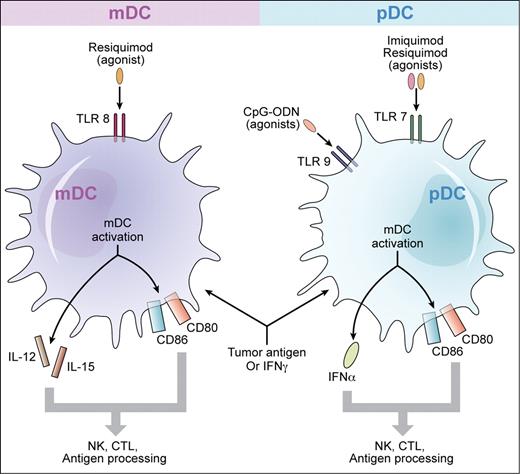
Toll-like receptor agonists plus tumor antigen, or interferon γ, may be therapeutically active for cutaneous T-cell lymphoma. The major populations of human dendritic cells include myeloid dendritic cells (mDCs) that express TLR8 and plasmacytoid dendritic cells (pDCs) that express TLRs 7 and 9. In the study by Kim et al, vaccination with CpG-ODNs, which are agonists for TLR9, was used to prime pDCs simultaneously with local radiation to active CTCL skin lesions. Other TLR agonists in clinical development for CTCL include imiquimod, an agonist for TLR7, and resiquimod, an agonist for TLR8. These TLR agonists may produce synergistic activation of the immune response with IFN γ. Similarly, there may be additive or synergistic stimulation of an antitumor response when a TLR agonist is used in combination with apoptotic tumor cells. On activation of mDCs, IL-12 and IL-15 are produced while activation of pDCs results in production of IFNα. There is also up-regulation of the co-stimulatory molecules CD80 and CD86. Induction of cytokines and up-regulation of co-stimulatory molecules are highly beneficial for the adaptive immune response leading to the development of antitumor cytotoxic T cells. Professional illustration by Kenneth X. Probst.
Toll-like receptor agonists plus tumor antigen, or interferon γ, may be therapeutically active for cutaneous T-cell lymphoma. The major populations of human dendritic cells include myeloid dendritic cells (mDCs) that express TLR8 and plasmacytoid dendritic cells (pDCs) that express TLRs 7 and 9. In the study by Kim et al, vaccination with CpG-ODNs, which are agonists for TLR9, was used to prime pDCs simultaneously with local radiation to active CTCL skin lesions. Other TLR agonists in clinical development for CTCL include imiquimod, an agonist for TLR7, and resiquimod, an agonist for TLR8. These TLR agonists may produce synergistic activation of the immune response with IFN γ. Similarly, there may be additive or synergistic stimulation of an antitumor response when a TLR agonist is used in combination with apoptotic tumor cells. On activation of mDCs, IL-12 and IL-15 are produced while activation of pDCs results in production of IFNα. There is also up-regulation of the co-stimulatory molecules CD80 and CD86. Induction of cytokines and up-regulation of co-stimulatory molecules are highly beneficial for the adaptive immune response leading to the development of antitumor cytotoxic T cells. Professional illustration by Kenneth X. Probst.
Importantly, the study of Kim and colleagues in this issue of Blood demonstrates that in situ vaccination of skin lesions of cutaneous T-cell lymphoma (CTCL) patients with subtherapeutic doses of the TLR9 agonist CpG oligodeoxynucleotides (ODNs) simultaneously with localized lesional radiation can lead to regression of distant, untreated clinical lesions.4 These results suggest that local radiation may render the malignant T-cell antigens more available for processing by antigen-presenting cells and that the priming of TLR9-bearing plasmacytoid dendritic cells (pDCs) by CpG-ODNs, even at subtherapeutic doses, appears to be adequate for the enhancement of processing of released tumor antigen leading to the generation of an anti-tumor response. The immunologic effects of this approach were evident on histologic examination of regressing lesions that demonstrated a decline in numbers of CD25+/Foxp3+ regulatory T cells along with an accompanying significant increase in pDCs. There was no change in these cellular populations within lesions of patients who had no response. Although the 6-mg dose of the CpG used in this study was previously demonstrated in a phase 1 trial to be subtherapeutic,2 it appears that the DC priming effect was nevertheless adequate to produce responses with the multimodality approach employed here.
There are numerous reasons why this combined approach of a dendritic cell activator along with a proapoptotic stimulus are most compelling as a therapeutic approach to CTCL. Clearly, maintenance of the host immune response is highly desirable for patients with CTCL. Suppression of the immune response in advanced-stage disease by agents that do not induce apoptosis of tumor cells, such as with cyclosporine or azathioprine, can lead to rapid progression of disease. Furthermore, for those who require systemic therapy, immune augmentative approaches are proving to be highly beneficial.5 It has long been known that IFNα, a product of pDCs, can produce substantial therapeutic benefit for all stages of CTCL.6 Moreover, IL-127 and IFNγ, both products of innate immune cells (the former from myeloid DCs and the latter largely from natural killer cells), have significant activity for CTCL.6 These cytokines can help drive and sustain a T helper type 1 (Th1) response that appears to be inhibited by the prevailing Th2 milieu that is typical of Sezary syndrome, the leukemic variant of CTCL. Therefore, agents that can activate the innate immune response with production of these cytokines are likely to ameliorate the immune abnormalities and to improve the disease manifestations.
In that regard, Wysocka and colleagues have demonstrated that several classes of TLR agonists have the capacity in vitro to potently activate innate immune cells of patients with advanced, refractory CTCL8,9 (see figure). The cells of patients with high tumor burden Sezary syndrome produce high levels of INFα in response to Type A CpG-ODNs. Significant activation of natural killer cells and CD8+ T-cells and a marked increase of natural killer cell cytolytic activity can also be observed. These effects can be synergistically enhanced if the patients' cells were initially primed with either IFNγ or IL-15. More remarkably, in vitro experimentation with members of the imidazoquinoline family, which are synthetic agonists for TLRs 7 and 8, and which appear to be significantly more potent than CpG-ODNs, broadly activated cellular immune responses of these patients. Similarly, marked synergism was observed with IFNγ priming of cells.
The in vitro potency of these TLR agonists has been translated into clinical benefit when either CpG-ODNs or imidazoquinolines have been administered to patients with CTCL. Kim et al, in a phase 1 study of a type B CpG administered subcutaneously, treated 28 highly refractory patients with advanced CTCL.2 Although the maximal tolerated dose was never reached, and the lower doses, including the 6-mg dose that produced responses when combined with local lesional radiation in the latest study, were ineffective at producing disease responses, overall a 32% response rate was observed, including several complete responses. Imiquimod, a TLR7 agonist in the imidazoquinoline family, when applied as a cream directly to CTCL skin lesions can induce local immune activation that can be associated with lesion regression.3 However, it has quite a low bioavailability leading to inconsistency of clinical response. Moreover, responses are critically dependent on numbers of resident pDCs within lesions that can be activated by imiquimod. A variety of factors can lead to diminished numbers and function of pDCs in the skin including the use of potent topical steroids and administration of ultraviolet light, each of which may induce apoptosis of dendritic cells. In addition, application of topical tacrilimus may inhibit the ability of resident pDCs to further activate surrounding normal T cells that are necessary for the induction of an adaptive immune response against the tumor cells.
Resiquimod, which is an agonist for TLR7 expressed on pDCs, as well as an agonist for TLR8 expressed on mDCs, is a promising member of the imidazoquinoline family that has yet to be put into clinical trial for CTCL.9 Its bioavailability as a topical gel is 10-fold greater than that of imiquimod cream and its potency may be up to 100 times greater. It could potentially be used as a single agent with potency great enough to induce systemic immune activation after cutaneous application. It may also be ideal to use it in combination with IFNγ with which it can synergize to broadly activate Th1 type cellular immunity.
Multimodality approaches like that used by Kim et al, employing a proapoptotic stimulus along with an immune adjuvant, such as a TLR agonist, are well suited for the therapy of CTCL. Other means of inducing high levels of apoptosis of tumor cells that are currently considered standard of care for CTCL are PUVA and photopheresis. Each of these has been demonstrated to produce higher response rates when combined with immune-boosting agents such as interferon α. Now that much more powerful TLR agonists such as resiquimod are available for clinical testing, this is the opportune time to undertake a study of multimodality therapy employing these exciting immune stimulants.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal