Abstract
Anaplastic large-cell lymphomas (ALCLs) bearing the t(2;5) translocation (ALK+ALCLs) are frequently characterized by skin colonization and associated with a poor prognosis. Using conditional transgenic models of anaplastic lymphoma kinase–positive (ALK+) lymphomas and human ALK+ALCL cell lines, in the present study, we show that high-mobility-group box-1 (HMGB-1), a proinflammatory cytokine, is released by ALK+ cells, and demonstrate extracellular HMGB-1–stimulated secretion of the IL-8 chemokine by HaCaT keratinocytes through the involvement of MMP-9, PAR-2, and the NF-κB pathway. Furthermore, we demonstrate that, in vitro, IL-8 is able to induce the invasiveness of ALK+ cells, which express the IL-8 receptors CXCR1 and CXCR2. In vitro and in vivo, HMGB-1 inhibition achieved by glycyrrhizin treatment led to a drastic reduction in ALK+ cell invasiveness. The pathophysiological relevance of our observations was confirmed by demonstrating that the HMGB-1 and IL-8 receptors are expressed in ALK+ALCL biopsies. We have also shown that IL-8 secretion is correlated with leukemic dissemination of ALK+ cells in a significant number of patients. The results of the present study demonstrate for the first time a relationship among the pro-inflammatory mediators HMGB-1, MMP-9, PAR-2, and IL-8. We propose that these mediators create a premetastatic niche within the skin, thereby participating in ALK+ lymphoma epidermotropism.
Introduction
Systemic anaplastic large-cell lymphoma (ALCL) is an aggressive peripheral T-cell lymphoma. There are 2 types of ALCL, classified as anaplastic lymphoma kinase (ALK)–positive (ALK+) or ALK-negative (ALK−) depending on whether the receptor tyrosine kinase ALK is expressed. ALK is activated most frequently through the nonrandom t(2;5) chromosome translocation, resulting in the fusion of the nucleophosmin (NPM) gene from the 5q35 locus to the 2p23 region that encodes ALK.1,2
ALK+ALCLs are characterized by frequent extranodal colonization, particularly in the skin (reported in 20%-30% of cases), and this is associated with a negative prognosis.1 The diagnosis of cutaneous dissemination in ALK+ALCL is not always easy and, in some cases, the original histopathological classification is one of “non-malignant inflammatory disease.”3 Interestingly, an insect bite could be the trigger for cutaneous ALK+ lymphoma metastases. Our group reported 5 cases of systemic ALK+ALCL in which skin lesions presented after an insect bite at the onset of the disease, postulating that bite-associated Ags could result in an influx of T lymphocytes, some of them bearing the t(2;5) translocation.4 The subsequent release of cytokines leading to skin inflammation at the site of the bite could act as a “second hit” eliciting activation of T lymphocytes, which would then express the oncogenic NPM-ALK protein and undergo uncontrolled proliferation and transformation.4
ALK+ALCL patients also present with an inflammatory syndrome characterized by high fever, lymphadenopathy or neutrophilia, release of various circulating inflammatory chemokines such as IL-8,5 and expression of skin-inflammatory biomarkers such as the alarmins heat-shock protein 90 (HSP90), HSP70,6 S100A8, or A11.7 Recent studies have highlighted a role for IL-8 in mediating cutaneous skin inflammation and human keratinocyte hyperplasia associated with acanthosis (thickening of the skin).8 IL-8 secreted by hyperplasic keratinocytes increases T-lymphocyte migration into the skin across both the vascular endothelium and the subendothelial matrix.9
The high-mobility-group box-1 (HMGB-1) alarmin, also called amphoterin, is a highly conserved component of eukaryotic nuclei.10 Interestingly, it can also be released from necrotic and tumoral cells, where it is considered an inflammatory cytokine. T- and B-cell non-Hodgkin lymphoma cells express and release high levels of HMGB-1.11 Its extracellular form binds to inflammatory receptors, such as the receptor for advanced glycation end products (RAGE) and TLR.12,13 HMGB-1 has also been shown to regulate matrix metallopeptidase-9 (MMP-9) levels via NF-κB–related pathways.14,15 In ALK+ALCL, we have demonstrated that MMP-9 is required for NPM-ALK+ cell invasiveness.16 Moreover, MMPs stimulate protease-activated receptors (PARs).17 Activation of PAR-2 results in the production of various cytokines and chemokines by keratinocytes,18 leading to epidermic inflammation via increased NF-κB p65 phosphorylation and secretion of chemokines including IL-8.19,20
Mouse models of ALK+ lymphomas have been developed to help in our understanding of the role of NPM-ALK in tumorigenesis21 ; however, previous models did not find the systemic inflammation common in ALK+ALCL patients. We recently developed a conditional mouse model for NPM-ALK–induced lymphomagenesis, which develops lymphoma that is associated with hyperplasia of the epidermis (acanthosis lesions).22 In 30% of these mice, a lymphomatous infiltration is observed in the dermis below the cutaneous lesions. In this model, we also observed an up-regulation of HMGB-1 mRNA in the lymph nodes and an increase in the serum of CXCL1 (also termed KC), the murine IL-8 functional homolog. Therefore, we investigated the role of these 2 pro-inflammatory cytokines and their downstream signaling in the dissemination of ALK+ cells. The results of the present study show for the first time that HMGB-1 expression and release by lymphoma cells amplifies the keratinocyte secretion of IL-8 via MMP-9, PAR-2, and NF-κB activation, and that this may result in ALK+ lymphoma epidermotropism.
Methods
Cell lines and cell culture conditions
Two t(2;5)(p23;q35)–positive (ALK+) ALCL cell lines were studied: Karpas-299 and SU-DHL-1.23,24 ALK+ALCL cells were maintained in IMDM and HaCaT cells (a human keratinocyte cell line)25 in DMEM at 37°C in a 5% CO2, humidified atmosphere. Media were supplemented with 15% FCS, 2mM l-glutamine, 1mM sodium pyruvate, and penicillin/streptomycin (all from Invitrogen) with or without the recombinant human proteins rhIL-8 (50 ng/mL; PeproTech France), rhHMGB-1 (250 ng/mL; R&D Systems Europe), and pro-rhMMP-9 (750 ng/mL) activated by 1mM p-aminophenylmercuric acetate at 37°C for 24 hours (R&D Systems Europe) and with or without the inhibitors glycyrrhizin (100mM), and GM6001 (10μM; Calbiochem-Merck).
ALK+ALCL supernatants (conditioned media) correspond to the medium from a 24 hours culture of ALK+ALCL cells. HaCaT keratinocytes were exposed to ALK+ALCL supernatants for an additional 24 hours before media were collected and analyzed.
Mice
The generation of transgenic mice expressing NPM-ALK under the tetracycline regulatory system was as described previously.22 Expression of the transgene was obtained by removing doxycycline (100 μg/mL; Sigma-Aldrich Chimie) from the drinking water. Animals used in the experiments were littermates.
Female SCID (C.B-17-Prkdcscid/lcrCrl) mice were obtained from the Centre d'élevage Janvier and used at 6 weeks of age. Karpas-299 cells (10 × 106) were subcutaneously injected into SCID mice. After 7 days, mice were treated (n = 10) or not (n = 10) with glycyrrhizin (IP injection of 10 mg/kg in 100 μL of PBS) for 7 additional days (Calbiochem-Merck). Tumor volume was assessed by calliper measurement. The extent of lung colonization by malignant cells was assessed by anti-ALK (rabbit monoclonal SP8) staining.
All mice were housed under pathogen-free conditions at the Inserm U1043 animal facility (Purpan Hospital, Toulouse, France) afterprotocols approved by Inserm.
Tumor and normal samples
The study was carried out in agreement with institutional review board–approved protocols and the procedures followed were in agreement with the Declaration of Helsinki of 1975, revised in 2000. Diagnosis of ALCL was based on morphologic and immunophenotypic criteria, as described in the latest World Health Organization classification.1,2 The percentage of malignant cells was assessed by ALK1 or CD30 staining and was greater than 80% for all selected cases. Frozen tumor samples from 15 ALK+ALCL and 3 reactive lymph nodes were retrieved from the tumor bank at Toulouse Centre Hospitalier Universitaire and used for RNA extraction. Sera from ALK+ALCL patients (n = 35) and 5 healthy donors were used for ELISA assays.
RNA extraction and real-time RT-PCR analysis
Total RNA from tumors, normal samples, and cell lines was prepared using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. The concentration of RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific). cDNA was synthesized from total RNA (2 μg) using M-MLV reverse transcriptase (Promega France).
All quantitative RT-PCRs (qRT-PCRs) were performed using an ABI 7300 Real-Time instrument (Applied Biosystems). Expression of the GAPDH, IL-8, CXCR1, CXCR2, PAR-2, and HMGB-1 human genes was estimated by qRT-PCR using the following primers: IL-8 forward primer: 5′-CTGGCCGTGGCTCTCTTG-3′ and reverse primer: 5′-CCTTGGCAAAACTGCACCTT-3′; CXCR1 forward primer: 5′-AGCCAGATCACCTTCCACACACAA-3′ and reverse primer: 5′-GCAAGGAGTTCTTGGCACGTCATT-3′; CXCR2 forward primer: 5′-AGCAGGAAGATGAGGACAACAGCA-3′ and reverse primer: 5′-ACAATACAGCAAACTGGCGGATGC-3′; PAR-2 forward primer: 5′-GGCCGCCATCCTGCTAG-3′ and reverse primer: 5′- TGTGCCATCAACCTTACCAATAA-3′; HMGB-1 forward primer: 5′-GCAGATGACAAGCAGCCTTA-3′ and reverse primer: 5′-TTTGCTGCATCAGGCTTTCC-3′. GAPDH was used as a reference (forward primer: 5′-CGGGAAGCTTGTGATCAATGG-3′ and reverse primer: 5′- GGCAGTGATGGCATGGACTG-3′). qRT-PCR was performed using SYBR Green real-time PCR master mix (Eurogentec). The PCR mixture (25 μL) consisted of 12.5 μL of master mixture, 0.75 μL for each PCR primer at 10μM, 5 μL of diluted cDNA (1:20) and 6 μL of DNase- and RNase-free water. qRT-PCR was performed using 40 cycles of 15 seconds at 90°C and 1 minute at 60°C. The relative fold changes in transcript expression were calculated by the ΔΔCT method and values are expressed as 2(−ΔΔCT). Detected target transcripts were normalized to the endogenous housekeeping gene GAPDH.
IL-8 and HMGB-1 quantification
IL-8 and HMGB-1 were detected in sera from ALK+ALCL patients and healthy donors using the human IL-8 Quantikine ELISA kit (R&D Systems Europe) or HMGB-1 ELISA (IBL) according to the manufacturers' instructions. IL-8 was also quantified in ALK+ALCL cell-line culture supernatants.
Western blotting
Expression of NF-κB p65, phospho-Ser536 NF-κB p65, HMGB-1, and β-actin was evaluated by Western blotting. Abs used were rabbit polyclonal anti–human NF-κB p65 at 1:750 (Cell Signaling Technology–Ozyme), rabbit polyclonal anti–human HMGB-1 at 1:1000 (Abcam), rabbit monoclonal anti–human phospho-Ser536 NF-κB p65 at 1:1000 (93H1; Cell Signaling Technology–Ozyme), and mouse monoclonal anti–human β-actin (Sigma-Aldrich Chimie) at 1:20 000, and was followed by goat anti–rabbit HRP or goat anti–mouse HRP. Visualization of the immunoreactive bands was performed by enhanced chemiluminescence (GE Healthcare Europe). Densitometric analysis was performed using GeneTools 3.06.04 software from Syngene.
siRNA transfections
siRNA transfections were performed using lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocols. The following siRNAs were used: synthetic si-control 1 (Ctrl siRNA; Eurogentec), PAR-2 siRNA (50nM; Santa Cruz Biotechnology), or RAGE siRNA (100nM; Santa Cruz Biotechnology). Twenty-four hours later, ALK+ALCL supernatant or recombinant human proteins were added to HaCaT cells. Cells and supernatants were collected 48 hours after transfection. RNA and protein extractions were performed for subsequent analysis by qRT-PCR or Western blotting using the AllPrep kit (QIAGEN).
Proliferation assay
Cells were seeded in 96-well plates at low density (5 × 104 cells/100 μL) and cultured in IMDM supplemented with 15% FCS with or without glycyrrhizin (100μM). Cell growth was monitored daily for 5 days using a CellTiter 96 Aqueous One solution Cell Proliferation Assay (Promega France) according to the manufacturer's instructions. The formation of formazan through cleavage of the MTS tetrazolium compound in metabolically active cells was measured by monitoring absorbance at 490 nm using a spectrophotometer. All assays were performed in triplicate.
Matrigel invasion assays
The Matrigel invasion assay measures the ability of ALCLs to invade a dense Matrigel matrix (BD Biosciences) that mimics the basement membranes. A total of 2 × 105 overnight serum-starved ALCL cells were labeled for 90 minutes with 0.5mM CellTracker Orange CMTMR (Invitrogen). After washing, cells were seeded in Labtek chambers and overlaid with Matrigel at 5 mg/mL. After 90 minutes at 37°C, medium containing 15% FCS with or without 50 ng/mL of IL-8 was added. After 24 hours, cells were analyzed by confocal microscopy (LSM510; Carl Zeiss) using a 20× objective. Optical Z sections were taken every 2.5 μm starting from the base and extending 100 μm into the Matrigel. To quantify invasion, CMTMR fluorescent cells in the sections above 20 μm were added and then divided by the sum of cells in all of the Z sections. Data represent the analysis of 200 cells per condition. Three-dimensional imaging and cell quantification were realized using Imaris Version 6.4.2 software (Bitplane AG).
Immunohistochemistry
Sections (5 μm) from formalin-fixed and paraffin-embedded human and mouse tissues were cut, deparaffinized, subjected to heat Ag retrieval, and stained with several mAbs or polyclonal Abs directed against B220/CD45R (rat mAb; clone RA3-6B2; 1:400), rabbit anti-ALK mAb (clone SP8; 1:100; Lab VisionCorporation), CXCR1 (clone 42 705; 1:500; R&D Systems France), and CXCR2 (clone 48 311; 1:200; R&D Systems France). CXCR1 and CXCR2 expression were detected in frozen sections and Ab binding was revealed using the streptavidin-biotin complex (ABC) alkaline phosphatase kit (Vector Laboratories). ALK and B220/CD45R expression were detected in fixed, paraffin-embedded sections and Ab binding was revealed using the ABC-peroxidase kit (Vector Laboratories). Ab binding was detected using a Leica DMR microscope equipped with the DFC300FX camera and a 200×/0.85 numerical aperture objective lens. Image processing was performed using IM50 software (Leica Microsystems).
Statistical analysis
Values were expressed as means ± SD. The nonparametrical Mann-Whitney test and/or unpaired t test or 2-way ANOVA was applied to analyze the differences between groups. Analyses were performed using Prism Version 4.00 software for Windows (GraphPad). P < .05, P < .01, and P < .001 were considered to be significant.
Results
Culture supernatant collected from ALK+ALCL cell lines up-regulates keratinocyte IL-8 secretion and NF-κB activation via HMGB-1 secretion
We compared cytokine production for a keratinocyte HaCaT cell line, ALK+ALCL cell lines (Karpas-299 and SU-DHL-1), and HaCaT cells exposed to Karpas-299 or SU-DHL-1 cell line culture supernatants. Initially, we used a cytokine Ab array (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) with visual interpretation of the results, which suggested an increased expression of RANTES, Serpin E1, sICAM-1, and MIF in the Karpas-299 supernatant (supplemental Figure 1C); sICAM-1, MIF, and IL-10 in the SU-DHL-1 supernatant (supplemental Figure 1D); and GROα, MIF, IL-1Rα, Serpin E1, and IL-8 in the HaCaT supernatant (supplemental Figure 1E). Interestingly, when HaCaT cells were exposed to supernatant collected from a culture of the ALK+ALCL cell lines Karpas-299 (supplemental Figure 1F) or SU-DHL-1 (supplemental Figure 1G), only IL-8 secretion increased. Therefore, we performed ELISA assays to accurately measure IL-8 secretion. As shown in Figure 1A, we confirmed that IL-8 was secreted by HaCaT cells but not by ALK+ALCL cell lines. Accordingly, qRT-PCR detected IL-8 mRNA only in keratinocytes (Figure 1A). ELISA assays performed on culture medium collected from HaCat cells exposed to ALK+ALCL cell line supernatants demonstrated a significant increase in IL-8 (Figure 1B). These data suggest that the observed IL-8 release by keratinocytes is stimulated by a soluble factor secreted by ALK+ALCL cells.
Culture supernatant collected from ALK+ALCL cell lines up-regulates IL-8 secretion and NF-κB activation by keratinocytesviaHMGB-1 secretion. Culture supernatants were collected from keratinocytes (HaCaT) or ALK+ALCL (Karpas-299 and Su-DHL-1) cells (A) and HaCaT cells exposed to Karpas-299 and SU-DHL-1 cell line supernatants for 24 hours; and (B) IL-8 production was measured by ELISA. IL-8 mRNA levels were also evaluated by qRT-PCR in Karpas-299 (Karpas), SU-DHL-1 and HaCat (keratinocytes) cell lines (A). (C) Western blotting was performed to monitor Ser536 phosphorylation of NF-κB p65 from keratinocytes exposed to Karpas-299 or SU-DHL-1 cell culture supernatants for 24 hours or those exposed to DMEM as a control. qRT-PCR (D) and Western blotting (E) were performed to determine IL-8 mRNA levels and NF-κB activation in keratinocytes treated by the rhHMGB-1 cytokine or not (PBS). Results are expressed as the mean ± SD of 3 independent experiments done in triplicate. *P < .05 and **P < .01 by Student t test.
Culture supernatant collected from ALK+ALCL cell lines up-regulates IL-8 secretion and NF-κB activation by keratinocytesviaHMGB-1 secretion. Culture supernatants were collected from keratinocytes (HaCaT) or ALK+ALCL (Karpas-299 and Su-DHL-1) cells (A) and HaCaT cells exposed to Karpas-299 and SU-DHL-1 cell line supernatants for 24 hours; and (B) IL-8 production was measured by ELISA. IL-8 mRNA levels were also evaluated by qRT-PCR in Karpas-299 (Karpas), SU-DHL-1 and HaCat (keratinocytes) cell lines (A). (C) Western blotting was performed to monitor Ser536 phosphorylation of NF-κB p65 from keratinocytes exposed to Karpas-299 or SU-DHL-1 cell culture supernatants for 24 hours or those exposed to DMEM as a control. qRT-PCR (D) and Western blotting (E) were performed to determine IL-8 mRNA levels and NF-κB activation in keratinocytes treated by the rhHMGB-1 cytokine or not (PBS). Results are expressed as the mean ± SD of 3 independent experiments done in triplicate. *P < .05 and **P < .01 by Student t test.
Because induction of secretion of the IL-8 protein could result from the stimulation of the NF-κB pathway, we investigated NF-κB activation in keratinocytes exposed to ALK+ALCL supernatants. Western blot analysis showed that, compared with keratinocytes cultured in IMDM control medium, the increase in IL-8 secretion paralleled the elevation of phosphorylation of NF-κB p65 on Ser536, a modification required for efficient transcriptional activation and nuclear function of NF-κB (Figure 1C).
Because T- and B-cell non-Hodgkin lymphoma cells release high levels of the pro-inflammatory cytokine HMGB-1,11 we investigated whether HMGB-1 could be the soluble factor inducing IL-8 release by HaCaT cells. We first analyzed HMGB-1 expression in Karpas-299 and SU-DHL-1 cells. By performing qRT-PCR, we observed HMGB-1 mRNA expression in these ALK+ALCL cell lines (supplemental Figure 2A). In addition, Western blotting performed on the culture supernatant revealed that both ALK+ALCL cell lines also secreted HMGB-1 (supplemental Figure 2B). To confirm that HMGB-1 was the soluble factor responsible for enhanced IL-8 release by keratinocytes in the ALK+ALCL culture supernatant, we cultured HaCaT cells in medium supplemented with rhHMGB-1. As expected, we observed that rhHMGB-1 significantly enhanced IL-8 mRNA expression (P = .0171; Figure 1D), with a concomitant elevation of phosphorylation of NF-κB p65 on Ser536 (Figure 1E).
MMP-9 secreted by ALK+ALCL cell lines enhances IL-8 expression by keratinocytes through PAR-2 and NF-κB activation
It has been demonstrated that: (1) HMGB-1 induces MMP-9 expression15 ; (2) MMP-9 is required for NPM-ALK+ cell invasiveness both in vitro and in vivo16 ; and (3) MMPs activate PARs.17 We therefore evaluated the impact of MMP-9 on IL-8 release by keratinocytes. Cells were cultured with rhMMP-9. As expected, qRT-PCR demonstrated that MMP-9 can also up-regulate IL-8 mRNA expression by keratinocytes (P < .001; Figure 2A). This effect was reduced in HaCaT cells when PAR-2 was knocked down by siRNA (siPAR-2; P < .001; Figure 2A). The PAR-2 mRNA knockdown was confirmed by qRT-PCR (fold inhibition: 2.5 and 3.5 for siPAR-2 without or with rhMMP-9, respectively; supplemental Figure 3A-B). To confirm that MMP-9 secreted by ALK+ cells could also partake in IL-8–enhanced secretion by keratinocytes, we cultured HaCaT cells in ALK+ALCL culture supernatant (also called conditioned medium) supplemented with GM6001, an MMP inhibitor. As shown in Figure 2B, qRT-PCR showed a significant decrease in IL-8 mRNA in keratinocytes cultured in conditioned medium from Karpas-299 (P < .001) and SU-DHL-1 cells (P < .001) in the presence of GM6001. In addition, we observed that the reduction in IL-8 mRNA expression by GM6001 was concomitant with a decrease in NF-κB p65 phosphorylation (using conditioned medium from Karpas-299; Figure 2C).
ALK+ALCL cells secrete MMP-9, which enhances IL-8 mRNA expression by keratinocytes through PAR-2 and NF-κB activation. (A) IL-8 mRNA levels were evaluated by qRT-PCR on PAR-2– or control siRNA–transfected HaCaT cells cultured in DMEM supplemented with (+) or without (−) rhMMP-9. (B) HaCaT cells exposed to culture supernatant from Karpas-299 (Karpas) and SU-DHL-1 cells supplemented with 10μM (+) or without (−) MMP inhibitor (GM6001) for 24 hours were collected and IL-8 mRNA measured by qRT-PCR. Results are expressed as the mean ± SD of 3 independent experiments done in triplicate. ***P < .001 by Student t test. (C) Western blotting was performed with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs to determine NF-κB activation in keratinocytes (HaCaT cell line) under the influence of culture supernatant from Karpas-299 cells supplemented with (+) or without (−) GM6001. β-actin was used as a loading control.
ALK+ALCL cells secrete MMP-9, which enhances IL-8 mRNA expression by keratinocytes through PAR-2 and NF-κB activation. (A) IL-8 mRNA levels were evaluated by qRT-PCR on PAR-2– or control siRNA–transfected HaCaT cells cultured in DMEM supplemented with (+) or without (−) rhMMP-9. (B) HaCaT cells exposed to culture supernatant from Karpas-299 (Karpas) and SU-DHL-1 cells supplemented with 10μM (+) or without (−) MMP inhibitor (GM6001) for 24 hours were collected and IL-8 mRNA measured by qRT-PCR. Results are expressed as the mean ± SD of 3 independent experiments done in triplicate. ***P < .001 by Student t test. (C) Western blotting was performed with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs to determine NF-κB activation in keratinocytes (HaCaT cell line) under the influence of culture supernatant from Karpas-299 cells supplemented with (+) or without (−) GM6001. β-actin was used as a loading control.
The PAR-2 receptor of keratinocytes is required for the effects of HMGB-1 on IL-8 synthesis and NF-κB activation
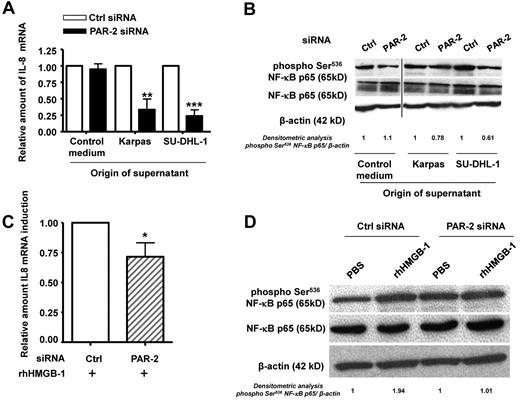
Because MMP-9–dependent IL-8 secretion and NF-κB activation were found to rely on PAR-2, we sought to evaluate whether this receptor is also involved in the downstream effects of HMGB-1, which is known to bind to the inflammatory receptor RAGE. RAGE was found to be weakly expressed by both unstimulated HaCat cells and HaCat cells exposed to the ALK+ALCL cell line supernatant (supplemental Figure 4A). The HMGB-1 effects on IL-8 secretion and NF-κB activation were not significantly affected when RAGE was knocked down by siRNA, suggesting that these could only be partially RAGE dependent in HaCaT cells cultured with rhHMGB-1 (supplemental Figure 4B) or exposed to ALK+ALCL culture supernatant (data not shown). We then investigated a potential central role for PAR-2 signaling in IL-8 up-regulation using siRNA (siPAR-2) to knock down PAR-2 in HaCaT cells exposed to the ALK+ALCL cell line supernatant. qRT-PCR showed a significant decrease in IL-8 mRNA in keratinocytes exposed to Karpas-299 and SU-DHL-1 supernatants (Figure 3A). Concomitantly, Western blotting demonstrated that after PAR-2 knockdown, there was a decrease in NF-κB p65 Ser536 phosphorylation (Figure 3B). The decrease in PAR-2 mRNA was confirmed using qRT-PCR (fold inhibition: 6.8 and 12 for Karpas-299 and SU-DHL-1, respectively; supplemental Figure 3C). These data suggest that PAR-2 is the required intermediate between the soluble factors secreted by ALK+ALCL cell lines and NF-κB–dependent expression of IL-8 by keratinocytes.
HMGB-1 induces IL-8 mRNA expression and NF-κB activation via PAR-2 receptors by keratinocytes. (A) IL-8 mRNA levels were evaluated by qRT-PCR on PAR2- or control siRNA–transfected keratinocytes (HaCaT cell line) exposed to Karpas-299 (Karpas) and SU-DHL-1 cell line (ALK+ALCL) supernatants for 24 hours. The level of IL-8 mRNA was normalized to that from HaCaT cells cultured in DMEM (control medium). (B) Protein lysates from HaCaT cells exposed or not to ALK+ALCL cell line supernatants were subjected to western-blotting with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs. β-actin was used as a loading control. A vertical line has been inserted to indicate a repositioned gel lane. (C-D) The same experiments as in panels A and B were performed on PAR2- or control siRNA–transfected HaCaT cells treated with rhHMGB-1 or not (PBS). IL-8 mRNA levels were normalized to the endogenous housekeeping gene GAPDH. Results are expressed as the fold increase compared with the control PBS condition (expression level = 1), and represent the mean ± SD of 3 independent experiments done in triplicate; *P < .05 by Student t test (C). Lysates from HaCaT cells were subjected to Western blotting with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs. β-actin was used as a loading control (D).
HMGB-1 induces IL-8 mRNA expression and NF-κB activation via PAR-2 receptors by keratinocytes. (A) IL-8 mRNA levels were evaluated by qRT-PCR on PAR2- or control siRNA–transfected keratinocytes (HaCaT cell line) exposed to Karpas-299 (Karpas) and SU-DHL-1 cell line (ALK+ALCL) supernatants for 24 hours. The level of IL-8 mRNA was normalized to that from HaCaT cells cultured in DMEM (control medium). (B) Protein lysates from HaCaT cells exposed or not to ALK+ALCL cell line supernatants were subjected to western-blotting with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs. β-actin was used as a loading control. A vertical line has been inserted to indicate a repositioned gel lane. (C-D) The same experiments as in panels A and B were performed on PAR2- or control siRNA–transfected HaCaT cells treated with rhHMGB-1 or not (PBS). IL-8 mRNA levels were normalized to the endogenous housekeeping gene GAPDH. Results are expressed as the fold increase compared with the control PBS condition (expression level = 1), and represent the mean ± SD of 3 independent experiments done in triplicate; *P < .05 by Student t test (C). Lysates from HaCaT cells were subjected to Western blotting with anti–NF-κB p65 and anti–phospho-Ser536 NF-κB p65 Abs. β-actin was used as a loading control (D).
We then added rhHMGB-1 to HaCaT cells transfected with siRNA directed against PAR-2 (siPAR-2). Knock-down was confirmed by qRT-PCR (fold inhibition: 9.6 and 11.6 for siPAR-2 without or with rhHMGB-1, respectively; supplemental Figure 3D). As shown in Figure 3C and D, IL-8 mRNA expression (P = .0257) was reduced and elevation of NF-κB p65 phosphorylation was not observed in HaCaT cells cultured with rhHMGB-1 after PAR-2 knockdown. A similar pattern was seen after the addition of rhMMP-9 (supplemental Figure 3B and data not shown), suggesting that HMGB-1 and MMP-9 are 2 soluble mediators secreted by ALK+ALCL cell lines that induce IL-8 expression in keratinocytes.
rhIL-8 enhances the invasiveness of ALK+ALCL cells
In humans, IL-8 elicits its effects by binding to 2 receptors, CXCR1 and CXCR2, on the surface of T lymphocytes.26 We used qRT-PCR to demonstrate that CXCR1 and CXCR2 mRNA is found in both Karpas-299 and SU-DHL-1 (data not shown). Flow cytometric analysis showed that both ALK+ALCL cell lines express the 2 IL-8 receptors on their surface (supplemental Figure 5A).
We then evaluated the in vitro response to exogenous rhIL-8 in terms of cell proliferation and invasion. As reported in supplemental Figure 5B, no change in proliferation was observed after rhIL-8 treatment of SU-DHL-1 cells. In contrast, a significant increase in invasion was observed in response to rhIL-8 (supplemental Figure 5C). These data show that recombinant IL-8 can increase invasiveness but not proliferation of ALK+ALCL cells in vitro.
Pharmacologic inhibition of released HMGB-1 impairs lymphoma-cell dissemination in vitro and in vivo
We then examined the effects of HMGB-1 inhibition on ALK+ALCL invasion using glycyrrhizin, an inhibitor of HMGB-1.27 First, Karpas-299 and SU-DHL-1 cells were treated daily for 5 days with glycyrrhizin to assess its effect on cell viability. As shown in Figure 4A, ALK+ALCL cell growth was not affected by the inhibitor. Second, we compared the invasiveness of ALK+ALCL cells under a gradient of control or conditioned medium collected from HaCaT cells exposed to ALK+ALCL culture supernatant with or without glycyrrhizin. As shown in Figure 4B, in ALK+ALCL cells treated with glycyrrhizin in HaCaT-conditioned medium, invasion was strongly reduced (fold inhibition: 12.3, P < .001, and 4.5, P < .01, for Karpas-299 and SU-DHL-1, respectively), demonstrating that HMGB-1 is required for ALK+ALCL cell invasiveness.
Pharmacologic inhibition of HMGB-1 impairs ALK+ cell dissemination in vitro and in vivo. Karpas-299 (Karpas) and SU-DHL-1 cells were incubated or not with 100mM glycyrrhizin. (A) The effect of glycyrrhizin on cell number was measured by following the absorbance at 490nm using the MTS assay. (B) ALK+ALCL cells cultured with supernatant from HaCaT cells previously exposed to Karpas-299 or SU-DHL-1 cell culture supernatants supplemented or not with 100mM glycyrrhizin were subjected to the 3D-Matrigel invasion assay described for Figure 5B. (C-E) Karpas-299 cells were subcutaneously injected into SCID mice. Tumor development progressed for 7 days, followed by 7 days of treatment with glycyrrhizin (one 10mg/kg IP injection/d) or PBS. (C) Tumor volume was measured every 2 days using callipers. (D) Histogram represents the mean ± SD of ALK+ cells infiltrating the lungs. The number of lymphoma cells was assessed by anti-ALK (rabbit monoclonal SP8) staining. (E) Histopathological examination of lung from xenografted NPM–ALK+ Karpas-299 mice shows that HMGB-1 inactivation leads to a decrease in ALK+ cells in the lungs. Mice were treated as described previously: lungs were harvested, fixed, and paraffin embedded for immunohistochemical analysis with anti-ALK (Sp8) to visualize invasion by ALK+ cells (original magnification, 400×).
Pharmacologic inhibition of HMGB-1 impairs ALK+ cell dissemination in vitro and in vivo. Karpas-299 (Karpas) and SU-DHL-1 cells were incubated or not with 100mM glycyrrhizin. (A) The effect of glycyrrhizin on cell number was measured by following the absorbance at 490nm using the MTS assay. (B) ALK+ALCL cells cultured with supernatant from HaCaT cells previously exposed to Karpas-299 or SU-DHL-1 cell culture supernatants supplemented or not with 100mM glycyrrhizin were subjected to the 3D-Matrigel invasion assay described for Figure 5B. (C-E) Karpas-299 cells were subcutaneously injected into SCID mice. Tumor development progressed for 7 days, followed by 7 days of treatment with glycyrrhizin (one 10mg/kg IP injection/d) or PBS. (C) Tumor volume was measured every 2 days using callipers. (D) Histogram represents the mean ± SD of ALK+ cells infiltrating the lungs. The number of lymphoma cells was assessed by anti-ALK (rabbit monoclonal SP8) staining. (E) Histopathological examination of lung from xenografted NPM–ALK+ Karpas-299 mice shows that HMGB-1 inactivation leads to a decrease in ALK+ cells in the lungs. Mice were treated as described previously: lungs were harvested, fixed, and paraffin embedded for immunohistochemical analysis with anti-ALK (Sp8) to visualize invasion by ALK+ cells (original magnification, 400×).
To evaluate the impact of HMGB-1 inhibition on the dissemination properties of ALK+ cells in vivo, Karpas-299 cells were subcutaneously engrafted into immunocompromised SCID mice and gave rise to rapidly growing tumors within 7 days. Tumor-bearing animals were then injected every day with IP doses of glycyrrhizin or PBS (control). Neither glycyrrhizin nor PBS treatment blocked tumor development (Figure 4C). To investigate the dissemination of lymphoma cells, immunohistochemistry was performed on tissue sections from lung using an anti-ALK Ab. Karpas-299 cells were found in all tested lung samples from control mice, confirming that subcutaneously xenografted ALK+ALCL cells were highly invasive. In contrast, the glycyrrhizin-injected mice showed a dramatic decrease in ALK+ cells present in lung samples (Figure 4D, P = .0028, and E).
Keratinocyte hyperplasia promotes skin migration of ALK+ cells in an NPM-ALK conditional mouse model
Because lymphomatous infiltration is observed in the dermis under skin lesions in 30% of conditional transgenic mice developing NPM-ALK lymphomas, we investigated whether cutaneous hyperplasia could induce tumoral cells to migrate to the skin. We analyzed the location of lymphoma cells in the skin of conditional transgenic mice maintained without doxycycline (thereby inducing expression of NPM-ALK) for 10, 15, 20, or 30 days after birth. Ten days after birth, spleen and skin architectures were normal (Figure 5A-B). As shown in Figure 5, we observed that the spleen was obliterated by sheets of ALK+ cells 15 days after birth (Figure 5C,E,G), and we noticed a hyperplasia of the epidermis 5 days later (compare Figure 5D, F, and H), suggesting that the lymphoma cells secreted cutaneous pro-inflammatory cytokines.
Concomitant increase of serum CXCL1/KC and skin hyperplasia are a prerequisite to NPM-ALK+ lymphoma cell epidermotropism. (A-H) Histological analysis of lymphoma cell dissemination in a conditional mouse transgenic model. Conditional NPM-ALK lymphoma mouse models develop ALK+ B-cell (B220+) lymphomas. Images show normal architecture and negative lymphoma cell staining of spleen (A) and skin 10 (B) and 15 days (D) after birth. Lymphoma cells positive for ALK staining are observed in the spleen 15 (C), 20 (E), and 30 (G) days after birth. Note that the skin presents a hyperplasia of the epidermis and is negative for lymphoma cells staining (B220−; F). In contrast, 30 days after birth, 30% of the animals display B220+ tumoral cells infiltrated below the skin lesions (H; right inset shows magnification of bracket). Original magnification was 400× for the spleen and 100× for the skin, except in panel H, where the original magnification was 50× and 640×, respectively (right inset).
Concomitant increase of serum CXCL1/KC and skin hyperplasia are a prerequisite to NPM-ALK+ lymphoma cell epidermotropism. (A-H) Histological analysis of lymphoma cell dissemination in a conditional mouse transgenic model. Conditional NPM-ALK lymphoma mouse models develop ALK+ B-cell (B220+) lymphomas. Images show normal architecture and negative lymphoma cell staining of spleen (A) and skin 10 (B) and 15 days (D) after birth. Lymphoma cells positive for ALK staining are observed in the spleen 15 (C), 20 (E), and 30 (G) days after birth. Note that the skin presents a hyperplasia of the epidermis and is negative for lymphoma cells staining (B220−; F). In contrast, 30 days after birth, 30% of the animals display B220+ tumoral cells infiltrated below the skin lesions (H; right inset shows magnification of bracket). Original magnification was 400× for the spleen and 100× for the skin, except in panel H, where the original magnification was 50× and 640×, respectively (right inset).
The conditional NPM-ALK lymphoma mouse model develops ALK+ B-cell (B220/CD45RA+) lymphoma.22 In these mice, abnormal ALK+ lymphoid cells are found in the blood and the skin. However, because the level of ALK expression is not homogenous, we evaluated the accumulation of lymphoma cells in the skin by staining with the B-cell marker B220/CD45RA, as described previously.22 We observed a massive cutaneous infiltration of lymphoma cells below the skin hyperplasia 30 days after birth (Figure 5H right inset shows magnification of bracket). These data suggest that skin hyperplasia is a prerequisite for ALK+ cell epidermotropism.
In an attempt to unravel the mechanism of ALK+ lymphoma metastasis to the skin, we used transcriptomic analysis to compare lymph nodes from NPM-ALK–transgenic mice with normal lymph nodes from age-matched wild-type littermate mice. HMGB-1 mRNA was overexpressed in lymphoma cells using a transcriptome array (mean value, 2.364 ± 0.259; data not shown). Moreover, an overproduction of serum CXCL1/KC, the murine IL-8 functional homolog, was detected only from mice with ALK+ tumors using a cytokine Ab array (pixel density: 2142e + 006 ± 47 279; data not shown). No CXCL1/KC serum secretion was observed in wild-type transgenic littermate mice or in NPM-ALK OFF healthy mice. In addition, we observed that in conditional transgenic mice maintained without doxycycline for 10, 15, 20, or 30 days after birth, the CXCL1/KC mRNA found in skin hyperplasia increased progressively over time (Figure 5I). These results strongly support the notion that HMGB-1 and IL-8 are involved in the migration of ALK+ cells into the skin during metastasis.
ALK+ALCL patients secrete HMGB-1 and express IL-8 receptors associated with increased IL-8 serum levels
We then proceeded to validate our data using lymph node biopsies from ALK+ALCL patients. The expression of HMGB-1 was determined using qRT-PCR on lymph node biopsies from 15 ALK+ALCL patients. To measure HMGB-1 levels in normal lymph nodes, the amount of HMGB-1 mRNA was determined in 3 lymph nodes from patients who underwent surgery because of a benign disease. These 3 reactive lymph nodes were used to calibrate the assay (expression level = 1; Figure 6A). We noted a significant increase in HMGB-1 mRNA in ALK+ALCL biopsies compared with the reactive lymph nodes (mean, 2.215 ± 0.342, P < .01; Figure 6A). A significant increase in serum HMGB-1 levels was also observed by performing ELISA (ALK+ALCL: mean, 41.74 ± 7.49; healthy donors: mean, 5.51 ± 1.41; P = .0035; Figure 6B). In addition, qRT-PCR and immunohistochemistry were also used to determine the levels of CXCR1 and CXCR2 in lymph nodes from ALK+ALCL patients. CXCR1 and CXCR2 mRNA were observed (Figure 6C), as well as the expression of both CXCR proteins (Figure 6D). Using immunochemistry, tumoral cells show a cytoplasmic staining for both CXCR proteins, with variations in the intensity of the staining, as illustrated in Figure 6D showing a case of strong staining (right image) alongside a weaker staining (left image). As expected, vessels admixed with lymphoma cells were positive for both CXCR proteins and were used as an internal positive control (Figure 6D asterisks). Finally, we measured serum IL-8 cytokine levels in ALK+ALCL patients using an ELISA assay (Figure 6E) and found that only samples from patients with leukemic dissemination expressed serum IL-8. Patients with serum IL-8 displayed both blood and BM colonization by NPM-ALK+ cells. These data suggest a close relationship among the proinflammatory cytokine HMGB-1, expression of the IL-8 receptor, and secretion of IL-8 in the dissemination of tumoral cells in a significant number of human ALK+ALCL patients.
HMGB-1, IL-8, and IL-8 receptor (CXCR1 and CXCR2) expression by ALK+ALCL patients. HMGB-1 (A) or CXCR1 and CXCR2 (C) mRNAs levels were evaluated by qRT-PCR on 15 ALK+ALCL biopsies. mRNA amounts were normalized to levels found in reactive lymph nodes. Results are expressed as the mean ± SD of independent experiments done in triplicate. **P < .01 by Student t test. (B,E) HMGB1 and IL-8 secretion was measured using an ELISA assay on sera from 35 ALK+ALCL patients with (n = 20) or without (n = 7) leukemic dissemination and from healthy patients (n = 10). The number of biopsies in each group corresponds to the n value. (D) CXCR1 and CXCR2 immunohistochemical staining of lymph nodes from 2 ALK+ALCL patients. Sections were stained with anti-CXCR1 or anti-CXCR2 Abs, and nuclei were counterstained with hematoxylin. Vessels were positive for both CXCR staining and were used as an internal control (asterisks). Staining intensity varies from tumoral cell to cell. Original magnification, 200×.
HMGB-1, IL-8, and IL-8 receptor (CXCR1 and CXCR2) expression by ALK+ALCL patients. HMGB-1 (A) or CXCR1 and CXCR2 (C) mRNAs levels were evaluated by qRT-PCR on 15 ALK+ALCL biopsies. mRNA amounts were normalized to levels found in reactive lymph nodes. Results are expressed as the mean ± SD of independent experiments done in triplicate. **P < .01 by Student t test. (B,E) HMGB1 and IL-8 secretion was measured using an ELISA assay on sera from 35 ALK+ALCL patients with (n = 20) or without (n = 7) leukemic dissemination and from healthy patients (n = 10). The number of biopsies in each group corresponds to the n value. (D) CXCR1 and CXCR2 immunohistochemical staining of lymph nodes from 2 ALK+ALCL patients. Sections were stained with anti-CXCR1 or anti-CXCR2 Abs, and nuclei were counterstained with hematoxylin. Vessels were positive for both CXCR staining and were used as an internal control (asterisks). Staining intensity varies from tumoral cell to cell. Original magnification, 200×.
Discussion
Inflammatory mediators play important roles in the development and progression of cancer. Therefore, agents modulating inflammation, such as aspirin and nonsteroidal anti-inflammatory drugs, decrease the incidence of some cancers,28 supporting the notion of inflammation-based cancers.
Recent studies have shown that abnormal persistence of endogenous damage-associated molecular pattern (DAMP) molecules, or alarmins, in chronic inflammation and tumor microenvironments underlies carcinogenesis and tumor progression.29 The alarmin family includes cytosolic calcium-binding proteins of the S100 family, HSPs, and HMGB proteins. It is becoming increasingly clear that the S100A8/A9 and HSP90 proteins, which are found in ALK+ALCL cells,6,7 are involved in many aspects of tumor growth and metastasis.16,29 Although human primary lymphomas often overexpress HMGB-1 compared with normal samples,11 until now, the role of HMGB-1 in ALK+ALCL development and extranodal spreading had not been studied. Using cells expressing NPM-ALK and conditional mouse models,22 we report for the first time that HMGB-1 is expressed and secreted by ALK+ tumoral cells. HMGB-1 promotes inflammatory responses and is involved in inflammation-associated skin tumors.30,31 From 20%-30% of ALK+ALCL extranodal colonization occurs in the skin,3 and HMGB-1 stimulates keratinocytes to release several chemokines, including IL-8, which recruits leucocytes into the skin. Therefore, extracellular HMGB-1 has emerged as a regulator of cell motility.32 In the present study, we have demonstrated that culture supernatants from 2 ALK+ALCL cell lines, Karpas-299 and SU-DHL-1, contain HMGB-1. Both the supernatants and rhHMGB-1 induce IL-8 secretion by keratinocytes. Conditioned medium from both keratinocytes and rhIL-8 can induce invasion of ALK+ cells. In vitro and in vivo, ALK+ cell invasiveness is blocked by glycyrrhizin, a pharmacologic compound known to bind to HMGB-1 and inhibit its cytokine functions.27 Therefore, we postulate that when ALK+ tumoral cells secrete HMGB-1 into the extracellular environment, this pro-inflammatory cytokine activates keratinocytes, which in turn release IL-8.
In human keratinocytes, both HMGB-1 and PAR-2, also known to induce skin inflammation, stimulate IL-8 secretion, leading to increased NF-κB p65 phosphorylation.19,33 Our data support a model in which ALK+ALCL–dependent secretion of HMGB-1 regulates IL-8 secretion through activation of NF-κB in keratinocytes. Both NF-κB p65 phosphorylation and IL-8 release were impaired by siPAR-2 in ALK+ cells. Therefore, we show for the first time a role for both HMGB-1 and PAR-2 in ALK+ tumoral cell activation of highly specialized cells, in this case keratinocytes, which are manipulated by the tumor to create an environment suited to maintaining metastasis development.
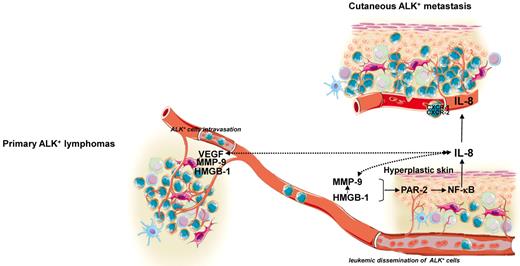
HMGB-1 and PAR-2 induce MMP expression and secretion15,34,35 and MMPs activate PARs.17 MMPs are key enzymes in the turnover of the extracellular matrix and are important regulators of cell invasion. In ALK+ALCL, MMP-9 is up-regulated and activated at the cell surface by the alarmin HSP90 to promote lymphoma cell invasion.16 MMP-9 was also demonstrated to be an effector and regulator of skin inflammatory responses.36 Indeed, IL-8 induces MMP expression,37 and MMP-9–mediated cleavage of IL-8 results in a 10-fold increase in the potency of the chemokine, providing an important positive feedback loop for normal or tumoral cell chemotaxis.38 In the present study, we observed an increase in IL-8 mRNA levels in keratinocytes treated with rhMMP-9. PAR-2 silencing in keratinocytes before rhMMP-9 treatment or MMP inhibition in keratinocytes cultured in medium conditioned by ALK+ALCL cells abolished IL-8 production. Our data reveal a close relationship among the pro-inflammatory mediators HMGB-1, MMP-9, PAR-2, and the IL-8 chemokine in the establishment of the metastatic microenvironment within the skin (Figure 7).
Schematic overview. Shown is a schematic diagram of the possible relationship among the pro-inflammatory mediator HMGB-1, MMP-9, PAR-2, and the chemokine IL-8 to establish a hyperplastic environment within the skin that might participate in ALK+ lymphoma cell epidermotropism and cutaneous metastasis development. Solid arrows indicate pathways established by our data; dashed arrows are currently supported only by circumstantial evidence and remain to be experimentally validated. Illustration done by Servier Medical Art (http://www.servier.fr/servier-medical-art).
Schematic overview. Shown is a schematic diagram of the possible relationship among the pro-inflammatory mediator HMGB-1, MMP-9, PAR-2, and the chemokine IL-8 to establish a hyperplastic environment within the skin that might participate in ALK+ lymphoma cell epidermotropism and cutaneous metastasis development. Solid arrows indicate pathways established by our data; dashed arrows are currently supported only by circumstantial evidence and remain to be experimentally validated. Illustration done by Servier Medical Art (http://www.servier.fr/servier-medical-art).
One important mechanism through which tumors can support their own growth has emerged in the last few years: the release of “danger signals” from necrotic or otherwise damaged areas. Zeh and Lotze have addressed this point by coining the phrase “cancer cells addicted to death.”39 Necrotic areas are a frequent occurrence in anaplastic lymphomas, which are fast-growing tumors that do not have time to mature their endothelial system to sustain growth at the center of the tumor. HMGB-1, which we found to be released from biopsies of these tumors in ALK+ALCL patients, is especially interesting, because initial serum HMGB-1 levels and their modulation during treatment of canine lymphoma seem to have a prognosis value.40 Leukemic and skin dissemination of ALK+ALCL have been identified as high-risk malignant neoplasms that require more aggressive therapy. Based on our observation that ALK+ALCL patients with leukemic involvement display elevated levels of serum IL-8, a protocol evaluating HMGB-1 levels in serum from ALCL patients could be implemented to evaluate its predictive value in human pathology. On a grander scale, HMGB-1 modulation of inflammatory signaling is emerging as a causative factor for skin carcinogenesis.41 The results of the present study suggest that the pro-inflammatory cytokine HMGB-1 released from ALK+ lymphoma cells could create a premetastatic niche into the skin. This epidermal inflammatory environment would then contribute to ALK+ tumor cell recruitment and favor the development of metastasis within the skin. Therefore, extracellular HMGB-1 might represent a very promising new target molecule in cutaneous ALK+ALCL, in which optimization of multitargeted therapies would increase the chances of eradicating ALK+ tumors.42
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr C. Deraison and N. Cénac (Centre de Physiopathologie de Toulouse Purpan–UMR 1043, Toulouse, France) for providing PAR-2 tools and for technical assistance; A. Quillet-Mary and E. Laprevotte (Cancer Research Center of Toulouse–UMR 1037, Toulouse, France) for providing CXCR1/2 tools and for technical assistance; and Dr T. Al Saati and F. Capilla (Plateau technique d'histopathologie expérimentale, Centre de Physiopathologie de Toulouse Purpan–UMR 1043, Toulouse, France), S. Allart and D. Sapede (Plateau Plateau Technique d'Imagerie Cellulaire, Centre de Physiopathologie de Toulouse Purpan–UMR 1043, Toulouse, France), M. March (Laboratoire d'Anatomie et Cytologie pathologiques, CHU Purpan, Toulouse, France), and C. Lopez (Cancer Research Center of Toulouse–UMR 1037, Toulouse, France) for technical support. F.M. dedicates this work to her friends: “Pour ce qui est de l'avenir, il ne s'agit pas de le prévoir mais de le rendre possible.” —Antoine de Saint-Exupéry
This work was supported by grants from Inserm and Ligue contre le cancer comité de l'Aude et de l'Ariège (to F.M.). E.D. was supported by a doctoral fellowship from the Association pour la Recherche sur le Cancer. M.F. was under a contract supported initially by La Fondation de France and then by La Fondation pour la Recherche Médicale. English proofreading was performed by Scientific Scripts (http://scientificscripts.com).
Authorship
Contribution: E.D., F.L., F.G.-I., and F.M. designed the research and analyzed the data; E.D., M.F., and F.L. performed the functional experiments; L.L. and G.D. diagnosed ALCL; N.P. performed the transcriptome array experiments; N.P. and A.M. analyzed the transcriptome array data; S.G. participated in discussions; and F.G.-I. and F.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabienne Meggetto, CRCT, UMR1037 Inserm, CHU-Purpan, BP-3028, 31024 Toulouse Cedex-3, France; e-mail: fabienne.meggetto@inserm.fr.