 4 (25.0) | 10 (29.4) | |
| Depth of gastric wall involvement, n (%)‖ | .831‡ | |||
| Submucosa or above | 9/22 (40.9) | 5/16 (31.2) | 10/27 (37.0) | |
| Muscularis propria or beyond | 13/22 (59.1) | 11/16 (68.8) | 17/27 (63.0) | |
| Initial pathologic features, n (%) | ||||
| DLBCL without MALT lymphoma | 30 (100) | 16 (100) | ||
| MALT lymphoma with foci of large-cell aggregations | 19 (55.9) | |||
| DLBCL with foci of CCL, LEL, or both | 15 (44.1) | |||
| Frontline treatment . | Pure (de novo) DLBCL . | DLBCL(MALT) . | P* . | |
|---|---|---|---|---|
| Chemotherapy, n = 30 . | Antibiotics, n = 16 . | Antibiotics, n = 34 . | ||
| Clinicopathologic characteristics | ||||
| Median age, y (range) | 65 (20-87) | 63 (34-88) | 55 (35-83) | .283† |
| Sex, male/female | 16/14 | 6/10 | 12/22 | .312‡ |
| Stage | .022‡ | |||
| IE, n (%) | 19 (63.3) | 9 (56.3) | 30 (88.3) | |
| IIE1, n (%) | 11 (36.7) | 7 (43.8) | 4 (11.7) | |
| Endoscopic features, n (%) | .537§ | |||
| Gastritis-like or multiple erosion on infiltrative mucosa | 7 (23.3) | 3 (18.8) | 11 (32.4) | |
| Ulceration or ulcerated mass | 18 (60.0) | 11 (68.8) | 18 (52.9) | |
| Erosions on giant nodular folds | 3 (10.0) | 1 (6.3) | 3 (8.8) | |
| Mixed | 2 (6.7) | 1 (6.3) | 2 (5.9) | |
| Location of tumor(s), n (%) | .790§ | |||
| Antrum | 12 (40.6) | 6 (37.5) | 10 (29.4) | |
| Angularis | 1 (3.3) | 0 (0) | 3 (8.8) | |
| Middle body, lower body, or both | 7 (23.3) | 5 (31.3) | 8 (23.5) | |
| Upper body, fundus, or both | 3 (10.0) | 1 (6.3) | 3 (8.8) | |
| ≥ 2 components | 7 (23.3) | 4 (25.0) | 10 (29.4) | |
| Depth of gastric wall involvement, n (%)‖ | .831‡ | |||
| Submucosa or above | 9/22 (40.9) | 5/16 (31.2) | 10/27 (37.0) | |
| Muscularis propria or beyond | 13/22 (59.1) | 11/16 (68.8) | 17/27 (63.0) | |
| Initial pathologic features, n (%) | ||||
| DLBCL without MALT lymphoma | 30 (100) | 16 (100) | ||
| MALT lymphoma with foci of large-cell aggregations | 19 (55.9) | |||
| DLBCL with foci of CCL, LEL, or both | 15 (44.1) | |||
LEL indicates lymphoepithelial lesions.
Comparison of discrete variables between pure (de novo) DLBCL and DLBCL(MALT).
P values (2-sided) were calculated using the Student t test.
P values (2-sided) were calculated using the Fisher exact test.
P values (2-sided) were calculated using 1-way ANOVA.
Gastric wall involvement was evaluated in 65 patients in total. Evaluation was by endoscopic ultrasonography in 64 patients and by histologic examination of surgical specimen in one gastric DLBCL(MALT) patient.
Tumor response to HPE therapy
Eradication of HP infection, defined as negative results of biopsy urease test, histology, and urease breath test (only performed after 2006) was achieved in 94.1% (32/34) of patients with DLBCL(MALT) and in all 100% (16/16) of patients with pure (de novo) DLBCL.
The pCR rate after antibiotic therapy was 58.0% (29/50; 95% confidence interval [CI], 43.8%-72.2%). Of the 48 HP-eradicated patients, the pCR rate was 68.8% (11/16; 95% CI, 43.2%-94.3%) for pure (de novo) DLBCL (Figure 1 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and 56.3% (18/32; 95% CI, 38.8%-74.4%) for DLBCL(MALT) (P = .404, χ2 test; Table 2).
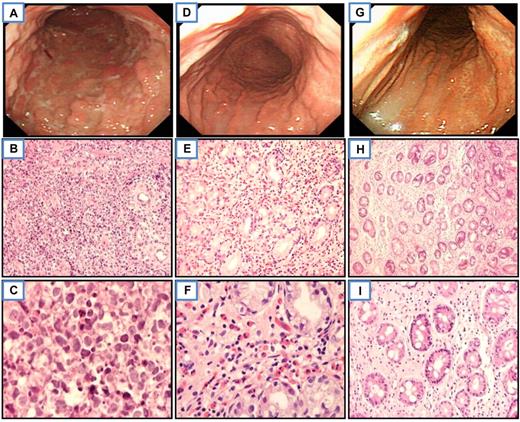
Endoscopic and histologic features of a representative gastric pure (de novo) DLBCL responsive to HPE therapy. Before treatment, multiple irregular-shaped ulcers with residual nodular mucosa at the entire gastric body (A) and diffuse large cells infiltrate in the lamina propria in histology (B-C; H&E, ×400 and ×1000, respectively). Six weeks after HP eradication, complete regression of all diffuse ulcerations with hyperemic and atrophic mucosa at the entire gastric body (D) and biopsy showed prominent eosinophils with scatter small lymphoid cells infiltrate in the lamina propria (E-F). Neither large cells nor features of MALT lymphoma were seen (H&E, ×400 and ×1000, respectively). Six months after HP eradication, nearly normal-appearing mucosa with some wide-based whitish scar at the body of the stomach (G) and biopsy shows scatter small lymphocytes infiltrate only the lamina propria (H-I; H&E, ×100 and ×400, respectively). All slides were observed with light microscopy. An Olympus BX60 microscope equipped with 10×/0.40, 40×/0.95, and 100×/1.35 objective lenses (Olympus) was used to visualize images. Pictures were taken with an Olympus DP70 camera, and Adobe Photoshop 6.0 was used to enlarge the images to their present magnification levels (Adobe Systems).
Endoscopic and histologic features of a representative gastric pure (de novo) DLBCL responsive to HPE therapy. Before treatment, multiple irregular-shaped ulcers with residual nodular mucosa at the entire gastric body (A) and diffuse large cells infiltrate in the lamina propria in histology (B-C; H&E, ×400 and ×1000, respectively). Six weeks after HP eradication, complete regression of all diffuse ulcerations with hyperemic and atrophic mucosa at the entire gastric body (D) and biopsy showed prominent eosinophils with scatter small lymphoid cells infiltrate in the lamina propria (E-F). Neither large cells nor features of MALT lymphoma were seen (H&E, ×400 and ×1000, respectively). Six months after HP eradication, nearly normal-appearing mucosa with some wide-based whitish scar at the body of the stomach (G) and biopsy shows scatter small lymphocytes infiltrate only the lamina propria (H-I; H&E, ×100 and ×400, respectively). All slides were observed with light microscopy. An Olympus BX60 microscope equipped with 10×/0.40, 40×/0.95, and 100×/1.35 objective lenses (Olympus) was used to visualize images. Pictures were taken with an Olympus DP70 camera, and Adobe Photoshop 6.0 was used to enlarge the images to their present magnification levels (Adobe Systems).
Results of HPE therapy in HP-positive, early-stage gastric DLBCL with and without histologic evidence of MALT origin
| Clinicopathologic characteristic . | Pure (de novo) DLBCL . | DLBCL(MALT) . | P* . |
|---|---|---|---|
| No. of patients | 16 | 34 | |
| HPE rate, % | 100 (16/16) | 94.1 (32/34) | 1.000† |
| pCR rate | |||
| All evaluable patients, % | 68.8 (11/16) | 52.9 (18/34) | .365† |
| HP-eradicated patients | 68.8 (11/16) | 56.3 (18/32) | .404† |
| HP-persistent patients | 0 (0/0) | 0 (0/2) | |
| Depth of gastric wall involvement | |||
| Submucosa or above, % | 100 (5/5) | 80 (8/10) | .524‡ |
| Muscularis propria or beyond, % | 54.5 (6/11) | 29.4 (5/17) | .248‡ |
| Time to pCR§ | |||
| Median (95% CI), mo | 2.1 (0.6-3.7) | 5.0 (2.8-7.5) | .024‖ |
| Follow-up time of complete responders¶ | |||
| Median (95% CI), y | 3.5 (0.7-6.3) | 11.1 (7.8-14.4) | |
| Relapse rate, %¶ | 0 (0/0) | 0 (0/0) |
| Clinicopathologic characteristic . | Pure (de novo) DLBCL . | DLBCL(MALT) . | P* . |
|---|---|---|---|
| No. of patients | 16 | 34 | |
| HPE rate, % | 100 (16/16) | 94.1 (32/34) | 1.000† |
| pCR rate | |||
| All evaluable patients, % | 68.8 (11/16) | 52.9 (18/34) | .365† |
| HP-eradicated patients | 68.8 (11/16) | 56.3 (18/32) | .404† |
| HP-persistent patients | 0 (0/0) | 0 (0/2) | |
| Depth of gastric wall involvement | |||
| Submucosa or above, % | 100 (5/5) | 80 (8/10) | .524‡ |
| Muscularis propria or beyond, % | 54.5 (6/11) | 29.4 (5/17) | .248‡ |
| Time to pCR§ | |||
| Median (95% CI), mo | 2.1 (0.6-3.7) | 5.0 (2.8-7.5) | .024‖ |
| Follow-up time of complete responders¶ | |||
| Median (95% CI), y | 3.5 (0.7-6.3) | 11.1 (7.8-14.4) | |
| Relapse rate, %¶ | 0 (0/0) | 0 (0/0) |
Comparison of discrete variables between pure (de novo) DLBCL and subtotal of DLBCL(MALT).
P values (2-sided) were calculated using the χ2 test.
P values (2-sided) were calculated using the Fisher exact test.
Only patients with successful HPE therapy were included.
P values (2-sided) were calculated using Kaplan-Meier analysis with log-rank test.
For complete responders only; analysis to compare the difference between pure (de novo) DLBCL and DLBCL(MALT) was not made because it was caused by timing of patient accrual but not treatment efficacy.
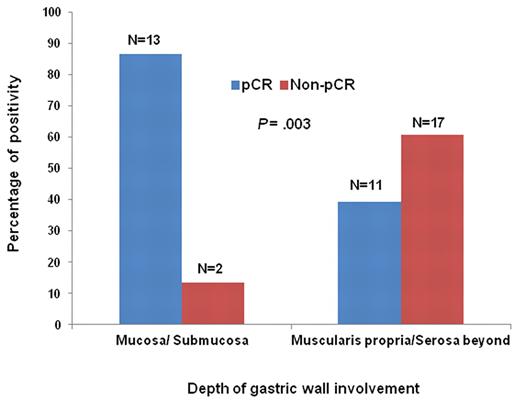
The median time to pCR after completion of HPE was 2.1 months (95% CI, 0.6%-3.7%) and 5.0 months (95% CI, 2.8%-7.5%) for pure (de novo) DLBCL and DLBCL(MALT), respectively (P = .024, log-rank test; Figure 2). The pCR rate of the 43 patients with known depth of invasion at baseline was 86.7% (13/15) for tumors limited to mucosa and submucosa and 39.3% (11/28) for those extending into muscularis propria or beyond (P = .003, χ2 test; Table 2 and Figure 3). Among patients with HP eradicated by antibiotics, the pCR rate of pure (de novo) DLBCL limited to mucosa and submucosa and extended into the muscularis propria or beyond was 100% (5/5) and 54.5% (6/11), respectively (P = .119, Fisher exact test), whereas that of DLBCL(MALT) was 80% (8/10) and 29.4% (5/17), respectively (P = .018, Fisher exact test).
Time to pCR of HP-eradicated patients. Time was calculated from the completion of antibiotic treatment to first evidence of pCR by Kaplan-Meier analysis (pure [de novo] DLBCL vs DLBCL[MALT]; P = .024, 2-sided, log-rank test).
Time to pCR of HP-eradicated patients. Time was calculated from the completion of antibiotic treatment to first evidence of pCR by Kaplan-Meier analysis (pure [de novo] DLBCL vs DLBCL[MALT]; P = .024, 2-sided, log-rank test).
Correlation of depth of gastric wall involvement and tumor response to HPE therapy. The number of cases of gastric pure (de novo) DLBCL and DLBCL(MALT) with available clinical staging (mucosa or submucosa invasion vs muscularlis, serosa, or perigastric lymph node involvement) in individual subgroups is indicated at the top of the corresponding histogram.
Correlation of depth of gastric wall involvement and tumor response to HPE therapy. The number of cases of gastric pure (de novo) DLBCL and DLBCL(MALT) with available clinical staging (mucosa or submucosa invasion vs muscularlis, serosa, or perigastric lymph node involvement) in individual subgroups is indicated at the top of the corresponding histogram.
Follow-up and chemotherapy for patients with and without frontline antibiotic therapy
As of December 2010, the median follow-up time for patients with pure (de novo) DLBCL was 3.9 years (95% CI, 3.7%-4.1%) and 10.8 years (95% CI, 8.9%-12.7%) for DLBCL(MALT). The different time periods, ie 2002 to 2009 for pure (de novo) DLBCL versus 1995 to 2009 for DLBCL(MALT), during which antibiotic or chemotherapy treatment was used for each disease category caused the discrepancy of follow-up time. Of the 50 patients who had frontline antibiotic treatment, all 29 complete responders remained lymphoma-free and alive, except for one patient who died of second primary non–small cell lung cancer 3.6 years after the diagnosis of gastric pure (de novo) DLBCL. All of the other 21 patients without pCR after antibiotics, including 5 pure (de novo) DLBCL patients and 16 DLBCL(MALT) patients, were immediately referred to have standard systemic chemotherapy at time of antibiotic failure. The chemotherapy regimens for all 3 groups of patients were similar and mainly consisted of cyclophosphamide, doxorubicin or epirubicin, vincristine, and prednisolone with or without rituximab. The pCR rate after systemic chemotherapy was 100% (5/5) and 93.8% (15/16) for antibiotic-failure pure (de novo) DLBCL patients and DLBCL(MALT) patients, respectively, and 86.7% (26/30) for frontline chemotherapy-treated pure (de novo) DLBCL patients. Two DLBCL(MALT) patients who achieved pCR after chemotherapy died because of pneumonia at the age of 75 years for one patient and hepatitis B reactivation–associated fulminant hepatitis in the other patient. The remaining chemotherapy nonresponding patients, including one DLBCL(MALT) patient and 4 pure (de novo) DLBCL patients, died of disease progression. The Kaplan-Meier analysis estimated 5-year overall survival rate was 88.9% and 78.3% for patients with pure (de novo) DLBCL receiving frontline antibiotics and chemotherapy, respectively (P = .551, log-rank test) and 94.1% for DLBCL(MALT) patients (Figure 4).
Overall survival of early-stage gastric pure (de novo) DLBCL patients receiving frontline antibiotics and chemotherapy, and gastric DLBCL(MALT) patients receiving frontline antibiotics. Overall survival was calculated from the date of initial treatment to the date of death because of any cause by Kaplan-Meier analysis (antibiotic-treated pure [de novo] DLBCL vs DLBCL[MALT]; P = .882, 2-sided, log-rank test; pure [de novo] DLBCL with antibiotics vs chemotherapy; P = .551, 2-sided, log-rank test). HPE indicates HPE therapy as a frontline treatment; and CT, chemotherapy as a frontline treatment.
Overall survival of early-stage gastric pure (de novo) DLBCL patients receiving frontline antibiotics and chemotherapy, and gastric DLBCL(MALT) patients receiving frontline antibiotics. Overall survival was calculated from the date of initial treatment to the date of death because of any cause by Kaplan-Meier analysis (antibiotic-treated pure [de novo] DLBCL vs DLBCL[MALT]; P = .882, 2-sided, log-rank test; pure [de novo] DLBCL with antibiotics vs chemotherapy; P = .551, 2-sided, log-rank test). HPE indicates HPE therapy as a frontline treatment; and CT, chemotherapy as a frontline treatment.
Discussion
Our study has shown that a substantial portion of patients with localized, HP-positive gastric pure (de novo) DLBCL achieved durable pCR after HPE therapy, an important observation that strongly supports previous anecdotal case reports that suggested that some HP-positive gastric pure (de novo) DLBCL may remain HP-dependent.13-15 We found that pure (de novo) DLBCL responded to HPE therapy more rapidly than DLBCL(MALT). Patients with gastric DLBCL(MALT) had a faster response from high-grade components to HPE therapy than low-grade components (data not shown). Our findings agree with a recent review of HPE treatment for primary high-grade gastric lymphoma that found 2 months was the median time to pCR for responders.27
In contrast, considering that DLBCL can be rapid-growing, our patients whose tumors showed stable disease or progression in the first follow-up endoscopic examination were immediately referred for conventional rituximab-based immunochemotherapy.28-30 All 5 patients with HP-independent pure (de novo) DLBCL achieved pCR after systemic chemotherapy, and they remained alive and recurrence-free at this report. Despite the excellent clinical outcomes of localized gastric pure (de novo) DLBCL after conventional chemotherapy, we advocate the importance of further exploration on the use of antibiotics as one of the frontline treatment options for HP-positive, early-stage gastric pure (de novo) DLBCL based on the following reasons: (1) eradication of HP can achieve drastic and durable pCR in HP-dependent DLBCL, (2) antibiotic therapy is well tolerated and associated with excellent life quality, and (3) HP-independent tumors can be effectively salvaged with systemic chemotherapy.31,32
In accordance with the results of previous reports, our study suggests that depth of tumor invasion is a significant predictor for the response of gastric DLBCL(MALT) to antibiotic treatment.33-36 Although the difference in pure (de novo) DLBCL did not reach statistic significance, it is most likely that this result was simply because of the limited number of patients in the current study. This issue can only be clarified by large-scale prospective studies.
In the current study, we have not only confirmed our previous hypothesis that the extent of large-cell transformation does not affect the response of early-stage DLBCL(MALT) to antibiotics but also further extended the hypothesis to cover gastric DLBCL without MALT features.7,8,37 Although the differential diagnosis between DLBCL(MALT) and pure (de novo) DLBCL cannot be absolutely accurate, the distinction between them may not be that important in making a treatment decision as both of them respond to antibiotics. Moreover, recent lines of evidence indicate that genetic aberrations in the large-cell components of gastric DLBCL(MALT) overlap with those of gastric pure (de novo) DLBCL, including frequent gains on chromosome arm 11q and losses on 6q,38,39 and the results of molecular studies have suggested that gastric DLBCL may be blastic variants of marginal zone lymphoma.40 Our results may be considered as a clinical evidence to support the hypothesis that will further blur the distinction between the 2 disease categories. But in clinical practice, it cannot be overemphasized that patients diagnosed as pure (de novo) DLBCL by endoscopic biopsy can still be cured by simple antibiotics because such potentially aggressive tumors may progress rapidly once refractory to antibiotics treatment and require aggressive immunochemotherapy.
Our findings indicate that the therapeutic efficacy of HPE for stage IE/IIE1 gastric pure (de novo) DLBCL is comparable to those for stage IE/IIE1 gastric DLBCL(MALT). However, our present study is a retrospective, explorative study that is not providing definitive conclusions about the use of antibiotics as the sole frontline therapy for early-stage gastric pure (de novo) DLBCL. These findings should be taken as investigational until validated by prospective studies.
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research grants NSC96-2321-B-002-013, NSC96-2321-B-002-014, NSC96-2314-B-002-164MY3, NSC 98-2314-B-002-087-MY3, and NSC 100-2321-B-002-032 from the National Science Council, Taiwan, and DOH100-TD-B-111-001 from the Department of Health, Taiwan.
Authorship
Contribution: S.-H.K., K.-H.Y., L.-T.C., and A.-L.C. contributed to the study design; S.-H.K., K.-H.Y., M.-S.W., C.-W.L., H.-P.W., L.-T.C., and A.-L.C. treated patients; all authors collected clinical data; S.-H.K., K.-H.Y., M.-S.W., C.-W.L., L.-T.C., and A.-L.C. were involved in data analysis and interpretation; S.-H.K., K.-H.Y., M.-S.W., L.-T.C., and A.-L.C. wrote the manuscript; and all authors revised and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li-Tzong Chen, National Institute of Cancer Research, National Health Research Institutes, No 36, Sheng-Li Road, 70456, Tainan, Taiwan; e-mail: leochen@nhri.org.tw; or Ann-Lii Cheng, Department of Internal Medicine and Department of Oncology, National Taiwan University Hospital, No 7, Chung-Shan South Road, Taipei, Taiwan; e-mail: alcheng@ntu.edu.tw.

![Figure 2. Time to pCR of HP-eradicated patients. Time was calculated from the completion of antibiotic treatment to first evidence of pCR by Kaplan-Meier analysis (pure [de novo] DLBCL vs DLBCL[MALT]; P = .024, 2-sided, log-rank test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-404194/4/m_zh89991289910002.jpeg?Expires=1769195573&Signature=mafEV~4xMhdunhnlpFKrv~x0gma6UmHcBbrb3hgCWjfLZozXY0DphnCv4AJ1QqKtwbRWTXsG75Hy83hDgurYwi8z8jwzFHT-M6zLOp8~h8rpn-LFjcdli1dWD59c~Nm6Q7QN4cfHFdd36VPtwsVcZh9JEpusCJmiQA1MOlQMlFNemjQFNcEL3QF-MSKpR7ZCRtQYGR5MrMbzskoKinqFwIxsqjYd8PWnBCfrPaEPBgJURK5xJLbSAPLv1JvbyYhkF~YRYgzK45WjSsp-El-5ApcaItgeAvE-VrO7kqoShi2QZjJj7LLrOZbbGL3eEhZxQiNf5ecijND5ywBgKaQszA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Overall survival of early-stage gastric pure (de novo) DLBCL patients receiving frontline antibiotics and chemotherapy, and gastric DLBCL(MALT) patients receiving frontline antibiotics. Overall survival was calculated from the date of initial treatment to the date of death because of any cause by Kaplan-Meier analysis (antibiotic-treated pure [de novo] DLBCL vs DLBCL[MALT]; P = .882, 2-sided, log-rank test; pure [de novo] DLBCL with antibiotics vs chemotherapy; P = .551, 2-sided, log-rank test). HPE indicates HPE therapy as a frontline treatment; and CT, chemotherapy as a frontline treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/21/10.1182_blood-2012-01-404194/4/m_zh89991289910004.jpeg?Expires=1769195573&Signature=LjJEBCagXdobYoE8yHOhqxXa4gegfIGeZEjgQOYTd5NLJ3bW7aMfv~wKkodOYij~IoiNGzImuP3Mzvg5Fnb3COhAfJynWVru2Fq4IUIpccaFV32cTtMyxgU1Ka4s7kRIoXQLxsf0TcwtJ1NolBe6F2-KZGKqzHP~RYhYL7WCldSeZolz46IKb0v~ocpd7SSLuXvfRpw8nLIl73pu97a99SmQJnodv8uJ1vbwoHqS6Vt8Ce1GMM6QVQXCncT5ZLHwe6-Ahal4JJ2GKp1P2HWnf5rzAF44RcrO6Dq2kjtl49ZoKG3MhgCH15vVjUSlrVWzDz8UaUTBRLzOgSkuwgG1jQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)