Abstract
EBV-associated smooth muscle tumors are found in immunocompromised patients, most commonly HIV/AIDS. We present a 12-year-old girl with the first documented case of EBV-related smooth muscle tumors in the presence of a rare classic NK cell deficiency. This sheds light on the role of NK cells in controlling EBV-related smooth muscle tumors.
Introduction
Natural killer (NK) cell deficiencies have provided insight into their role against a variety of infections.1 As part of the innate immune response, these cells provide direct cytotoxicity, cytokine production, and costimulation of immune cells.1,2 Patients with isolated NK cell deficiencies are exceedingly rare but are highly susceptible to herpes viral infections. Epstein-Barr virus (EBV) is an oncogenic herpetic virus and is associated with smooth muscle tumors (SMTs) in HIV and transplant patients. EBV has never been linked to an isolated NK cell deficiency.3-6
Classic NK deficiency is defined as a deficiency in NK cell numbers and function with normal NKT cell (CD56+/CD3+) numbers in the presence of otherwise intact immunity.1 We report, to our knowledge, the first case of classic NK deficiency discovered in the presence of bilateral EBV+ adrenal SMTs.
Methods
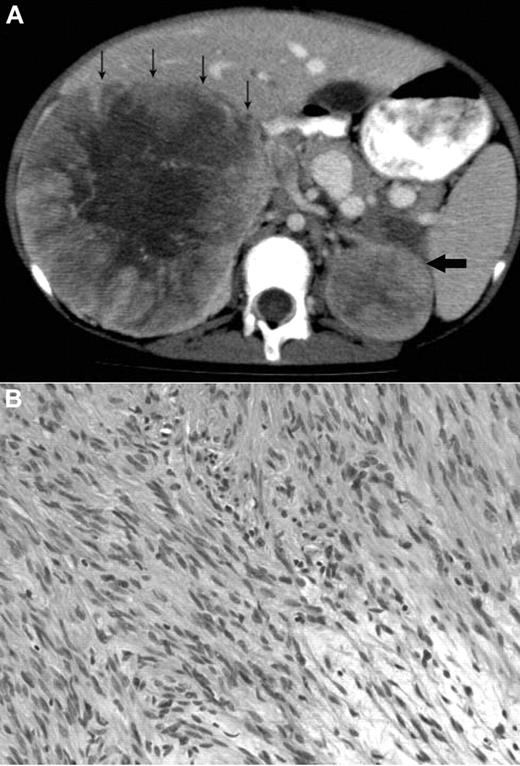
A 12-year-old Metis, Canadian aboriginal group, female presented with a 2-month history of abdominal and back pain with decreased energy. Her medical history included presumed fetal alcohol syndrome with moderate to severe developmental delay, cutis laxa with aortic root dilation, anemia secondary to celiac disease, and seizures. She did not have recurrent or unusual infections. Physical examination at diagnosis showed palpable masses confirmed to be bilateral adrenal masses on abdominal CT scan (Figure 1A). The parents provided written informed consent for the studies in accordance with the Declaration of Helsinki and publication of results per Children's Hospital of Philadelphia Institutional Review Board approval requirements.
Radiologic and histologic findings. (A) Axial CT view of the upper abdomen after intravenous contrast injection and oral contrast administration: Large solid mass in the right suprarenal region with faint central calcification and central stellar hypodensity. Small arrows indicate the anterior border of this mass. The smaller more homogeneous solid lesion in the left suprarenal region is indicated by one large arrow. The right-sided lesion displaces the inferior vena cava anteriorly and medially, and there is visibility of an intracaval defect corresponding to a clot. (B) H&E stain showing elongated spindle cells arranged in a storiform pattern admixed with scattered chronic inflammatory cells (original magnification ×400). The images were captured digitally through a Nikion Eclipse i80 microscope (Nikon) with a 40×/0.75 objective at room temperature without flourochromes. The camera was a Pixelink PL-SW Microscopy (Canimpex Enterprise Ltd) with acquisition software of Pixelink Capture SE Version 4 software for Windows XP (Canimpex Enterprise Ltd). No subsequent image reprocessing occurred.
Radiologic and histologic findings. (A) Axial CT view of the upper abdomen after intravenous contrast injection and oral contrast administration: Large solid mass in the right suprarenal region with faint central calcification and central stellar hypodensity. Small arrows indicate the anterior border of this mass. The smaller more homogeneous solid lesion in the left suprarenal region is indicated by one large arrow. The right-sided lesion displaces the inferior vena cava anteriorly and medially, and there is visibility of an intracaval defect corresponding to a clot. (B) H&E stain showing elongated spindle cells arranged in a storiform pattern admixed with scattered chronic inflammatory cells (original magnification ×400). The images were captured digitally through a Nikion Eclipse i80 microscope (Nikon) with a 40×/0.75 objective at room temperature without flourochromes. The camera was a Pixelink PL-SW Microscopy (Canimpex Enterprise Ltd) with acquisition software of Pixelink Capture SE Version 4 software for Windows XP (Canimpex Enterprise Ltd). No subsequent image reprocessing occurred.
Complete blood count and differential were normal except for mild and intermittent thrombocytopenia. Serology was positive for EBV-VCA IgG, negative for EBV-VCA IgM, and negative for HIV on more than one occasion. CT revealed right and left adrenal masses 21 × 12 × 11 cm and 6 × 5 × 4.5 cm in size, respectively. Staged tumor mass resections were performed because of initial severe malnutrition related to her celiac disease. The patient has had no recurrence at 26 months after the final resection. Experimental investigations were performed after written consent though an institutional review board–approved protocol at the Children's Hospital of Philadelphia.
Results and discussion
The microscopic findings in the left and right adrenal tumors were diagnostic of EBV-SMTs7,8 (Figure 1B). Elongated spindle cells with eosinophilic cytoplasm were present along with inflammatory cells, including lymphocytes and histiocytes. The endothelial cell lining was in a hemangiopericytoma-like pattern. Immunoperoxidase staining demonstrated positive vimetin, smooth muscle actin, and desmin, all specific findings in SMTs.8 Lastly, in situ hybridization for EBV early RNAs produced strong and diffuse reactivity in addition to positive nuclear staining of EBV early RNAs. An initial tumor biopsy showed a translocation t(4;21) (q21:q11.2) but was not replicated in subsequent cytogenetic cultures and is of uncertain significance. Constitutional chromosomes were normal.
The presence of SMT in an HIV-negative person demanded further immunologic evaluation. The immunologic and serologic investigations are summarized in supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The patient had intermittent borderline lymphopenia but normal T and B lymphocyte numbers, normal IgG levels, and specific IgG to both protein and polysaccharide vaccine antigens. T lymphocyte proliferation to mitogens, antigens, and cytotoxic T lymphocyte activity against allogeneic EBV B cells were normal. There was, however, a consistent and marked deficiency in cytotoxicity by 51Cr-release assay and absolute numbers of CD3−/CD16+/CD56+ NK cells by flow cytometry on 4 separate evaluations spanning 18 months. This deficiency remains now 26 months after successful treatment of her EBV-SMT. Further NK function evaluations, performed when the patient was well, demonstrated no detectable NK cell activity against K562 erythroleukemia and 721.221 B lymphoblastoid cell targets, and activity was only marginally induced after short-term IL-2 stimulation (Figure 2A-B). There was also undetectable antibody-dependent cellular cytotoxicity against anti-CD20 mAb (rituximab) opsonized Raji cells as targets (Figure 2C). The phenotype of the few NK cells present in the patient was evaluated and, in the majority, was similar to control. However, potential differences that were noted were an increase in CD117 expression that could suggest immaturity, or incomplete development (Figure 2D). CD56dim and CD56bright cells were distinguishable.
Deficient patient NK cell numbers and cytolytic functions. Evaluation of the NK cell cytotoxicity by 4-hour 51Cr-release assay against (A) K562, (B) 721.221, or (C) Raji target cells using PBMCs. For the Raji cells, lysis was evaluated with or without the addition of rituximab to opsonize the cells as indicated in the legend. For K562 and 721.221 target cells, 1000 U/mL IL-2 was added to the assay for its duration as indicated in the legends. Statistical significance was calculated using the Mann-Whitney U test. (D) Phenotypic evaluation of NK cells demonstrating forward versus side scatter gating strategy (left), CD56 versus CD3 with the square gate denoting classic NK cells (middle-left), the presence of CD16 on the gated CD56+/CD3− NK cells using 2 different clones of anti-CD16, B73.1, and 3G8 (middle right), and the expression of CD56 as a percentage of maximal expression on the gated NK cells (right). Results for the patient (top) and a control (bottom) are shown. Evaluation of NK cell subsets and functional markers are for gated CD56+/CD3− classic NK cells only. Fluorescence intensity for an IgG isotype control (black) is shown compared with that for the antigen-specific antibody for a control (blue) and the patient (red). The control IgG for the patient is shown but was similar to that for the control donor. All results are representative of at least 3 independent repeats. NKp44, NKG2C, and IL-15RB were performed on a sample separate from the others.
Deficient patient NK cell numbers and cytolytic functions. Evaluation of the NK cell cytotoxicity by 4-hour 51Cr-release assay against (A) K562, (B) 721.221, or (C) Raji target cells using PBMCs. For the Raji cells, lysis was evaluated with or without the addition of rituximab to opsonize the cells as indicated in the legend. For K562 and 721.221 target cells, 1000 U/mL IL-2 was added to the assay for its duration as indicated in the legends. Statistical significance was calculated using the Mann-Whitney U test. (D) Phenotypic evaluation of NK cells demonstrating forward versus side scatter gating strategy (left), CD56 versus CD3 with the square gate denoting classic NK cells (middle-left), the presence of CD16 on the gated CD56+/CD3− NK cells using 2 different clones of anti-CD16, B73.1, and 3G8 (middle right), and the expression of CD56 as a percentage of maximal expression on the gated NK cells (right). Results for the patient (top) and a control (bottom) are shown. Evaluation of NK cell subsets and functional markers are for gated CD56+/CD3− classic NK cells only. Fluorescence intensity for an IgG isotype control (black) is shown compared with that for the antigen-specific antibody for a control (blue) and the patient (red). The control IgG for the patient is shown but was similar to that for the control donor. All results are representative of at least 3 independent repeats. NKp44, NKG2C, and IL-15RB were performed on a sample separate from the others.
Extended evaluation of NK cell subsets failed to identify any absence in NK cell differentiation markers and demonstrated normal perforin expression (Figure 2D). However, severely decreased NKG2D activation receptors were found, an important activation receptor involved in mediating tumor control. Cytotoxicity of NK subsets was not increased, but IL-2 expression of certain receptors was. In summary, this demonstrates an abnormal quantity of NK cells in the periphery, despite a normal phenotype. It is unclear whether the deficiency results from abnormal development from NK cell precursors, or decreased survival of matured cells.
NK cells work in concert with T cells to provide optimal defense against EBV.9 Inadequate responses in immunodeficient patients permit not only EBV replication but also EBV-mediated cellular proliferation. We speculate that the severe deficiency of NK cells in our patient, despite normal cytotoxic CD8+ T cells, was sufficient to compromise the initial immune response, therefore contributing to the development of an EBV-SMT as seen in HIV, organ transplant, and congenital T-cell deficiency patients.3-8,12 The NK cell deficiency appeared to be quantitative because the few cells present were phenotypically normal and low-level activity could be detected after IL-2 stimulation. It has been shown that EBV transformation can increase levels of indoleamine 2,3-dioxygenase in some cells, leading to down-modulation of NKG2D from NK cells.13 It is possible that an increased level of indoleamine 2,3-dioxygenase activity on the SMTs resulted in a down-regulation of NKG2D during interaction of the NK and tumor cells. These findings suggest a severe clinically relevant selective impairment in NK cell development and underscore the importance of NK cells in controlling EBV oncogenicity.
EBV-related SMTs have been described in the setting of HIV/AIDS and organ transplant but not NK cell deficiencies.7,10-12,14,15 Congenital immunodeficiency, such as ataxia telangiectasia and T-cell deficiency, have been linked to EBV-SMT; however, these cases are extremely rare.3-5 Our case demonstrates that a broader range of immunodeficiency syndromes may be associated with the development of EBV-SMTs and highlights the utility of immune defenses outside of the adaptive system. Adrenal gland involvement in EBV-SMTs is surprising given the limited smooth muscle present.6 Nevertheless, 8 cases of unilateral adrenal EBV-SMTs in patients with HIV or AIDS have been documented since 1995,7,15,16 although only 2 involved children. Bilateral adrenal involvement has only been reported in one case of an 11-year-old girl with HIV.6 In light of the present observations, it is worth noting that NK cell functional defects are known in HIV/AIDS, and it is possible that a unifying theme in susceptibility to EBV-SMT can be impaired NK cell defense.17
Treatment of EBV-SMTs in patients with HIV/AIDS has included tumor resection, chemotherapy, and radiation therapy, although the optimal management is unknown.18 In our case, resection of the tumor has been sufficient treatment to date.
We report the first case of bilateral adrenal EBV-SMT in a child with a quantitative classic NK deficiency. This supports the role NK cells play in controlling herpes viral infections. Furthermore, EBV-SMTs have been attributed to congenital immunodeficiencies in few cases despite numerous occurrences in HIV and transplant patients. Therefore, if other causes have been excluded, NK cell deficiency, either qualitative or quantitative, should be considered when EBV-SMT is identified. Although we are unable to prove this definitely, predisposition is probable given that the NK cell deficiency has persisted after successful treatment of EBV-SMTs. Finally, although the most common diagnosis for bilateral adrenal tumors in children is neuroblastoma, our case indicates that clinicians should be aware of the possibility of EBV-SMTs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their patient and her family.
J.S.O. was supported by the National Institutes of Health (grant AI067946).
National Institutes of Health
Authorship
Contribution: R.K.S. collected data and wrote the manuscript; A.C.I. designed and performed research, collected, analyzed, and interpreted data, and critically reviewed the manuscript; R.F., P.S., and B.M. collected data and reviewed and approved the final manuscript; L.M.-S. performed research and reviewed and approved the final manuscript; J.S.O. designed and performed research, analyzed and interpreted data, and critically reviewed the manuscript; and C.V.F. designed research, collected data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Conrad V. Fernandez, Department of Pediatrics, IWK Health Centre, Dalhousie University, 5850 University Ave, Halifax, NS, Canada B3K 6R8; e-mail: conrad.fernandez@iwk.nshealth.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal