The splenic marginal zone (MZ) is comprised of specialized populations of B cells, dendritic cells, and macrophages that are uniquely arrayed outside the white pulp follicles to screen the blood for bacterial and other particulate Ags. Mechanisms responsible for MZ B-cell formation, localization, retention, and function are understood to include antigenic specificity, transcription factors, integrins, and surface receptors for soluble ligands such as S1P. Here, we add to this repertoire by demonstrating that the receptor for CXCL12, CXCR7, is expressed on MZ but not on follicular B cells. Treatment of mice with CXCR7 inhibitors led to disruption of MZ architecture, reduced numbers of MZ B cells, and altered granulocyte homeostasis associated with increasing serum levels of CXCL12. CXCR7 thus appears to function as a scavenger receptor for CXCL12 on MZ B cells.
Introduction
Localization and retention of B cells in the marginal zone (MZ) depends on antigenic specificity,1,2 interactions between integrins and their receptors,3 and signaling by the lysophospholipid, S1P, through the S1P1 receptor.4 Although MZ B cells are considered nonrecirculatory, exposure to cognate Ag or bacterial products causes them to relocalize from the MZ to the splenic white pulp,4 providing for efficient delivery of captured Ags to follicular dendritic cells.5
Although no direct evidence links chemokine involvement to MZ B-cell localization and retention, the MZs are strikingly reduced or absent in mice deficient in a series of factors that may act downstream of chemokine receptors.6 Recent studies, however, showed that transcripts for the chemokine receptor, CXCR7, were expressed at much higher levels by mouse MZ than follicular (FO) B cells.7,–9 CXCR7 has variously been reported to function as the primary receptor and scavenger for CXCL12,8,10,11 a modulator of CXCR4 activity, or to act directly on different tumor types.11,12 This prompted us to determine whether CXCR7 and its ligands, CXCL11 and CXCL12,11,13 might influence MZ B-cell biology by taking advantage of recently developed CXCR7-specific mAbs and small molecule inhibitors. We found that CXCR7 was expressed on MZ but not on FO B cells and that treatment with inhibitors disrupted the normal organization of the MZ, altered MZ B-cell numbers, and increased serum levels of CXCL12, resulting in altered homeostasis of granulocytes.
Methods
Flow cytometry
Splenic single-cell suspensions were blocked with a Fc receptor–blocking mAb, 2.4G2, and stained with fluorochrome-labeled Abs specific for B220, CD19, CD23, CD21, Gr-1, CD3, CD11b, αL, α4, β1, and α4β7 (purchased from BD Biosciences or eBioscience). In some experiments, cells were stained with anti-CXCR7 mAb (11G8; provided by ChemoCentryx) followed by FITC-conjugated secondary Ab. An isotype control Ab was included as a negative control. The cells were analyzed by FACSCalibur or LSRII (BD Biosciences), and the data were analyzed by FlowJo Version 7.5.5 software (TreeStar).
Animals, treatment, serum CXCL12, and histologic analysis
FVB and B6 mice (2-6 months old) were purchased from The Jackson Laboratory. FVB mice have unusually large MZs,14 whereas B6 mice are functionally deficient in CXCL11 because of a deletion in the coding sequence.15 Mice were injected subcutaneously with CXCR7 antagonists CCX754 (100 mg/kg twice daily) or CCX771 (30 mg/kg daily; provided by ChemoCentryx) for 2-7 days. The specificity, half-life, and effect of the inhibitors have been reported previously.11,16,17 Control mice were injected with equal volumes of vehicles (10% Captisol). The spleens were analyzed by flow cytometry and confocal microscopy, as described previously, with the use of labeled mAb to IgM, metallophilic macrophages (MOMA-1), and marginal sinus epithelial cells (MAdCAM-1).18 The slides were imaged at 20°C with the use of ×10 and ×20 oil-immersion lenses on a Leica SP2 4-chanel confocal microscope. Images were subsequently processed using Adobe Photoshop. Serum levels of CXCL12 were quantified by ELISA (Mouse CXCL12/SDF-1α; Quantikine ELISA kit; R&D Systems) and CCX754 levels by ChemoCentryx. All animal studies were performed under protocols of LIP-4 and LIP-6 approved by National Institute of Allergy and Infectious Diseases Institutional Animal Care and Use Committee.
Results and discussion
CXCR7 is differentially expressed on subsets of B lineage cells
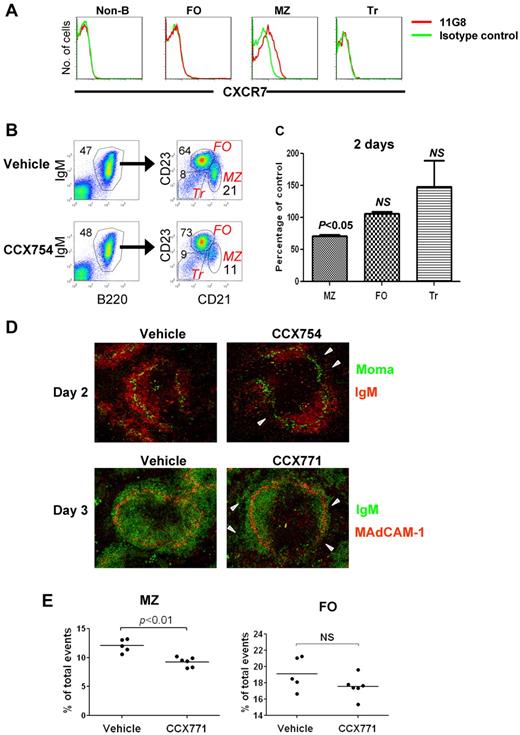
Although previous studies indicated that CXCR7 transcripts and protein were expressed by a variety of lymphoid and nonlymphoid cell types in mice and humans,8,9,19 recent analyses performed with the CXCR7-specific mAb 11G8 and other Abs showed that CXCR7 protein is not expressed by human peripheral blood T or B cells, natural killer cells, or monocytes or by mouse peripheral blood leukocytes.20 Nonetheless, earlier reports of high levels of CXCR7 transcripts in mouse MZ B cells and their precursors8,9 prompted us to reexamine this issue with the use of mAb 11G8 in flow cytometric studies of spleen cells from FVB and B6 mice. We found that CXCR7 was expressed by MZ B cells but little, if at all, by other B-cell subsets or non-B cells (Figure 1A).
Analysis of CXCR7 expression and function in MZ B cells. (A) Splenocytes were analyzed for expression of CXCR7 by indicated cell population by flow cytometry. Non-B indicates CD19− cells; FO, follicular B cells; MZ, MZ B cells; Tr, transitional B cells (refer to panel B for gating schemes). (B) The percentages of splenic B-cell subsets in mice treated with CCX754 or vehicle for 2 days. The numbers indicate percentages of cells falling in each gate. (C) Changes in the indicated cell subsets after CCX754 treatment for 2 days. Data represent 3 independent experiments, totaling 9 mice (means ± SEM). (D) Splenic frozen sections of indicated mice were stained with fluorescent Abs (indicated). The triangles in the right panel indicate disorganized MZ B-cell band. Original magnification ×10 for all images. (E) Percentages of MZ and FO B cells in mice treated with CCX771 or vehicle for 3 days. Each symbol represents a mouse. Data are representative of 3 separate experiments with similar results. Horizontal bars represent the median values.
Analysis of CXCR7 expression and function in MZ B cells. (A) Splenocytes were analyzed for expression of CXCR7 by indicated cell population by flow cytometry. Non-B indicates CD19− cells; FO, follicular B cells; MZ, MZ B cells; Tr, transitional B cells (refer to panel B for gating schemes). (B) The percentages of splenic B-cell subsets in mice treated with CCX754 or vehicle for 2 days. The numbers indicate percentages of cells falling in each gate. (C) Changes in the indicated cell subsets after CCX754 treatment for 2 days. Data represent 3 independent experiments, totaling 9 mice (means ± SEM). (D) Splenic frozen sections of indicated mice were stained with fluorescent Abs (indicated). The triangles in the right panel indicate disorganized MZ B-cell band. Original magnification ×10 for all images. (E) Percentages of MZ and FO B cells in mice treated with CCX771 or vehicle for 3 days. Each symbol represents a mouse. Data are representative of 3 separate experiments with similar results. Horizontal bars represent the median values.
Inhibition of CXCR7 results in disruption of MZ structure and reduced populations of MZ B cells
Treatment of FVB mice with specific high-affinity small molecule antagonists of CXCR7 (CCX754 and CCX771) caused significant reduction of MZ B cells as early as 1 day after injection with reductions persisting through 7 days of treatment (Figure 1B-C; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The reductions in MZ B cells correlated with high concentrations of serum CCX754 (supplemental Figure 2). Confocal images of spleens from treated mice showed segmental losses in the continuity and numbers of B cells occupying the MZ (Figure 1D arrowheads in top right panel) and fragmentation into the red pulp (Figure 1D bottom panel; supplemental Figure 3A-B). Reductions in MZ B cells were associated with reductions in ER-TR9+ MZ macrophages (supplemental Figure 3B), consistent with previous documentations of their codependence for residence in the MZ.21 Similar changes in the proportions of MZ B cells were seen in spleens of both FVB (Figure 1E) and B6 (supplemental Figure 4) mice. Because B6 mice are functionally deficient in CXCL11,15 the effects of CXCR7 blockade seen in both strains can be attributed specifically to inhibition of CXCL12 ligation. Effects of the inhibitors on MZ B-cell numbers could not be attributed to apoptosis because increased cell death was not observed among purified MZ B cells treated in vitro with a series of CXCR7 inhibitors: CCX771, CCX754, CCX733, and CCX704 (data not shown). We also ruled out the possibility that observed changes in MZ B-cell numbers after treatment with CCX754 resulted from MZ B cells, assuming the phenotype of FO B cells because studies that used CD1d as an alternative marker for identifying MZ B cells yielded the same results (supplemental Figure 6). Although the fate of drug-treated MZ B cells remains to be determined, these results indicate that ligation of CXCL12 by CXCR7 on MZ B cells is critical to the stability of MZ structure and local retention of MZ B cells.
Blocking CXCR7 increases the levels of CXCL12 in peripheral blood and alters mobilization of neutrophils
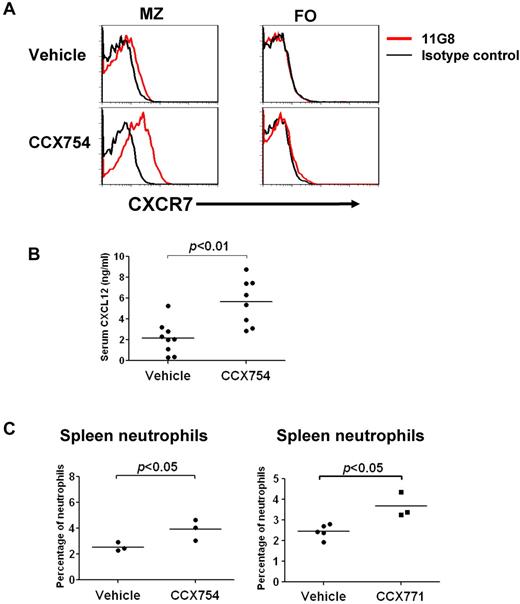
CXCR7 has been described as a scavenger for CXCL12 and CXCL11, mediating ligand internalization and degradation, with the ability to eliminate CXCL12 from its environment, modulating the activity of the other CXCL12 receptor, CXCR4.10 This suggested that blockade of CXCR7 in vivo might result in increased levels of CXCL12. Indeed, treatment with CCX754 was associated with significantly increased levels of CXCR7 expression on the surface of MZ B cells but not FO B cells (Figure 2A), an indication of interrupted internalization and recycling of CXCR7.10,22 The levels of CXCL12 in sera from mice treated for 2 days with the inhibitor CCX754 increased > 2-fold (Figure 2B; P < .01), suggesting that MZ and other CXCR7-expressing cells act as sinks for CXCL12 in vivo.
CXCR7 blockers affect CXCL12 availability and neutrophil mobilization. (A) Increased expression of CXCR7 on the cell surface of MZ but not FO B cells in mice treated with CCX754 for 2 days. Data are representative of ≥ 3 similar experiments. (B) Increased serum levels of CXCL12 in mice treated with CCX754 for 2 days. Data are pooled from 3 independent experiments. Each symbol represents a mouse. (C) Frequencies of neutrophils in the spleen of indicated groups were analyzed by flow cytometry. Neutrophils were gated as CD19−Gr-1briCD11b+. Each dot represents a mouse. Data are representative of ≥ 3 separate experiments with similar results. Horizontal bars represent the median values (B and C).
CXCR7 blockers affect CXCL12 availability and neutrophil mobilization. (A) Increased expression of CXCR7 on the cell surface of MZ but not FO B cells in mice treated with CCX754 for 2 days. Data are representative of ≥ 3 similar experiments. (B) Increased serum levels of CXCL12 in mice treated with CCX754 for 2 days. Data are pooled from 3 independent experiments. Each symbol represents a mouse. (C) Frequencies of neutrophils in the spleen of indicated groups were analyzed by flow cytometry. Neutrophils were gated as CD19−Gr-1briCD11b+. Each dot represents a mouse. Data are representative of ≥ 3 separate experiments with similar results. Horizontal bars represent the median values (B and C).
CXCL12 is a potent chemoattractant for T cells but limits mobilization of granulocytes from the BM. This led us to examine the frequencies of T cells and neutrophils among spleen cells and blood of mice treated with the inhibitors CCX754 and CCX771; no significant changes were seen for T cells (data not shown). In contrast, the proportions of granulocytes in the spleens but not the blood of mice treated with either inhibitor were significantly increased (Figure 2C; data not shown), suggesting that heightened expression of CXCL12 promoted granulocyte entry, retention, or survival in the spleen.
Blockade of CXCR7 does not alter expression of integrins or follicular shuttling of MZ B cells
Integrins are involved in MZ B-cell localization and positioning.3 CCX771 had no effect on expression of αL, α4, β1, or α4β1 integrins on MZ B cells (supplemental Figure 5), indicating that CXCR7 and integrins are 2 independent systems involved in the regulation of MZ B-cell retention. MZ B cells were shown to constantly relocate to the follicle, a process associated with the Ag presentation function of MZ B cells.5 Although S1P1 plays a critical role in mediating MZ B-cell shuttling to the follicle,5 CXCR7 appeared to be dispensable for this process because follicular shuttling of residual MZ B cells in CCX771-treated mice was not affected as assessed by an in vivo labeling assay5 (supplemental Figure 6). With increasing numbers of new receptors discovered to be responsible for retention of MZ B cells, including the recently reported Cannabinoid receptor 2,23 it will be interesting to determine whether and how these seemingly different systems interplay under different pathologic conditions, including microbial infections.
Previous studies of conventional Cxcr7−/− mice and a conditional Cxcr7 knockout mouse showed moderate reductions in the MZ B-cell compartment,8 although precise positioning of MZ B cells in the MZ and red pulp was not clearly defined.8 Our data are consistent with this report and extend these findings in showing that CXCR7 is required for MZ B-cell alignment to the MZ. CXCR7 is a novel marker for mouse splenic MZ B cells, which requires engagement of the receptor by CXCL12 for their retention in the MZ. In addition, CXCR7 expressed by MZ B cells and possibly other cells, is a sink for CXCL12. Because CXCR7 is part of a regulatory network that involves CXCL12, CXCR4, and CXCR7, we examined whether CXCR7-mediated MZ B-cell retention depends on the function of CXCR4, which is also expressed by MZ B cells in a CXCR7-independent fashion (supplemental Figure 7A). Our preliminary data indicate that treatment of mice with AMD3100, a selective antagonist of CXCR4, abrogated CCX754-induced mobilization of MZ B cells and granulocytes in the spleen (supplemental Figure 7B), arguing that a chemokine receptor network that involves CXCR4, CXCR7, and CXCL12 regulates MZ B-cell localization. Further studies are required to determine the mechanisms governing this network in MZ B cells and the roles of CXCR7 in innate and adaptive immunity and possibly in the development of MZ B-cell lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Philip M. Murphy at National Institute of Allergy and Infectious Diseases for expert advice and Dr Mark E. T. Penfold of ChemoCentryx Inc, for reagents and valuable advice. The small molecule CXCR7 antagonists used in some experiments are proprietary property of ChemoCentryx Inc. They also thank Alfonso Macias for technical assistance.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
National Institutes of Health
Authorship
Contribution: H.W. designed, performed, and interpreted the experiments and wrote and edited the paper; N.B., S.C., and D.-M.S. performed experiments; C.-F.Q. and M.M. performed confocal microscopy experiments; and H.C.M. designed experiments, interpreted the data, and wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The Laboratory of Immunopathology has recently merged with the Laboratory of Immunogenetics at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The new name is the Laboratory of Immunogenetics, Virology and Cellular Immunology Section.
Correspondence: Herbert C. Morse III or Hongsheng Wang, Laboratory of Immunogenetics, Virology and Cellular Immunology Section, NIAID, 5640 Fishers Ln, Twinbrook 1, Rockville, MD 20852; e-mail: hmorse@niaid.nih.gov or wanghongs@niaid.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal