Abstract
Natural killer (NK) cells play a crucial role in early immunity after hematopoietic stem cell transplantation because they are the first lymphocyte subset recovering after the allograft. In this study, we analyzed the development of NK cells after intrabone umbilical cord blood (CB) transplantation in 18 adult patients with hematologic malignancies. Our data indicate that, also in this transplantation setting, NK cells are the first lymphoid population detectable in peripheral blood. However, different patterns of NK-cell development could be identified. Indeed, in a group of patients, a relevant fraction of NK cells expressed a mature phenotype characterized by the KIR+NKG2A− signature 3-6 months after transplantation. In other patients, most NK cells maintained an immature phenotype even after 12 months. A possible role for cytomegalovirus in the promotion of NK-cell development was suggested by the observation that a more rapid NK-cell maturation together with expansion of NKG2C+ NK cells was confined to patients experiencing cytomegalovirus reactivation. In a fraction of these patients, an aberrant and hyporesponsive CD56−CD16+p75/AIRM1− NK-cell subset (mostly KIR+NKG2A−) reminiscent of that described in patients with viremic HIV was detected. Our data support the concept that cytomegalovirus infection may drive NK-cell development after umbilical CB transplantation.
Introduction
Natural killer (NK)–cell function is finely regulated by an array of receptors transducing either inhibitory or activating signals.1–3 Among receptors negatively regulating NK-cell function, a crucial role is played by those interacting with MHC class I molecules. These receptors allow NK cells to spare autologous normal cells and to kill cells in which MHC class I expression is down-regulated (eg, by tumor transformation or viral infection) or cells expressing non-self MHC class I alleles unable to engage these receptors (eg, allogenic transplantation settings). In humans, HLA class I–specific receptors include killer Ig-like receptors (KIRs) specific for determinants shared by groups of HLA-A, -B, and -C allotypes4 and CD94/NKG2A heterodimer specific for the nonclassic, class I molecule HLA-E5 . Human NK cells also express on their cell surface non–MHC-specific inhibitory receptors, including p75/AIRM1 (siglec-7) that is expressed virtually by all NK cells.6
Among receptors that trigger NK-cell function, the main non–MHC-specific activating receptors are NKp46, NKp30, NKp44 (collectively termed natural cytotoxicity receptors, NCRs), NKG2D, DNAM-1, and CD16.2 In addition, human NK cells can express HLA-class I–specific activating receptors, including KIR2DS1 and KIR2DS4,7,8 and CD94/NKG2C.5
Human NK cells mainly differentiate in BM; however, recent studies suggest that different sites, such as secondary lymphoid compartments,9 may represent sites of NK-cell development. Two main subsets of mature NK cells characterized by distinct functional and phenotypic properties have been described: the CD56bright CD16−/low and the CD56dim CD16+ subsets. CD56bright NK cells typically express high levels of CD94/NKG2A but low levels of KIRs. They are infrequent in peripheral blood but dominate in secondary lymphoid compartments. These cells produce high amounts of immunoregulatory cytokines but are poorly cytotoxic. In contrast, the CD56dim subset, largely represented in peripheral blood, is characterized by high surface expression of KIRs, high cytotoxicity against tumor and virus-infected targets, and rapid production of cytokines on activation.10 A third subset, characterized by a CD56−CD16+ surface phenotype, represents only a few percent of NK cells in healthy persons. However, expansion of this subset has been described in patients with viremic HIV and hepatitis C,11,12 as well as in recipients of HSC transplant.13,14
The relation existing between the 2 main NK-cell subsets has been long debated. Several recent reports suggest that CD56bright cells are the precursors of CD56dim NK cells.15,16 NK cells are the first peripheral blood lymphocytes to appear after HSCT, and they are initially represented by CD56bright cells, whereas CD94/NKG2A+ CD56dim cells are detected later. Full maturation of CD56dim cells involves the expression of combinations of KIRs, resulting in a relatively stable KIR repertoire. Notably, the KIR repertoire is mainly determined genetically, but it is also influenced by the HLA class I genotype.4,17,18
Umbilical CB transplantation (UCBT) is being widely used to treat patients affected by many disorders of both hematologic and nonhematologic origin.19 In comparison to BM transplantation (BMT), UCBT offers the advantages of an easier procurement with no risk for the donor. In addition, there is lower risk of transmitting infections and of developing both acute and chronic GVHD. UCBT also permits transplantation in the absence of full HLA compatibility in the donor/recipient pair. Moreover, UCBT from unrelated donors offers the advantage of a rapid availability of cryopreserved cells, the median time for a successful donor search being < 1 month.20 Despite the lower incidence of acute and chronic GVHD in UCB transplant recipients, the risk of leukemia relapse is not increased. This clinical finding suggests that other cells, different from T cells, such as NK cells, may be responsible for a clinically meaningful GVL effect. This said, although in the past 10 years CB has been frequently used as a source of HSCs, it is noteworthy that few studies have focused on the phenotype and function of NK cells recovering after UCBT.13,21,22 In comparison to BMT, UCBT presents also some disadvantages, such as delayed hematopoietic recovery, increased risk of graft failure, and increased risk of infections because of slow immune reconstitution. This has been mainly attributed to the reduced number of nucleated cells in the graft and to the naive state of T and B lymphocytes.19,23 Consequently, UCBT is associated with a higher risk of morbidity and mortality especially in adult patients. To improve the outcome of UCBT and to overcome the limitation of the low number of transplanted cells, different approaches have been attempted.19,20 Among these, the direct intrabone injection of CB cells has been shown to be particularly promising. In fact, preliminary results obtained in a phase 1/2 trial showed an accelerated platelet recovery and lower rate of graft failure even when the number of nucleated cells in the graft was low.24
The aim of the present study was to analyze the phenotype and the function of NK cells recovering in adults given UCB transplant for hematologic malignancies. We show important functional and phenotypic/developmental diversities in NK cells maturing in different groups of patients which are possibly correlated with human CMV (HCMV) infection.
Methods
Patients and samples
Eighteen adult patients with hematologic malignancies, mostly acute myeloid leukemia, were included in this study. All patients received CB-derived hematopoietic cells that were administered with the intrabone method.24 Patients received transplants at the San Martino Hospital, Genoa, Italy. All patients received a combination of cyclosporin (Novartis Pharma), mycophenolate mofetil (Roche), and an antithymocyte globulin (Genzyme) as GVHD prophylaxis. Cyclosporin was started intravenously from day 7 before transplantation at a daily dose of 1 mg/kg. The dose of cyclosporine was adjusted to maintain a serum trough level between 150 and 300 μg/L. After engraftment cyclosporin was given orally and tapered until 1 year after UCBT. Mycophenolate mofetil was administered orally at a dosage of 15 mg/kg twice a day from day 1 to day 28 after transplantation. Antithymocyte globulin was given before transplantation at a dose of 2-3 mg/kg on days −3 and −2. No patients received steroids for GVHD prophylaxis. Details on patient characteristics and outcome are summarized in Table 1. All patients gave their informed consent to be included in this study, which was approved by ethical committees of the San Martino Hospital and the University of Genoa and was conducted in accordance with the tenants of the Declaration of Helsinki. Peripheral blood samples were collected from patients at 1, 3, 6, and 12 months after transplantation.
Patient characteristics and outcome
| Pt no. . | Age, y . | Sex . | Diagnosis . | Disease stage . | Conditioning . | CD34+ infused, × 105/kg . | Cells infused, × 107/kg . | HLA-I match . | CMV serology R/D . | Acute GVHD . | Chronic GVHD . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | F | AML | First CR | CY + TBI | 2.75 | 3.4 | 4/6 | Pos/neg | No | No | Alive |
| 2 | 44 | M | AML | First CR | CY + TBI | 1.7 | 2.0 | 4/6 | Pos/neg | Grade I | No | Alive |
| 3 | 46 | F | AML | First CR | CY + TBI | 2.7 | 3.87 | 4/6 | Neg/neg | Grade I | Grade I | Relapsed, dead after 29 mo after UCBT |
| 4 | 35 | M | MDS | NA | CY + TBI | 1.0 | 2.19 | 4/6 | Pos/pos | No | No | Alive |
| 5 | 34 | F | AML | Second CR | CY + TBI | 1.1 | 2.32 | 4/6 | Pos/neg | Grade II | No | Alive |
| 6 | 38 | M | AML | Second CR | CY + TBI | 1.16 | 3.2 | 4/6 | Pos/neg | no | No | Alive |
| 7 | 39 | M | AML | Disease present | CY + TBI | 3.4 | 3.56 | 4/6 | Pos/pos | no | No | Relapsed, dead after 6 mo after UCBT |
| 8 | 38 | F | AML | First CR | CY + TBI | 4.1 | 5.5 | 5/6 | Pos/neg | Grade I | Grade I | Alive |
| 9 | 21 | M | AML | First CR | CY + TBI | 2.16 | 2.89 | 4/6 | Neg/NA | No | Grade I | Alive |
| 10 | 50 | F | AML | Second CR | CY + TBI | 0.99 | 3.9 | 4/6 | Pos/neg | Grade I | Grade I | Alive |
| 11 | 63 | M | CML | RF | THIO TREO FLU | 0.68 | 2.13 | 4/6 | Pos/neg | Grade I | Grade I | Relapsed, dead after 23 mo after UCBT |
| 12 | 41 | M | AML | Disease present | CY + TBI | 0.77 | 3.33 | 4/6 | Pos/neg | Grade I | Grade I | Alive |
| 13 | 39 | F | AML | RF | CY + TBI | 1.1 | 3.9 | 4/6 | Pos/pos | No | No | Alive |
| 14 | 33 | F | AML | RF | CY + TBI | 1.89 | 3.43 | 4/6 | Neg/NA | Grade I | No | Alive |
| 15 | 35 | M | ALL | RF | CY + TBI | 1.04 | 3.12 | 4/6 | Pos/neg | No | No | Relapsed, dead before 3 mo after UCBT |
| 16 | 46 | M | AML | First CR | CY + TBI | 1.26 | 4.08 | 4/6 | Pos/neg | Grade II | Grade II | Relapsed, dead before 3 mo after UCBT |
| 17 | 60 | F | ALL | Second CR | FLU + TBI | 0.83 | 3.02 | 4/6 | Pos/neg | Grade I | NA | Relapsed, dead before 3 mo after UCBT |
| 18 | 27 | M | AML | RF | CY + TBI | 1.46 | 2.66 | 4/6 | Neg/pos | Grade I | Grade III | GVHD, dead before 3 mo after UCBT |
| Pt no. . | Age, y . | Sex . | Diagnosis . | Disease stage . | Conditioning . | CD34+ infused, × 105/kg . | Cells infused, × 107/kg . | HLA-I match . | CMV serology R/D . | Acute GVHD . | Chronic GVHD . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | F | AML | First CR | CY + TBI | 2.75 | 3.4 | 4/6 | Pos/neg | No | No | Alive |
| 2 | 44 | M | AML | First CR | CY + TBI | 1.7 | 2.0 | 4/6 | Pos/neg | Grade I | No | Alive |
| 3 | 46 | F | AML | First CR | CY + TBI | 2.7 | 3.87 | 4/6 | Neg/neg | Grade I | Grade I | Relapsed, dead after 29 mo after UCBT |
| 4 | 35 | M | MDS | NA | CY + TBI | 1.0 | 2.19 | 4/6 | Pos/pos | No | No | Alive |
| 5 | 34 | F | AML | Second CR | CY + TBI | 1.1 | 2.32 | 4/6 | Pos/neg | Grade II | No | Alive |
| 6 | 38 | M | AML | Second CR | CY + TBI | 1.16 | 3.2 | 4/6 | Pos/neg | no | No | Alive |
| 7 | 39 | M | AML | Disease present | CY + TBI | 3.4 | 3.56 | 4/6 | Pos/pos | no | No | Relapsed, dead after 6 mo after UCBT |
| 8 | 38 | F | AML | First CR | CY + TBI | 4.1 | 5.5 | 5/6 | Pos/neg | Grade I | Grade I | Alive |
| 9 | 21 | M | AML | First CR | CY + TBI | 2.16 | 2.89 | 4/6 | Neg/NA | No | Grade I | Alive |
| 10 | 50 | F | AML | Second CR | CY + TBI | 0.99 | 3.9 | 4/6 | Pos/neg | Grade I | Grade I | Alive |
| 11 | 63 | M | CML | RF | THIO TREO FLU | 0.68 | 2.13 | 4/6 | Pos/neg | Grade I | Grade I | Relapsed, dead after 23 mo after UCBT |
| 12 | 41 | M | AML | Disease present | CY + TBI | 0.77 | 3.33 | 4/6 | Pos/neg | Grade I | Grade I | Alive |
| 13 | 39 | F | AML | RF | CY + TBI | 1.1 | 3.9 | 4/6 | Pos/pos | No | No | Alive |
| 14 | 33 | F | AML | RF | CY + TBI | 1.89 | 3.43 | 4/6 | Neg/NA | Grade I | No | Alive |
| 15 | 35 | M | ALL | RF | CY + TBI | 1.04 | 3.12 | 4/6 | Pos/neg | No | No | Relapsed, dead before 3 mo after UCBT |
| 16 | 46 | M | AML | First CR | CY + TBI | 1.26 | 4.08 | 4/6 | Pos/neg | Grade II | Grade II | Relapsed, dead before 3 mo after UCBT |
| 17 | 60 | F | ALL | Second CR | FLU + TBI | 0.83 | 3.02 | 4/6 | Pos/neg | Grade I | NA | Relapsed, dead before 3 mo after UCBT |
| 18 | 27 | M | AML | RF | CY + TBI | 1.46 | 2.66 | 4/6 | Neg/pos | Grade I | Grade III | GVHD, dead before 3 mo after UCBT |
R/D indicates recipient/donor; AML, acute myeloid leukemia; CR, complete remission; CY, cyclophosphamide; TBI, total body irradiation; UCBT, umbilical CB transplantation; MDS, myelodysplastic syndrome; NA, not available; CML, chronic myeloid leukemia; RF, refractory; THIO, thiotepa; TREO, treosulfan; FLU, fludarabine; and ALL, acute lymphocytic leukemia.
HCMV serology was assessed before transplantation with the use of enzyme-linked immunoassay for virus-specific IgM and IgG. HCMV infection was monitored by determination as HCMV pp65-positive cells/2 × 105 polymorphonuclear cells. Patients were preemptively treated in the presence of ≥ 2 pp65-positive polymorphonuclear cells/2 × 105 (or on first confirmed positivity, when a single positive cell was detected). Preemptive therapy was based on administration of intravenous ganciclovir (5 mg/kg twice a day), replaced by foscarnet (90 mg/kg twice a day) in case of ganciclovir-induced neutropenia (< 0.5 × 109 neutrophils/L) or sustained increase of HCMV levels in blood during therapy with ganciclovir. Antiviral treatment was stopped in the presence of virus clearance from blood (ie, after 2 consecutive negative results). HCMV relapse episodes were treated similarly.
Cord blood samples and peripheral blood samples from healthy adults, used as controls in the study, were kindly provided by the Liguria Cord Blood Bank and by the Centro Trasfusionale San Martino, respectively.
Cells, monoclonal Abs, assays, RT-PCR analysis, KIR gene profile, and KIR ligand analyses
See supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Patients characteristics, outcome, and lymphocyte reconstitution
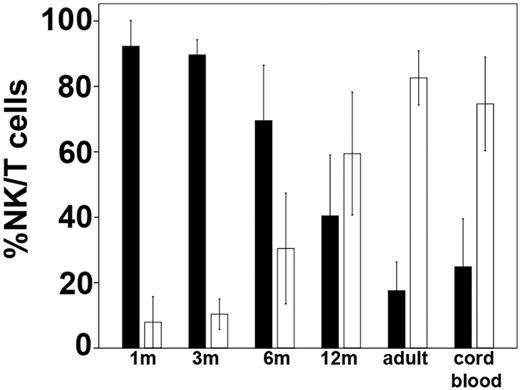
Of the 18 patients enrolled in this study, 4 died within 3 months from transplantation (3 because of relapse and 1 because of GVHD) and were thus excluded from the analysis. The other 14 patients were evaluated for ≥ 1 year after UCBT (except patient 7, 6 months). Reconstitution of lymphocyte subsets was assessed by cytofluorimetric analysis at different time intervals after UCBT. As shown in Figure 1, NK cells represented the main lymphocyte population in blood after 1 month, because T and B cells were virtually absent (data not shown). This finding is in line with previous reports in other types of allogeneic HSCT settings, including BMT25–27 and intravenous UCBT.21,28 The NK/T ratio remained high until 6 months after UCBT, until at 12 months it markedly decreased when T cells outnumbered NK cells (Figure 1).
T- and NK-cell recovery after intrabone UCBT. The relative percentage of peripheral blood NK cells (■) and T cells (□) are shown at 1, 3, 6, and 12 months after UCBT in comparison to adult and CB. Values represent mean ± SD (n = 14 for transplant recipients, n = 15 for CB, and n = 60 for healthy adults).
T- and NK-cell recovery after intrabone UCBT. The relative percentage of peripheral blood NK cells (■) and T cells (□) are shown at 1, 3, 6, and 12 months after UCBT in comparison to adult and CB. Values represent mean ± SD (n = 14 for transplant recipients, n = 15 for CB, and n = 60 for healthy adults).
Maturation of NK-cell subsets during NK-cell reconstitution
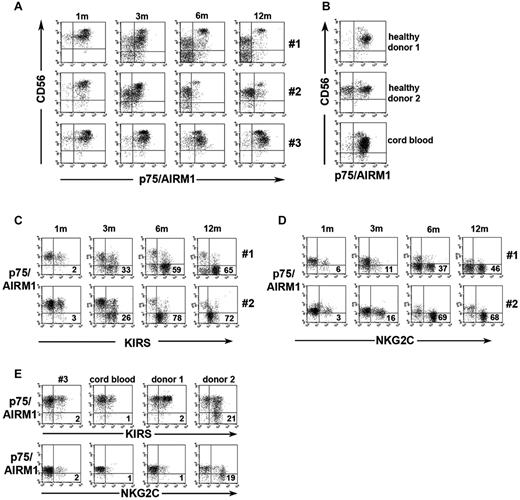
An early expansion of CD56bright NK cells occurs after HSCT. These cells are considered the precursors of CD56dim NK cells.25–27 Thus, in our patients, we analyzed the maturational stages of NK cells by evaluating CD56bright CD16−/low and CD56dim CD16+ NK-cell subsets in freshly isolated cells. In Figure 2A, 3 representative patients are shown. In line with previous data,25–27 during the first 3 months the percentage of CD56bright cells (Figure 2A) was higher compared with NK cells derived from adult peripheral blood or CB (Figure 2B). However, at later intervals, remarkable differences were detected in different patients in both phenotypic and functional characteristics of NK cells. Thus, in a fraction of patients (patients 1, 2, 4, 5, 6, 7, 8, 9, 10, and 11; Table 1) the frequency of CD56bright cells gradually decreased, reaching values comparable to controls at 12 months after UCBT (13% ± 6% vs 10% ± 7% adults or 11% ± 7% CB), whereas in the remaining patients (patients 3, 12, 13, and 14; Table 1) the percentage of CD56bright NK cells remained high even at 12 months after UCBT (30% ± 12%).
Different size and distribution of donor-derived CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ subsets in NK cells reconstituting after intrabone UCBT. (A) Fresh NK cells, collected at the indicated time intervals from recipients of a transplant, were analyzed by double immunofluorescence and FACS analysis for the expression of CD56 and CD16. Three different patients are shown. Dotted lines in the plots separate CD56bright from CD56dim NK-cell subsets. The percentage of each subset is indicated in the different quadrants. (B) For comparison, the distribution and relative percentage of the CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ NK-cell subsets are shown for NK cells isolated from representative healthy adult peripheral blood (left) and CB (right). (C-D) The differential development of the CD56negCD16+ (C) and the CD56bright (D) NK-cell subsets are shown as 95% CIs for the mean at 1, 3, 6, and 12 months after UCBT in the 3 groups of patients (Top, group 1 ●; middle, group 2 ■; bottom, group 3 ▴). In each panel the 95% CI for the mean is reported for healthy adult (□) and CB (○) NK-cell subset. (E) The percentage of CD56−CD16+ in group 1 (black histograms) and group 2 (white histograms) patients is shown as 95% CI at 6 and 12 months after UCBT. Statistical significance is indicated (*P < .05).
Different size and distribution of donor-derived CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ subsets in NK cells reconstituting after intrabone UCBT. (A) Fresh NK cells, collected at the indicated time intervals from recipients of a transplant, were analyzed by double immunofluorescence and FACS analysis for the expression of CD56 and CD16. Three different patients are shown. Dotted lines in the plots separate CD56bright from CD56dim NK-cell subsets. The percentage of each subset is indicated in the different quadrants. (B) For comparison, the distribution and relative percentage of the CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ NK-cell subsets are shown for NK cells isolated from representative healthy adult peripheral blood (left) and CB (right). (C-D) The differential development of the CD56negCD16+ (C) and the CD56bright (D) NK-cell subsets are shown as 95% CIs for the mean at 1, 3, 6, and 12 months after UCBT in the 3 groups of patients (Top, group 1 ●; middle, group 2 ■; bottom, group 3 ▴). In each panel the 95% CI for the mean is reported for healthy adult (□) and CB (○) NK-cell subset. (E) The percentage of CD56−CD16+ in group 1 (black histograms) and group 2 (white histograms) patients is shown as 95% CI at 6 and 12 months after UCBT. Statistical significance is indicated (*P < .05).
In addition, starting from month 6, a fraction of patients (patients 1, 4, 5, and 6) was characterized by the dramatic expansion of a peculiar NK-cell subset characterized by the CD56−CD16+ surface phenotype. In these patients this subset reached a mean value of 43% ± 14% of NK cells 1 year after UCBT (eg, patient 1, Figure 2A). Notably, CD56−CD16+ NK cells were poorly represented in adult controls (2% ± 2%; n = 60), whereas, as previously described, they were consistently detectable in CB (Figure 2B),29,30 but their percentage was considerably lower than in patients 1, 4, 5, and 6 (Figure 2B-C).
Taken together, on the basis of the CD56 and CD16 phenotype detected during NK-cell development after UCBT (see above and Figure 2C-D), 3 groups of patients could be operationally identified. Group 1 patients (1, 4, 5, and 6) were characterized by decrements in the CD56bright subset and expansion of the CD56−CD16+subset (see patient 1, Figure 2A,C-E). Group 2 patients (2, 7, 8, 9, 10, and 11) showed a similar decrease in the CD56bright subset but lacked substantial expansion of the CD56−CD16+ subset (eg, patient 2, Figure 2A). Group 3 patients (3, 12, 13, and 14), in which this subset was barely detectable, were characterized by the persistence of high levels of CD56bright cells (eg, patient 3, Figure 2A). Notably, the percentage of CD56−CD16+ cells was significantly different in group 1 patients as compared with group 2 patients, both at 6 and 12 months after transplantation (Figure 2C,E).
The assignment of the various patients to different groups according to the distribution of the CD56bright, CD56dim, and CD56− subsets was supported also by k-mean cluster analysis of our data (not shown).
Expression of HLA-specific inhibitory receptors
The reciprocal expression of CD94/NKG2A and KIR by NK cells freshly isolated from 3 patients representative of groups 1, 2, and 3 (patients 1, 2, and 3), respectively, at different time points after UCBT is shown in Figure 3A. One month after transplantation, CD94/NKG2A+ KIR− NK cells were largely predominant in all patients, whereas CD94/NKG2A− KIR+ NK cells were present in low proportions. An increment of this subset occurred only at month 3 in all patients irrespective of their subgroup attribution. At month 6, major increments of CD94/NKG2A− KIR+ NK cells occurred in groups 1 and 2 but not in group 3 (even 12 months after UCBT). Notably, in group 1 and 2 patients the proportions of NKG2A− KIR+ NK cells were comparable or even higher than those of adult controls, whereas in group 3 they were similar to those of CB (Figure 3B-C). In addition, the proportion of NKG2A+ NK cells within the CD56dim subset was sharply decreased (Figure 3D top and middle) in patients belonging to groups 1 and 2 but not in group 3, in which it remained stable even 12 months after transplantation (Figure 3D bottom).
Reciprocal expression of NKG2A and KIR in different groups of patients undergoing UCBT. Peripheral blood NK cells were analyzed by FACS for the expression of NKG2A in combination with KIRs at 1, 3, 6, and 12 months after transplantation. (A) Three patients representative of the different groups are shown. The percentage of NKG2A+KIR−, NKG2A+KIR+, and NKG2A−KIR+ NK-cell subsets are indicated in the corresponding quadrants at each time point. (B) For comparison, NKG2A in combination with KIRs is shown for 2 representative healthy donor NK cells (middle and right) and for CB NK cells (left). (C) The percentage of KIR+NKG2A− and (D) the percentage of NKG2A+ CD56dim NK cells are shown as 95% CI for the mean at the different time points (top, group 1 ●; middle, group 2 ■; bottom, group 3 ▴). (C-D) The 95% CI for the mean is reported for healthy adult (□) and CB (○) NK-cell subsets in each panel.
Reciprocal expression of NKG2A and KIR in different groups of patients undergoing UCBT. Peripheral blood NK cells were analyzed by FACS for the expression of NKG2A in combination with KIRs at 1, 3, 6, and 12 months after transplantation. (A) Three patients representative of the different groups are shown. The percentage of NKG2A+KIR−, NKG2A+KIR+, and NKG2A−KIR+ NK-cell subsets are indicated in the corresponding quadrants at each time point. (B) For comparison, NKG2A in combination with KIRs is shown for 2 representative healthy donor NK cells (middle and right) and for CB NK cells (left). (C) The percentage of KIR+NKG2A− and (D) the percentage of NKG2A+ CD56dim NK cells are shown as 95% CI for the mean at the different time points (top, group 1 ●; middle, group 2 ■; bottom, group 3 ▴). (C-D) The 95% CI for the mean is reported for healthy adult (□) and CB (○) NK-cell subsets in each panel.
Phenotypic signature of CD56−CD16+ NK cells is similar to that detected in HIV or HCV-infected patients
An expansion of CD56−CD16+ NK cells was reported previously in different conditions, including HSCT13,14 or HIV or hepatitis C virus (HCV) infections.12 In patients with HIV, this subset was characterized by the unusual NKG2A−, NKG2C+, KIR+ p75/AIRM1− phenotype. Therefore, we further analyzed NK cells in group 1 patients at 6 months after UCBT (Figure 2), that is, when CD56−CD16+ cells represent a large fraction of NK cells. As shown in Figure 4 (upper square), CD56−CD16+ cells were primarily composed of KIR+ cells, whereas NKG2A+ cells accounted for a minor fraction. These cells were also p75/AIRM1−, thus displaying major similarities with CD56− NK cells isolated from HIV viremic patients.31 Similar phenotypic characteristics were detected in the minor CD56− subset derived from group 2 patients (supplemental Table 1). Notably, the CD56− NK subset present in CB displayed marked differences being characterized by normal levels of p75/AIRM1 and low percentage of KIR+ cells.
Surface phenotype of CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ NK-cell subsets reconstituting after intrabone UCBT. Purified NK cells were stained with anti-CD56 and anti-CD16 mAbs to identify 3 subsets: CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+. Each of the 3 gated populations was assessed for the expression of the indicated surface Ags. In each histogram the percentage of positive cells is shown. The number in italics indicates the percentage of CD56dim or CD56−CD16+ NK cells expressing the CD94dim phenotype. A representative patient of group 1 and 1 of group 3 are shown in the first and second panels, whereas a CB and a healthy adult are shown in the third and fourth panels, respectively. The phenotype of CD56−CD16+ is not reported for group 3 patients and adult donors because in these 2 cases this NK subset was substantially undetectable.
Surface phenotype of CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+ NK-cell subsets reconstituting after intrabone UCBT. Purified NK cells were stained with anti-CD56 and anti-CD16 mAbs to identify 3 subsets: CD56brightCD16−/low, CD56dimCD16+, and CD56−CD16+. Each of the 3 gated populations was assessed for the expression of the indicated surface Ags. In each histogram the percentage of positive cells is shown. The number in italics indicates the percentage of CD56dim or CD56−CD16+ NK cells expressing the CD94dim phenotype. A representative patient of group 1 and 1 of group 3 are shown in the first and second panels, whereas a CB and a healthy adult are shown in the third and fourth panels, respectively. The phenotype of CD56−CD16+ is not reported for group 3 patients and adult donors because in these 2 cases this NK subset was substantially undetectable.
The KIRhigh, CD16high, NKG2Alow signature is compatible with a fully mature NK-cell phenotype.32 Thus, to gain better information on the maturation status of these cells, we analyzed the expression of CD62L, CD94, and CD57, because the differential expression of these surface markers characterizes different steps of NK-cell maturation. In particular, expression of CD62L33 and the high-density CD9416 characterize NK cells at early-intermediate stages of development, whereas the expression of CD57 is associated with terminally differentiated NK cells.34,35 As shown in Figure 4, at 6 months after UCBT the CD56−CD16+ subset had lower CD62L expression and higher percentage of CD94dim NK cells compared with CD56dim NK cells. In addition, a substantial cell fraction expressed CD57.
Down-regulation of p75/AIRM1 expression and progressive expansion of p75/AIRM1−KIR+ NKG2C+ NK cells
In groups 1 and 2, at 6 months, p75/AIRM1 was brightly expressed only by CD56bright NK cells, whereas it was down-regulated in CD56dim and virtually absent in CD56− NK cells. In contrast, p75/AIRM1 was expressed by virtually all NK cells, in group 3, as well as in healthy adults and in CB (Figure 4).
The appearance of CD56dimp75/AIRM1− and CD56−p75/AIRM1− subsets was analyzed at different time points after UCBT in the 3 representative patients (patients 1, 2, and 3). At 1 month, p75/AIRM1 was expressed on virtually all NK cells in all patients (Figure 5A). After 3 months, however, down-regulation of p75/AIRM1 occurred on a discrete subset of CD56dim NK cells, in groups 1 and 2. A small CD56−p75/AIRM1− NK subset was also detectable. After 6 months, the CD56dim p75/AIRM1− subset sharply increased in both groups 1 and 2, whereas a remarkable increment of CD56−p75/AIRM1− occurred only in group 1 (see also Figure 4). No down-regulation of p75/AIRM1 was detectable in NK cells of group 3. Altogether these data indicate that, in group 1, similar to HIV viremic patients,31 the loss of p75/AIRM1 preceded that of CD56. In group 2, down-regulation of p75/AIRM1 in CD56dim NK cells was not followed by a substantial expansion of CD56− NK cells.
Progressive expansion of p75/AIRM1−KIR+ and p75/AIRM1−NKG2C+ NK cells. (A) Freshly isolated NK cells were double-stained with anti-CD56 and anti-p75/AIRM1 mAbs. Three representative donors from groups 1, 2, and 3 are shown at different time intervals after transplantation. (B) For comparison, NK cells isolated from 2 representative healthy adult donors and 1 CB sample are shown. (C-D) Freshly isolated NK cells were double-stained with anti-p75/AIRM1 and anti-KIR (C) or anti-NKG2C (D) mAbs at 1, 3, 6, and 12 months after UCBT. In the lower right quadrant the percentage of p75/AIRM1−KIR+ and of p75/AIRM1−NKG2C+ cells is indicated. A representative patient from group1 (top) and one from group 2 (bottom) are shown. (E) The same analysis was performed for NK cells from a patient belonging to group 3, a CB, and 2 healthy donors. In the double fluorescence analyses against NKG2C, a different anti-p75/AIRM1 mAb displaying lower reactivity had to be used for technical reasons.
Progressive expansion of p75/AIRM1−KIR+ and p75/AIRM1−NKG2C+ NK cells. (A) Freshly isolated NK cells were double-stained with anti-CD56 and anti-p75/AIRM1 mAbs. Three representative donors from groups 1, 2, and 3 are shown at different time intervals after transplantation. (B) For comparison, NK cells isolated from 2 representative healthy adult donors and 1 CB sample are shown. (C-D) Freshly isolated NK cells were double-stained with anti-p75/AIRM1 and anti-KIR (C) or anti-NKG2C (D) mAbs at 1, 3, 6, and 12 months after UCBT. In the lower right quadrant the percentage of p75/AIRM1−KIR+ and of p75/AIRM1−NKG2C+ cells is indicated. A representative patient from group1 (top) and one from group 2 (bottom) are shown. (E) The same analysis was performed for NK cells from a patient belonging to group 3, a CB, and 2 healthy donors. In the double fluorescence analyses against NKG2C, a different anti-p75/AIRM1 mAb displaying lower reactivity had to be used for technical reasons.
In healthy subjects or in CB virtually all NK cells expressed p75/AIRM1 (Figure 5B healthy donor 1). Only a small fraction of healthy donors (15 of 60 donors analyzed) displayed a detectable subset of CD56dimp75/AIRM1− NK cells (Figure 5B healthy donor 2).
In both groups 1 and 2, p75/AIRM1− NK cells emerging after transplantation were characterized by the expression of KIRs (Figure 5C). Notably, KIRs were both self-HLA-I and non–self-HLA-I reactive (supplemental Figures 1-2). Analysis of single KIR expressing NK cells showed that, in most instances, these cells expressed self-KIRs, although in some cases cells derived from C2/C2 persons expressed the non–self-KIR KIR2DL2/3 only (see donor 2, supplemental Figures 1-2). A progressive expansion of a large KIR+, NKG2C+ NKG2A−, p75/AIRM1− subset of NK cells occurred in groups 1 and 2, whereas it was virtually undetectable in group 3 as well as in CB and in healthy adult NK cells (Figure 5D-E; supplemental Figure 1).
Patients experiencing HCMV reactivation display rapid NK-cell maturation and expansion of p75/AIRM1−NKG2C+KIR+ cells
The expansion of NKG2C+ NK cells has been associated with HCMV seropositivity.36,37 Thus, we verified whether in our patients the expansion of p75/AIRM1−NKG2C+KIR+ NK cells could be correlated with HCMV reactivation. Indeed, all patients belonging to groups 1 and 2, but none of those of group 3, experienced HCMV reactivation during the first 3 months after transplantation (Table 2). Remarkably, in group 3 patients, different from the HCMV-infected patients, a slow NK-cell maturation occurred. Thus, the percentage of CD56bright NK cells remained high (Figure 2), and there was a delayed acquisition of the KIR+, NKG2A− phenotype (Figure 3A,C).
Possible correlation between NK-cell development and HCMV infection
| . | p75−KIRS+, % . | p75−NKG2C+, % . | p75−CD56dim, % . | CD56−CD16+, % . | KIRS+NKG2A−, % . | HCMV pp65-antigenemia . |
|---|---|---|---|---|---|---|
| Group 1 | 56 ± 18 | 40 ± 12 | 78 ± 15 | 35 ± 7 | 54 ± 15 | All pts positive |
| Group 2 | 50 ± 17 | 51 ± 15 | 78 ± 14 | 15 ± 5 | 53 ± 18 | All pts positive |
| Group 3 | 5 ± 2 | 3 ± 1 | 14 ± 5 | 7 ± 2 | 13 ± 4 | No pts positive |
| CB | 2 ± 1 | 2 ± 1 | 5 ± 2 | 16 ± 6 | 9 ± 4 | NA |
| Healthy adult | 6 ± 6 | 3 ± 2 | 11 ± 6 | 2 ± 1 | 36 ± 14 | NA |
| . | p75−KIRS+, % . | p75−NKG2C+, % . | p75−CD56dim, % . | CD56−CD16+, % . | KIRS+NKG2A−, % . | HCMV pp65-antigenemia . |
|---|---|---|---|---|---|---|
| Group 1 | 56 ± 18 | 40 ± 12 | 78 ± 15 | 35 ± 7 | 54 ± 15 | All pts positive |
| Group 2 | 50 ± 17 | 51 ± 15 | 78 ± 14 | 15 ± 5 | 53 ± 18 | All pts positive |
| Group 3 | 5 ± 2 | 3 ± 1 | 14 ± 5 | 7 ± 2 | 13 ± 4 | No pts positive |
| CB | 2 ± 1 | 2 ± 1 | 5 ± 2 | 16 ± 6 | 9 ± 4 | NA |
| Healthy adult | 6 ± 6 | 3 ± 2 | 11 ± 6 | 2 ± 1 | 36 ± 14 | NA |
Values are reported as mean ± SD. Data are referred to 6 months after transplantation. All patients from groups 1 and 2 experienced HCMV reactivation, with the exception of patient 9 in whom primary infection occurred.
HCMV indicates human CMV; pts, patients; and NA, not available.
CD56− NK cells express low levels of activating receptors and have altered effector functions
In further experiments, we focused on NK cells derived from group 1 patients at 6 months after transplantation (corresponding to the emerging of major phenotypic differences). First, we compared the CD56− NK subset with the CD56bright and CD56dim subsets for the expression of the main activating NK receptors and for the content of perforin and granzyme B. As shown in Figure 6, CD56− NK cells expressed lower levels of NKp46, NKp30, and NKG2D compared with CD56dim NK cells, whereas perforin and granzyme B were comparable. Different from patients of group 1, CD56−CD16+ and CD56dim NK cells isolated from CB displayed similar levels of activating receptors.
CD56−CD16+ NK cells express low levels of activating receptors and display compromised effectors functions. (A) Freshly isolated NK cells were stained with anti-CD56 and anti-CD16 mAbs to distinguish 3 different subsets as in Figure 4. Each subset was assessed for the surface expression of the main activating receptors and for intracellular expression of perforin and granzyme B. (B) The same NK cells were cultured overnight in the presence or in the absence of rhIL-15 and then incubated with either medium alone (not shown) or with K562 for 3 hours. After incubation, different NK-cell subsets were analyzed for CD107a expression. In parallel the different NK-cell subsets were evaluated for intracellular IFN-γ in response to K562 or phorbol 12-myristate 13-acetate plus ionomycin. The percentage of positive cells and relative mean fluorescence intensity are indicated in each histogram. A representative patient from group 1 at 6 months after transplantation and, for comparison, a representative CB and a healthy donor are shown. (C) CD107a expression and (D) intracellular IFN-γ production, after incubation with K562, are shown for CD56−, CD56dim, and CD56bright NK-cell subsets from group 1 patients (n = 4; left) at 6 months after transplantation and from healthy adult controls (n = 30; right). (E) Freshly isolated NK cells were cultured overnight in medium alone and then incubated with the FcγR+ p815 murine cell line for 3 hours in the presence or absence of anti-CD16, anti-NKp46, or anti-NKG2C mAbs. CD107a expression is shown for CD56− and CD56dim NK-cell subsets in group 1 patients (n = 4; left) at 6 months after transplantation and in healthy adult controls (n = 6; right). (C-E) The 95% CIs for the mean and statistical significance are indicated (*P < .05).
CD56−CD16+ NK cells express low levels of activating receptors and display compromised effectors functions. (A) Freshly isolated NK cells were stained with anti-CD56 and anti-CD16 mAbs to distinguish 3 different subsets as in Figure 4. Each subset was assessed for the surface expression of the main activating receptors and for intracellular expression of perforin and granzyme B. (B) The same NK cells were cultured overnight in the presence or in the absence of rhIL-15 and then incubated with either medium alone (not shown) or with K562 for 3 hours. After incubation, different NK-cell subsets were analyzed for CD107a expression. In parallel the different NK-cell subsets were evaluated for intracellular IFN-γ in response to K562 or phorbol 12-myristate 13-acetate plus ionomycin. The percentage of positive cells and relative mean fluorescence intensity are indicated in each histogram. A representative patient from group 1 at 6 months after transplantation and, for comparison, a representative CB and a healthy donor are shown. (C) CD107a expression and (D) intracellular IFN-γ production, after incubation with K562, are shown for CD56−, CD56dim, and CD56bright NK-cell subsets from group 1 patients (n = 4; left) at 6 months after transplantation and from healthy adult controls (n = 30; right). (E) Freshly isolated NK cells were cultured overnight in medium alone and then incubated with the FcγR+ p815 murine cell line for 3 hours in the presence or absence of anti-CD16, anti-NKp46, or anti-NKG2C mAbs. CD107a expression is shown for CD56− and CD56dim NK-cell subsets in group 1 patients (n = 4; left) at 6 months after transplantation and in healthy adult controls (n = 6; right). (C-E) The 95% CIs for the mean and statistical significance are indicated (*P < .05).
To evaluate their functional capability, the different NK subsets were further assessed for degranulation (CD107a expression) and IFN-γ release on stimulation with K562 target cells. As shown in Figure 6, CD56bright and CD56dim NK cells, isolated from patient 1, displayed a substantial mobilization of CD107a, which was further increased after overnight culture in the presence of recombinant human IL-15 (rhIL-15). Remarkably, degranulation of CD56− NK cells was reduced compared with CD56bright and CD56dim NK cells and could not be efficiently induced by treatment with rhIL-15 (Figure 6B-C). The analysis of IFN-γ production further confirmed the impairment of effector functions in the CD56− NK-cell subset from group 1 patients (Figure 6B,D). Moreover, experiments of reverse Ab-dependent cellular cytotoxicity with anti-NKp46, anti-CD16, or anti-NKG2C mAb indicated that the defective response was not confined to activating receptors displaying reduced surface expression (NKp46) but affected also receptors that were not reduced such as CD16 or reduced only in part such as NKG2C (Figure 6E). This functional/phenotypic asset persisted up to 1 year after the allograft (not shown). However, NK cells were not exhausted or nonreversibly dysfunctional as suggested by their ability to produce IFN-γ on stimulation with phorbol 12-myristate 13-acetate plus ionomycin (Figure 6B).
Different from NK cells derived from patients who received transplants, no major differences existed between the CD56− and CD56dim subsets isolated from CB. As shown in Figure 6, both these subsets had a limited functional capability, although rhIL-15 could increase degranulation in agreement with previous studies on CB-NK cell–mediated cytotoxicity.29,30
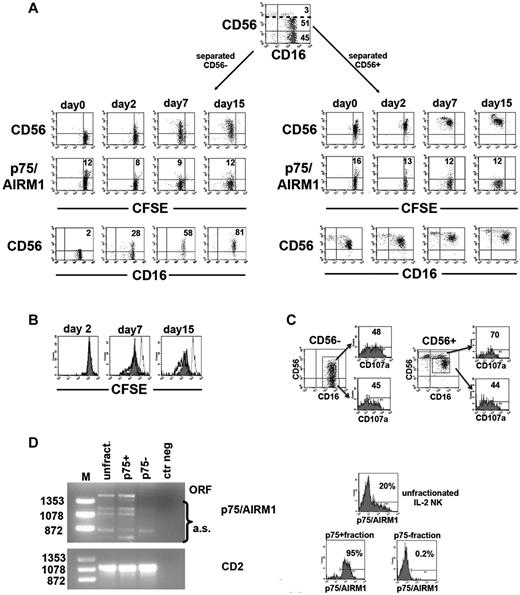
IL-2 restores CD56 surface expression and induces proliferation and effector functions in CD56− NK cells
In these experiments, NK cells freshly isolated from a group 1 patient were magnetically separated into CD56+ and CD56−. The 2 subsets were comparatively analyzed for their surface phenotype and proliferative capability (by CFSE dilution) on culture with rhIL-2 (or rhIL-15). As shown in Figure 7A (left), purified CD56− NK cells gradually expressed the CD56 Ag. In contrast, p75/AIRM1 expression was not induced either in CD56− or in CD56dim (right) subsets. As shown in Figure 7A, these experiments also showed that CD56− NK cells could proliferate in response to rhIL-2, although less efficiently than CD56dim NK cells (Figure 7B). In addition, they also increased their cytolytic function as indicated by the expression of CD107a on interaction with K562. As shown in Figure 7C, degranulation was comparable to that of CD56dim NK cells. Similar results were obtained in another patient belonging to group, 1 as well as in NK cells cultured in the presence of rhIL-15 (not shown).
Effect of rhIL-2 on NK cells lacking CD56 and/or p75/AIRM1. Peripheral blood NK cells from a group 1 patient were labeled with CFSE, magnetically separated into CD56− and CD56+ subsets, and then cultured in the presence of rhIL-2. (A) The 2 NK-cell subsets were analyzed by FACS at the indicated time intervals to assess their CD56 or p75/AIRM1 expression and their proliferation capability. The percentage of CD56+CD16+ NK cells detected in cultures containing purified CD56− cells are indicated (bottom left). CFSE dilution of NK cells stained with anti-CD56 or anti-p75/AIRM1 is also shown (top and middle). Numbers in the middle panels refer to the percentage of p75/AIRM1+ NK cells. (B) CFSE dilution at different time intervals is comparatively shown for the purified CD56− (gray profile) and CD56dim (thick open profile) purified NK-cell fractions. In each histogram the CFSE staining of NK cells at day 0 is depicted (thin open profile). (C) After 7 days of culture in the presence of rhIL-2, cultures containing purified CD56− or CD56+ NK cells were evaluated for CD107a expression after incubation with K562. The percentage of CD107a+ cells is shown for the CD56− and CD56+ NK cells derived from cultures containing the purified CD56− fraction (left) and for the CD56bright and CD56dim NK cells derived from the purified CD56+ fraction (right). (D) RT-PCR analysis of p75/AIRM1 transcripts was performed on total RNA extracted from the indicated NK-cell populations. In the p75/AIRM1 analysis the PCR products corresponding to the open reading frame (ORF) or to the alternative splicing forms (a.s.) are indicated. Molecular weights are labeled on the left. RT-PCR analysis of CD2 transcripts are shown as controls. On the right, the surface expression of p75/AIRM1 is shown on the analyzed NK-cell populations before (top histogram, unfractionated) and after FACS sorting (lower histograms, p75/AIRM1+ and p75/AIRM1−). The percentage of positive cells is indicated.
Effect of rhIL-2 on NK cells lacking CD56 and/or p75/AIRM1. Peripheral blood NK cells from a group 1 patient were labeled with CFSE, magnetically separated into CD56− and CD56+ subsets, and then cultured in the presence of rhIL-2. (A) The 2 NK-cell subsets were analyzed by FACS at the indicated time intervals to assess their CD56 or p75/AIRM1 expression and their proliferation capability. The percentage of CD56+CD16+ NK cells detected in cultures containing purified CD56− cells are indicated (bottom left). CFSE dilution of NK cells stained with anti-CD56 or anti-p75/AIRM1 is also shown (top and middle). Numbers in the middle panels refer to the percentage of p75/AIRM1+ NK cells. (B) CFSE dilution at different time intervals is comparatively shown for the purified CD56− (gray profile) and CD56dim (thick open profile) purified NK-cell fractions. In each histogram the CFSE staining of NK cells at day 0 is depicted (thin open profile). (C) After 7 days of culture in the presence of rhIL-2, cultures containing purified CD56− or CD56+ NK cells were evaluated for CD107a expression after incubation with K562. The percentage of CD107a+ cells is shown for the CD56− and CD56+ NK cells derived from cultures containing the purified CD56− fraction (left) and for the CD56bright and CD56dim NK cells derived from the purified CD56+ fraction (right). (D) RT-PCR analysis of p75/AIRM1 transcripts was performed on total RNA extracted from the indicated NK-cell populations. In the p75/AIRM1 analysis the PCR products corresponding to the open reading frame (ORF) or to the alternative splicing forms (a.s.) are indicated. Molecular weights are labeled on the left. RT-PCR analysis of CD2 transcripts are shown as controls. On the right, the surface expression of p75/AIRM1 is shown on the analyzed NK-cell populations before (top histogram, unfractionated) and after FACS sorting (lower histograms, p75/AIRM1+ and p75/AIRM1−). The percentage of positive cells is indicated.
Regulation of p75/AIRM1 expression
In view of the particular pattern of expression of p75/AIRM1 molecule, we investigated whether it was regulated at the transcriptional level. To this end we analyzed the p75/AIRM1 transcript in different polyclonally activated NK-cell populations derived from patients belonging to one or another of the 3 different groups and cultured in the presence of rhIL-2. RT-PCR analysis indicated that p75/AIRM1 transcripts were more expressed in the samples characterized by higher percentages of p75/AIRM1+ cells (not shown). Next, the p75/AIRM1 open reading frame was amplified in a polyclonal NK-cell population derived from a patient belonging to group 1. In these experiments, unfractionated NK cells were compared with the separated p75/AIRM1+ and p75/AIRM1− fractions. The intensity of the p75/AIRM1-specific PCR band was higher in the unfractionated and the p75/AIRM1+ fractions compared with the p75/AIRM1− fraction, in which the transcript was almost undetectable. Therefore, data on p75/AIRM1 transcripts were substantially in line with the surface expression of the molecule (Figure 7D).
Discussion
Our present study represents the first analysis on NK cells reconstituting after direct intrabone infusion of unrelated CB progenitors in adult patients with hematologic malignancies. We could identify 3 groups of patients in which different time intervals were required for NK-cell development, generating subsets characterized by distinct phenotypic and functional patterns.
Importantly, our data suggest that the developmental difference may be largely influenced by HCMV (re)activation. Indeed, a more rapid NK-cell maturation was detected in 2 groups of patients, in which HCMV replication was documented by pp65-antigenemia early after transplantation. In addition, in some of these patients, an aberrant NK-cell subset was generated during immune reconstitution. In agreement with the hypothesis of a virus-related effect, striking similarities existed between this NK subset and those previously described in HCV- or HIV-infected subjects.
One of the 3 groups of patients identified was characterized by the presence of a CD56−CD16+ NK subset that was maximally expanded at 6 months after UCBT and conserved at 12 and even at 24 months (as assessed in 2 monitored patients; not shown). In contrast, this subset was virtually undetectable in group 3 patients and poorly represented in group 2 patients (Figure 2A,C,E).
Notably, CD56−CD16+ NK cells are infrequent in healthy adults, but they are detectable in CB, where they were thought to represent an immature subset, capable of further differentiating into mature CD56+CD16+ cells.29,30 CD56−CD16+ NK cells were described also in peripheral blood of adult recipients given intravenous UCB transplant13 and in patients undergoing T cell–depleted HLA-haploidentical HSCT.14 In these settings, CD56− NK cells developed early after HSCT but declined 4 months after transplantation. They displayed a normal functional potential that appeared to correlate with a GVL effect.14 High proportions of CD56− NK cells were also detected in subjects chronically infected with HIV or HCV11,12,38,39 and, more recently, in patients infected with hantavirus.39 In HIV and HCV infections, CD56− NK cells displayed an altered functional capability with markedly impaired effector functions.12
In the present study, the CD56−CD16+ subset in group 1 patients was not detected at early stages after UCBT but reached high levels after 6-12 months (Figure 2). In addition, their phenotypic characteristics resembled those of CD56− NK cells from HIV-infected patients11 rather than those of CB NK cells (Figure 4). Their similarities with CD56− NK cells present in patients with HIV extended also to the impairment in functional capabilities. This may be consequent, at least in part, to the low expression of major activating receptors such as NCRs and NKG2D (Figure 6), as also reported in chronically HIV-infected patients.11 However, experiments of reverse Ab-dependent cellular cytotoxicity (Figure 6E) indicated that these cells display impaired response not only by NCRs (which are down-modulated) but also by CD16 (which is expressed at comparable levels in CD56− and CD56dim subsets). It is conceivable that CD56− NK cells may be generated when T-cell immunity is impaired. This occurs both in T-depleted HSCT and in UCBT (in which T-cell recovery is delayed because of the naive status of T cells and the administration of immune-suppressive drugs) or in virus-induced immunodeficiencies.
One fundamental question about the origin of hypofunctional CD56− NK cells is whether they may represent an aberrant population arising from progenitors under given selective pressures40 or whether they are exhausted cells (anergy from chronic stimulation?) derived from mature NK cells. Their chemokine receptor profile (not shown) and their response to rhIL-2 or rhIL-15 (Figure 7A-C) suggests their potential capability of migrating to peripheral tissues where they could recover their function in the presence of appropriate cytokines.
The phenotypic signature of CD56− NK cells present in group 1 patients (CD16+NKG2A−KIR+CD94lowCD62LlowCD57+/−) is similar to that of mature NK cells at late stages of differentiation.16,33,34 Thus, it is conceivable that they may derive from CD56dim mature NK cells down-regulating both CD56 expression and effector functions. In line with this hypothesis is also the pattern of expression of p75/AIRM1 Ag that was impaired not only in CD56− but also in CD56dim NK cells. Lack of p75/AIRM1 expression was not confined to group 1 patients but was found also in CD56dim NK cells of group 2 patients (in which there was no expansion of CD56− cells), whereas it was not detected in group 3. In this context, in HIV-infected patients, p75/AIRM1− CD56dim NK cells preceded the expansion of CD56− p75/AIRM1− NK cells.31
The presence of an expanded KIR+ NKG2A− NKG2C+ p75/AIRM1− NK-cell subset in group 1 and 2 patients suggested that these patients may have experienced a HCMV infection. Indeed, previous data indicated that NKG2C is expressed at high frequency in NK cells of otherwise healthy HCMV+ subjects. Notably, in adults, these cells are usually KIR+ NKG2A−,36 whereas in children the expression of NKG2C is not accompanied by loss of NKG2A and up-regulation of KIRs.41 Other studies have shown that HCMV-infected fibroblasts could induce the expression of NKG2C on cocultured NK cells.37 It is of note that reactivation of HCMV is common in patients given HSC transplant, especially after UCBT, whereby the absence of memory T cells in the graft and the delayed recovery of T cells significantly increase the risk of viral infections.19,28 According to this view, NK cells may markedly contribute to the control of HCMV infection after UCBT. In another report, NKG2C+ NK cells have been proposed to play a crucial role in the resolution of HCMV infection in a T cell–deficient patient.42
Remarkably, both group 1 and 2 patients experienced HCMV infection within the first 3 months after UCBT, whereas group 3 patients did not (Table 2). Patients belonging to group 3 displayed a delayed NK-cell maturation compared with HCMV-infected patients. In addition, although group 1 and 2 patients were characterized by a “KIR-dominant” or “high-KIR” inhibitory receptor repertoire, that of group 3 was “NKG2A-dominant” or “low-KIR” (Figure 3A,C), according to previous studies.18,43 Notably, in group 3, the frequency of CD56bright NK cells decreased 1-2 years after UCBT; however, the percentage of KIR+NKG2A− did not substantially change (data not shown). Thus, patients who never had HCMV reactivation maintain an NK phenotype similar to CB-NK cells.30 The correlation between HCMV reactivation and a rapid NK-cell development/maturation suggests a major role for the virus infection itself. HCMV could provide (either directly or indirectly) a stimulatory signal through which developing NK cells undergo a rapid maturation toward a fully differentiated stage. At the present it is difficult to explain why only some of the HCMV-infected patients expand the CD56− subset. Differences in CD56 expression might be related, for example, to the different capability of CB donors to produce cytokines, such as IL-15, that can restore CD56 expression (Figure 7). HCMV has also been reported to promote virus-specific, as well as nonspecific T-cell recovery in children receiving T cell–depleted HSC transplant.44
HCMV reactivation/infection also occurs in patients with HIV at late stages of disease. Moreover, patients with HIV display high levels of NKG2C+ and low levels of NKG2A+ NK cells.45,46 Thus, one may speculate that the main similarities between HIV viremic patients and group 1 and 2 patients could reflect, in both cases, the modulating properties of HCMV.
It cannot be excluded that down-regulation of p75/AIRM1 and the subsequent loss of CD56 may be related to HCMV infection both in HIV+ and in patients who received UCB transplant and that the p75/AIRM1− NK-cell subset might be directly involved in antiviral responses. The lack of activation Ags (eg, CD25, CD69, NKp44) or proliferation markers such as Ki67 (not shown) may suggest that p75/AIRM1− cells represent a subset of long-living NK cells that have down-regulated their activation markers after clearance of the virus. In this context, the lack of p75/AIRM1 seems to represent a stable phenotype, largely regulated at the transcriptional level (Figure 7D) and not revertible by in vitro cytokine stimulation (Figure 7).
In future studies it will be important to evaluate whether the p75/AIRM1− NKG2C+ KIR+ CD57+ CD56dim/neg NK-cell subset detected after UCBT contains “memory” NK cells as suggested by studies in mice after CMV infection.47,48 In line with this hypothesis, a recent study by Lopez-Verges et al showed that CD56dim NK cells coexpressing CD57 and NKG2C (possibly representing memory cell”) expand during acute HCMV infection in patients who received certain solid-organ transplants.49 Another issue that needs to be investigated is the role of HCMV infection on the GVL effect related to NK-cell alloreactivity in this particular transplantation setting. Interestingly, a recent study reported a correlation between early HCMV reactivation and reduction of leukemia relapse after allogenic HSCT in adult patients50 ; thus, it cannot be excluded that HCMV might also favor alloreactive responses mediated by NK cells. Finally, it will be also important to understand to what extent our present data might be influenced by the mode of administration of UCB progenitors (intrabone vs the more conventional intravenous route).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr G. Reggiardo (Data Management Unit, Medi Service, Genoa, Italy) for helpful suggestions in statistical analyses, Dr Benvenuto Federica and Dr Ivaldi Federico (Center of Excellence for Biomedical Research, Genoa, Italy) for cell sorting, and the staff at the Stem Cell Center (Azienda Ospedaliera San Martino, Genoa, Italy) for their help in providing samples.
This work was supported by the Associazione Italiana Ricerca sul Cancro (IG project 10643, A.M.), (grant 10225, L.M.), (grant 5544, F.F.), and (Special Project 5 × 1000 number 9962, A.M., L.M., and F.L.); Ministero dell'Istruzione, Università e Ricerca (MIUR-FIRB 2003 project RBLA039LSF-001/003, A.M. and L.M.); Ministero della Salute (RF2006-Ricerca Oncologica-Project of Integrated Program 2006-08, agreement number RO strategici 3/07, A.M. and L.M.), (Ricerca Finalizzata 2007, M.F.), and (G35J11000180001, F.F.); Compagnia di San Paolo Torino (F.F.); Progetto CARIGE Cellule Staminali (F.F.); and Associazione Italiana Leucemie, Sezione Ligure.
Authorship
Contribution: M.D.C. designed research, performed experiments, and wrote the paper; M.F. performed experiments and wrote the paper; M.P. and F.F. designed research, recruited study subjects, and contributed to data analysis and paper writing; F.L. critically revised the paper; L.M. provided economic support and revised the paper; and A.M. designed research, interpreted data, provided economic support, and wrote the paper.
Conflict-of-interest disclosure: A.M. is a founder and shareholder of Innate-Pharma (Marseille, France). The remaining authors declare no competing financial interests.
Correspondence: Alessandro Moretta, Dipartimento di Medicina Sperimentale, Sezione di Istologia, Via G.B. Marsano 10, 16132 Genova, Italy; e-mail: alemoret@unige.it.
References
Author notes
F.F. and A.M. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal