In this issue of Blood, Futosi and colleagues investigate the effects of the multikinase inhibitor dasatinib on mature human neutrophils, helping to explain the increased infection rate in chronic myelogenous leukemia (CML) patients resistant to or intolerant of imatinib. Through careful analysis of neutrophil function, the authors also develop a pathway for the development of kinase inhibitors as anti-inflammatory drugs.1
Dasatinib is a potent adenosine triphosphate and competitive inhibitor of tyrosine kinases. It has been highly effective in patients with imatinib-resistant CML and Philadelphia chromosome-positive acute lymphoblastic leukemia.2 The deregulated activity of the oncogenic tyrosine kinase Bcr-Abl is linked to malignant transformation in CML, which has been successfully treated with imatinib.3 In addition to Bcr-Abl and Abl, dasatinib also inhibits different tyrosine kinases including cKit, platelet-derived growth factor receptor, Eph receptors, and Src- and Btk-family members in malignant and hematopoietic cells.2 Tyrosine kinase inhibitors are also used to treat other diseases including pulmonary fibrosis, asthma, and rheumatoid arthritis. Dasatinib is efficient and safe, although side effects such as rash, fluid retention, bleeding episodes, gastrointestinal symptoms, and myelosuppression are commonly observed.4 Furthermore, treatment with dasatinib increases the risk of infection.5
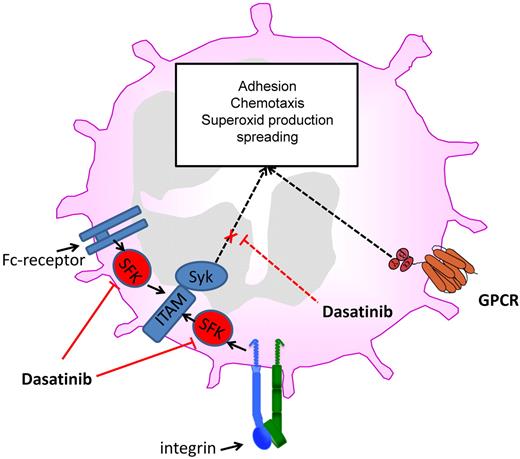
Dasatinib inhibits different neutrophil functions by blocking Src family kinases and downstream signaling.
Dasatinib inhibits different neutrophil functions by blocking Src family kinases and downstream signaling.
Neutrophils belong to the innate immune system and are recruited to sites of inflammation and tissue injury. Recruitment of neutrophils is tightly regulated, and a disruption of this process leads to an altered immune response. Reduced neutrophil recruitment can result in a reduced ability to fight invading microorganisms, whereas an increased neutrophil recruitment may lead to the development of chronic inflammatory disease. The classic neutrophil recruitment cascade consists of capturing, rolling, slow rolling, arrest, postadhesion strengthening, crawling, and paracellular or transcellular transmigration.6 The first contact of neutrophils with endothelial cells is mediated by the interaction of selectins with their counter-receptors that results in neutrophil rolling. During rolling on inflamed endothelium, engagement of G-protein–coupled receptors and adhesion receptors induces the activation of different signaling pathways leading to neutrophil activation.6 Intracellular signaling pathways ultimately couple these receptors to the activation of integrins (inside-out signaling) and subsequently the activated integrins may bind to their counter-receptor.7 After engagement integrins can also transmit signals into the cell (outside-in signaling; see figure).8 This process activates intracellular signaling pathways that mediate postadhesion strengthening, migration, respiratory burst, phagocytosis, and polarization. The important role of different tyrosine kinases for the initiation and regulation of neutrophil recruitment and functional responsiveness is well established.9 Especially the Src family kinases and Btk, both targets of dasatinib (see figure), are critically involved in various neutrophil activation pathways.
In the present study, the authors studied the effect of dasatinib on functional responses of human und murine neutrophils using in vitro and ex vivo assays.1 The potency of dasatinib to inhibit both integrin-dependent and Fc-receptor–dependent neutrophil activation at low concentrations (IC50 ranging from 9-50nM depending on the stimulus) is striking. These receptors share the immunoreceptor tyrosine activation motif (ITAM) mode of activating neutrophils via Src family kinases and Syk (see figure).8 Low nanomolar concentrations of dasatinib inhibited the phosphorylation of Syk and also reduced Syk-dependent cell functions including spreading, adhesion, and exocytosis of secondary granules. However, dasatinib did affect PMA-mediated spreading, suggesting that the drug does not influence the cytoskeletal machinery. As Src family kinases are upstream of Syk in integrin-mediated outside-in signaling,8 it is likely that dasatinib inhibits the activation of Src family kinases (not investigated by Abram and Lowell in this study8 ) and only indirectly reduces phosphorylation of Syk and other downstream targets. Further studies have to address the question of which of the Src family kinases are inhibited in neutrophils by dasatinib, because some protein tyrosine kinases have a unique function in neutrophil activation, whereas other protein tyrosine kinases have overlapping or redundant functions.9,10
Interestingly, low nanomolar concentrations of dasatinib partially inhibited fMLP-induced responses, whereas G-protein–coupled receptor-mediated functions were predominantly not affected by dasatinib. Chemotaxis, adhesion, and migration are all β2-integrin–dependent processes. However, dasatinib only reduced chemotaxis and adhesion but did not affect migration through membranes, suggesting that several protein tyrosine kinases are involved in the different steps. Future studies have to identify the protein tyrosine kinases involved in the different steps. One strength of the current study is its use of dasatinib in clinically relevant concentrations, supporting the notion that some neutrophil functions are inhibited during dasatinib treatment. Here, oral administration of 5 mg/kg of dasatinib to mice significantly reduced tumor necrosis factor-α–induced leukocyte adhesion to FCS-coated surface.
The fact that dasatinib reduces neutrophil adhesion but has no major effect on phagocytosis or killing bacteria suggests that the increased infection rate in patients treated with dasatinib is because of reduced neutrophil recruitment into injured tissue.
Futosi et al attribute the effects of dasatinib to its inhibition of Src family kinases, a critical nonreceptor kinase directly involved in integrin- and Fc-receptor signaling as well as in stabilization of neutrophil adhesion.1 It will be interesting in the future to study bosutinib, which also inhibits Src family kinases but likely not neutrophil Btk, together with dasatinib. These studies will show how much neutrophil inhibition is specifically related to Src family kinase inhibition, and whether bosutinib carries a lower infection rate than dasatinib. The study by Futosi et al provides further proof that neutrophil nonreceptor protein tyrosine kinases, such as the Src family kinases or Syk, may be excellent targets for therapy of chronic inflammatory diseases.1 However, the use of kinase inhibitors in chronic inflammatory disease may require greater selectivity for the target kinase to limit the off-target kinase–induced side effects.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■