Abstract
Sickle cell trait (HbAS) is known to be protective against Plasmodium falciparum malaria, but it is unclear when during the course of infection this protection occurs and whether protection is innate or acquired. To address these questions, a cohort of 601 children 1-10 years of age were enrolled in Kampala, Uganda, and followed for 18 months for symptomatic malaria and asymptomatic parasitemia. Genotyping was used to detect and follow individual parasite clones longitudinally within subjects. Children with HbAS were protected against the establishment of parasitemia, as assessed by the molecular force of infection at older but not younger ages (at 2 years of age: incidence rate ratio [IRR] = 1.16; 95% confidence interval [95% CI], 0.62-2.19; P = .6; at 9 years of age: IRR = 0.50; 95% CI, 0.28-0.87; P = .01), suggesting an acquired mechanism of protection. Once parasitemic, children with HbAS were less likely to progress to symptomatic malaria, with protection again being the most pronounced at older ages (at 2 years of age: relative risk [RR] = 0.92; 95% CI, 0.77-1.10; P = .3; at 9 years of age: RR = 0.68; 95% CI, 0.51-0.91; P = .008). Conversely, the youngest children were best protected against high parasite density (at 2 years of age: relative density = 0.24; 95% CI, 0.10-0.54; P = .001; at 9 years of age: relative density = 0.59; 95% CI, 0.30-1.19; P = .14), suggesting an innate mechanism of protection against this end point.
Introduction
Malaria caused by Plasmodium falciparum continues to be a major cause of child morbidity and mortality in sub-Saharan Africa.1 The high mortality associated with malaria has exerted strong selective pressure on the human genome. Indeed, the high prevalence of the sickle hemoglobin gene (HbS), the result of a single point mutation (Glu → Val) in the sixth codon of the β globin chain,2 in subSaharan Africa is generally thought to be because of the survival advantage conferred by its heterozygous form, known as sickle cell trait (HbAS). HbAS has been shown to offer 70%-90% protection against severe malaria3-8 and 50% protection against uncomplicated malaria compared with individuals not carrying the sickle hemoglobin gene (HbAA).9-11
Although the protective effect of HbAS has been well known for more than 60 years,12-14 the point(s) during the course of infection at which HbAS offers protection against malaria remains unclear. HbAS may protect against the establishment of bloodstream infections, help to control parasite density once parasitemia is established, and/or prevent progression to symptomatic malaria. To our knowledge, no studies have investigated whether HbAS protects against the establishment of parasitemia, decreasing the incidence of patent bloodstream infections, defined as the force of infection. A reduced prevalence of asymptomatic parasitemia was seen in HbAS children in some,15 but not other,9,16 prior studies. Multiple studies showed lower parasite densities during symptomatic malaria in HbAS versus HbAA individuals, suggesting that HbAS may enhance control of infection once parasitemia is established.4,6,9,10,17-20 However, to our knowledge, no studies have investigated the effect of HbAS on the progression from patent parasitemia to symptomatic malaria.
Both innate and acquired mechanisms have been hypothesized to mediate protection conferred by HbAS. Early studies found that the biochemical and physical properties of HbAS erythrocytes led to decreased intraerythrocytic growth,21 decreased erythrocyte invasion,22 and increased sickling of infected erythrocytes.23 Recent evidence suggests that protection by HbAS may be also mediated, at least in part, by acquired immunity. Relative protection against uncomplicated malaria compared with HbAA individuals has been shown to increase with age in HbAS children.24,25 In addition, some studies have found more robust humoral immune responses against certain P falciparum Ags in HbAS individuals.19,26,27 The contribution of protection conferred by HbAS that is mediated by biochemical or other innate mechanisms versus acquired immunity remains unknown.
To better understand when during the course of infection HbAS confers protection and to distinguish between innate and acquired mechanisms of protection, a cohort of children in Kampala, Uganda was followed closely for 18 months. Using frequent samples from this cohort, we identified patent P falciparum infections and followed these episodes clinically and at the level of the individual strain using genotyping. The effects of HbAS on the establishment of blood-stage infections, control of parasitemia, and progression to symptomatic malaria were evaluated. Interaction with age was analyzed for these outcomes to investigate an acquired component of protection.
Methods
Study site and participants
The study was conducted in a cohort of 601 children living in the Mulago III Parish of Kampala, Uganda, a densely populated urban slum where malaria was mesoendemic. The details of the study cohort were described previously.11,28,29 Between November 2004 and April 2005, 601 children 1-10 years of age were randomly selected from a census performed in a geographically defined area30 and enrolled in a randomized trial of combination antimalarial therapies. Hemoglobin type was determined at enrollment by hemoglobin electrophoresis, and children with sickle cell disease (HbSS) were excluded from the study. Parents or guardians of study participants were asked to bring their children to a designated study clinic for all medical care. Each time a child presented to the clinic with a fever, defined as the subjective history of a fever within the last 24 hours or a tympanic temperature greater than 38°C, thick and thin blood smears were performed. Parasite density was estimated by counting the number of asexual parasites per 200 WBCs and calculating parasites per microliter, assuming a WBC count of 8000/mL. A smear was judged to be negative if no parasites were seen after review of 100 high-powered fields. Malaria was diagnosed if a child had fever and a thick blood smear positive for parasites at any density. Study participants were randomized to receive 1 of 3 combination therapies (amodiaquine plus sulfadoxine-pyrimethamine, amodiaquine plus artesunate, or artemether-lumefantrine) when diagnosed with their first episode of uncomplicated malaria, and were actively followed for 28 days to ensure response to therapy. The same assigned treatment was given for all subsequent episodes of uncomplicated malaria. Children had monthly blood smears performed for assessment of asymptomatic parasitemia, which was not treated.
Genotyping by capillary electrophoresis
To distinguish parasite clones within individual samples and between consecutive samples from the same subject, the highly diverse polymorphic surface Ag merozoite surface protein 2 (msp2) was genotyped using capillary electrophoresis for all positive blood smears. Using these methods, we showed previously that the probability of 2 genotypes matching by chance is less than 3%.31 Briefly, DNA was extracted from stored blood spots on filter paper using chelating resin (Chelex; Bio-Rad).32 DNA was then amplified using nested PCR with fluorescent second-round primers specific for the msp2 allelic families FC27 and IC3D7.31 Amplified PCR products were denatured and sized on a 3730xl DNA analyzer (Applied Biosystems). Alleles were determined with GeneMapper Version 4.0 software (Applied Biosystems). Two alleles from consecutive samples in the same subject were considered to represent the same strain if they belonged to the same allelic family and the amplicon size was within 1 base. Laboratory artifacts were distinguished from true alleles by a single investigator who was blinded to the status of the samples. Questionable alleles were labeled as such by the investigator and were automatically removed if the same size allele was not identified as a true allele in contiguous samples from the same subject. Failed reactions were repeated once, after which they were classified as having failed genotyping. Strains were considered persistent if they were detected in consecutive samples, allowing for one “skip,” meaning that an allele was assumed to be present and not detected if detected in 2 of 3 contiguous samples with the intervening sample negative for parasites, having failed genotyping, or with genotyping successful but the allele in question not detected.33 Strains with matching alleles were considered incident events if they were not detected for at least 2 consecutive samples. Of 2295 incident strains, only 203 (9%) matched alleles of prior strains in the same individual, with a median time between matching alleles of 148 days (interquartile range, [108-255]), suggesting that the majority of these matching alleles were truly newly acquired parasites.
To evaluate whether a significant proportion of incident strains were missed by genotyping only smear-positive samples, genotyping was performed on 100 randomly selected samples associated with negative blood smears in both the HbAS and HbAA groups. Of these 200 samples, only 2 had detectable genotypes and both were in the HbAS group. In 1 of these samples, 3 alleles were detected, 2 of which had been detected in the prior and subsequent sample from that subject and therefore already assumed to be present at that time point. The third allele was already present in the subsequent sample from that subject and therefore already counted as an incident strain. In the second sample with a detectable genotype, 1 allele was present that was already detected in a subsequent sample and therefore already counted as an incident strain. Because genotyping of 200 smear-negative samples did not alter results significantly, no further smear-negative samples were genotyped and only data from smear-positive samples were analyzed for this study.
Statistical analysis
For all analyses, the exposure of interest was hemoglobin type, classified as a binary variable: HbAS or HbAA. Interaction with age, considered as a continuous variable, was evaluated in all analyses. The primary definition of malaria was fever and any parasitemia. A secondary definition using a parasite threshold of 5000 parasites/μL was also explored, but results were similar; therefore, only data using the primary definition are reported.
To assess the association between hemoglobin type and infection dynamics, molecular force of infection, multiplicity of infection, and duration of infection were evaluated as continuous variables. Molecular force of infection was defined as the number of incident parasite strains detected by genotyping per person-year at risk.34 Time at risk excluded the 14 days after treatment for a malaria episode. Multiplicity of infection was defined as the number of unique strains detected by genotyping in a given sample. Duration of infection was defined as the number of days between the first and last positive blood smear during which a parasite strain was detected. We estimated that an asymptomatic infection extended 15 days before and 15 days after a blood smear and that infection began 4 days before a child presented with symptomatic malaria and ended with therapy unless detected in subsequent smears. The association between hemoglobin type and molecular force of infection was estimated as an incidence rate ratio (IRR) using negative binomial regression with robust inference. The association between hemoglobin type and multiplicity of infection was estimated as a relative difference using generalized estimating equations with robust inference, accounting for repeated measures within individuals.35 Multiplicity of infection might theoretically be underestimated more in samples containing low parasite density. Therefore, potential confounding of the association between hemoglobin type and multiplicity of infection by parasite density was assessed but was not present, and unadjusted results are reported. The association between hemoglobin type and duration of infection was estimated as a difference in days using generalized estimating equations with robust inference.
To assess the association between hemoglobin type and control of parasitemia, we evaluated parasite density in positive blood smears. Associations were estimated as differences in log parasite density using generalized estimating equations with robust inference.
To assess the association between hemoglobin type and the probability of developing malaria, we evaluated the risk of being parasitemic in a given month and, once parasitemic, the probability of developing symptomatic malaria that month. Months were defined by calendar time, and all months in which at least 1 blood smear was taken were included in our analysis. Parasitemic episodes included symptomatic malaria, defined at the beginning of this section, and asymptomatic parasitemia, defined as the presence of a positive blood smear in the absence of fever at least 5 days before and 28 days after a patient received treatment for malaria. Outcomes were assessed monthly; it was uncommon (12 of 9644 person-months) to have more than 1 new episode of malaria within a calendar month. Associations were estimated as RRs using generalized estimating equations with robust inference.
Statistical analysis was performed using Stata Version 9 (StataCorp) and R Version 2.9.0 software (R Foundation for Statistical Computing). P < .2 was considered significant for interaction terms36 ; P < .05 was considered significant for all other analyses.
Ethical permission
All study participants or their parents or guardians provided individual written informed consent in accordance with the Declaration of Helsinki. Approval was granted by the Uganda National Council of Science and Technology, the Makerere University Research and Ethics Committee, the University of California, San Francisco Committee on Human Research, and the University of California, Berkeley Committee for Protection of Human Subjects.
Results
Cohort characteristics
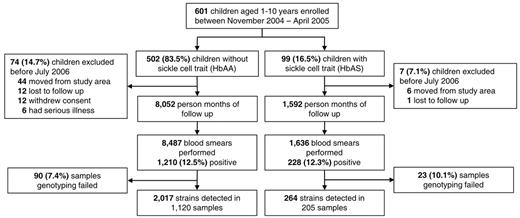
Of 601 children enrolled in the study, 99 (16.5%) had HbAS (Figure 1). Variables previously associated with risk of malaria in this cohort, such as age, use of an insecticide-treated bed net, and distance of residence from a mosquito breeding site,11 were similar between HbAA and HbAS children (Table 1). Between November 2004 and June 2006, children were followed for a total of 9644 months of person-time, with a median of 16.8 months per child. A total of 377 children (62.7%) had at least one blood smear positive for malaria parasites and 350 (58.2%) were diagnosed with at least one episode of malaria (Figure 1).
Distribution of study participants from enrollment to the end of follow-up.
Cohort characteristics in HbAS versus HbAA children
| Characteristic, n (%) . | HbAA (n = 502) . | HbAS (n = 99) . | P . |
|---|---|---|---|
| Mean age at enrollment, y (SD) | 5.9 (2.62) | 5.8 (2.73) | .69 |
| Bed net use | 217 (43.2%) | 41 (41.4%) | .74 |
| Insecticide-treated bed net use* | 31 (6.2%) | 7 (7.1%) | .34 |
| Living within 200 m of the swamp | 206 (41.0%) | 45 (45.5%) | .42 |
| Treatment arm | .79 | ||
| AQ + SP | 93 (18.5%) | 20 (20.2%) | |
| AQ + AS | 101 (20.1%) | 18 (18.2%) | |
| AL | 92 (18.3%) | 18 (18.2%) | |
| Severe malaria† | 4 (0.01%) | 0 (0.0%) | |
| Never had malaria | 212 (42.2%) | 43 (43.4%) | |
| Age distribution, y | .99 | ||
| 1-2 | 87 (17%) | 18 (18%) | |
| 3-4 | 110 (22%) | 20 (20%) | |
| 5-6 | 100 (20%) | 19 (19%) | |
| 7-8 | 115 (23%) | 23 (23%) | |
| 9-10 | 90 (18%) | 19 (19%) |
| Characteristic, n (%) . | HbAA (n = 502) . | HbAS (n = 99) . | P . |
|---|---|---|---|
| Mean age at enrollment, y (SD) | 5.9 (2.62) | 5.8 (2.73) | .69 |
| Bed net use | 217 (43.2%) | 41 (41.4%) | .74 |
| Insecticide-treated bed net use* | 31 (6.2%) | 7 (7.1%) | .34 |
| Living within 200 m of the swamp | 206 (41.0%) | 45 (45.5%) | .42 |
| Treatment arm | .79 | ||
| AQ + SP | 93 (18.5%) | 20 (20.2%) | |
| AQ + AS | 101 (20.1%) | 18 (18.2%) | |
| AL | 92 (18.3%) | 18 (18.2%) | |
| Severe malaria† | 4 (0.01%) | 0 (0.0%) | |
| Never had malaria | 212 (42.2%) | 43 (43.4%) | |
| Age distribution, y | .99 | ||
| 1-2 | 87 (17%) | 18 (18%) | |
| 3-4 | 110 (22%) | 20 (20%) | |
| 5-6 | 100 (20%) | 19 (19%) | |
| 7-8 | 115 (23%) | 23 (23%) | |
| 9-10 | 90 (18%) | 19 (19%) |
AQ indicates amodiaquine; SP, sulfadoxine-pyrimethamine; AS, artesunate; and AL, artemether-lumefantrine.
All children were given long-lasting, insecticide-treated bed nets in the last 2 months of follow-up, between May and June of 2006.
Children received quinine for treatment of severe malaria. Four children had only 1 episode of complicated malaria and no episodes of uncomplicated malaria and were not randomized to a treatment arm.
Sickle cell trait protects against establishment of blood-stage infection
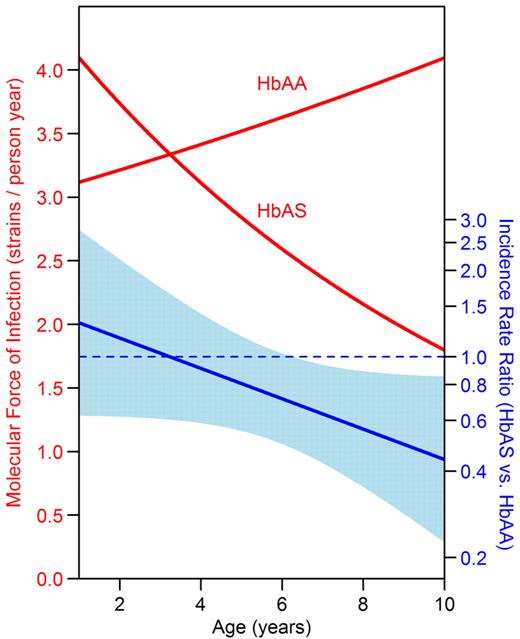
To better understand when during the course of infection HbAS is protective, we characterized the infection dynamics of P falciparum from the time patent parasitemia was first detected until parasitemia was cleared using genotyping to discriminate parasite strains. Genotyping was successful for 1325 of 1438 samples (92%), which contained 177 unique alleles. To assess differences in the ability of P falciparum to establish patent blood-stage infection in HbAA and HbAS children, we estimated the force of infection with the molecular force of infection, defined as the number of new strains detected in an individual per year.34 The molecular force of infection decreased with advancing age in HbAS children (IRR = 0.91 per 1-year increase in age; 95% CI, 0.81-1.03; P = .14), but not in HbAA children (IRR = 1.03 per 1-year increase in age; 95% CI, 0.97-1.09; P = .28; Figure 2, P value for interaction = .08). Therefore, there was no protection against force of infection conferred by HbAS in younger children (at 2 years of age: IRR = 1.16; 95% CI, 0.62-2.19; P = .64), whereas the molecular force of infection was significantly lower in those with HbAS after age 6 (at 9 years of age: IRR = 0.50; 95% CI, 0.28-0.87; P = .01). Consistent with the lower molecular force of infection in HbAS children, the multiplicity of infection was 20% lower in HbAS children for both episodes of malaria and episodes of asymptomatic parasitemia (Table 2).
HbAS children had a lower molecular force of infection than HbAA children at older ages. The y-axis on the left denotes the number of incident P falciparum strains detected in the blood per year, or the molecular force of infection. The y-axis on the right denotes the IRR of the molecular force of infection in HbAS versus HbAA children. The light blue band indicates the 95% CI for the IRR; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using negative binomial regression.
HbAS children had a lower molecular force of infection than HbAA children at older ages. The y-axis on the left denotes the number of incident P falciparum strains detected in the blood per year, or the molecular force of infection. The y-axis on the right denotes the IRR of the molecular force of infection in HbAS versus HbAA children. The light blue band indicates the 95% CI for the IRR; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using negative binomial regression.
MOI and parasite density in HbAS versus HbAA children
| Outcome . | HbAA, mean (range) . | HbAS, mean (range) . | Relative MOI (95% CI) . | P . |
|---|---|---|---|---|
| MOI* | ||||
| Overall | 2.64 (1-15) | 2.12 (1-11) | 0.79 (0.65-0.95) | .01 |
| Symptomatic malaria | 2.61 (1-15) | 2.17 (1-8) | 0.80 (0.68-0.95) | .01 |
| Asymptomatic parasitemia | 2.68 (1-15) | 2.08 (1-11) | 0.78 (0.60-1.02) | .07 |
| Parasite density† | Geometric mean (IQR) | Geometric mean (IQR) | Relative density (95% CI) | P |
| Overall | 4208 (640-38 480) | 1719 (232-13 580) | 0.42 (0.26-0.68) | < .001 |
| Symptomatic malaria | 11 955 (3000-65 520) | 5661 (1100-42 040) | 0.49 (0.29-0.85) | .01 |
| Asymptomatic parasitemia | 708 (192-2280) | 544 (96-2140) | 0.76 (0.49-1.20) | .24 |
| Outcome . | HbAA, mean (range) . | HbAS, mean (range) . | Relative MOI (95% CI) . | P . |
|---|---|---|---|---|
| MOI* | ||||
| Overall | 2.64 (1-15) | 2.12 (1-11) | 0.79 (0.65-0.95) | .01 |
| Symptomatic malaria | 2.61 (1-15) | 2.17 (1-8) | 0.80 (0.68-0.95) | .01 |
| Asymptomatic parasitemia | 2.68 (1-15) | 2.08 (1-11) | 0.78 (0.60-1.02) | .07 |
| Parasite density† | Geometric mean (IQR) | Geometric mean (IQR) | Relative density (95% CI) | P |
| Overall | 4208 (640-38 480) | 1719 (232-13 580) | 0.42 (0.26-0.68) | < .001 |
| Symptomatic malaria | 11 955 (3000-65 520) | 5661 (1100-42 040) | 0.49 (0.29-0.85) | .01 |
| Asymptomatic parasitemia | 708 (192-2280) | 544 (96-2140) | 0.76 (0.49-1.20) | .24 |
IQR indicates interquartile range.
Multiplicity of infection (MOI) was defined as the number of unique parasite genotypes detected in a positive blood smear.
Parasite density was measured via thin smear as parasites per microliter.
Sickle-cell trait protects against high parasite densities
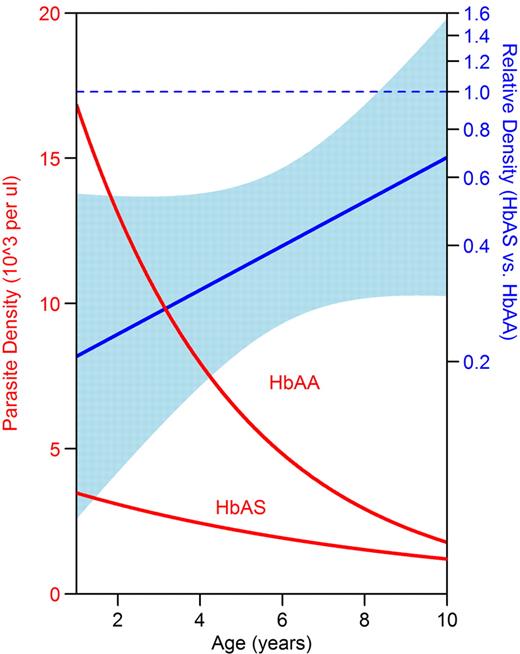
Parasite density was lower in HbAS children during episodes of malaria and asymptomatic parasitemia, although the difference did not reach statistical significance during asymptomatic parasitemia (Table 2). Parasite densities decreased significantly with advancing age in both HbAA and HbAS children, but decreased more rapidly with age in HbAA children (Figure 3, P value for interaction = .13). As a result, parasite density was significantly lower in HbAS compared with HbAA children less than 8 years of age (at 2 years of age: relative density = 0.24; 95% CI, 0.10-0.54; P = .001), whereas the difference in parasite density did not reach statistical significance in older children (at 9 years of age: relative density = 0.59; 95% CI, 0.30-1.19; P = .14).
HbAS children had lower parasite densities than HbAA children at younger ages. The y-axis on the left denotes geometric mean parasite density as measured by blood smear. The y-axis on the right denotes relative parasite density of HbAS versus HbAA children. The light blue band indicates the 95% CI for the relative density; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using generalized estimating equations with robust inference.
HbAS children had lower parasite densities than HbAA children at younger ages. The y-axis on the left denotes geometric mean parasite density as measured by blood smear. The y-axis on the right denotes relative parasite density of HbAS versus HbAA children. The light blue band indicates the 95% CI for the relative density; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using generalized estimating equations with robust inference.
Sickle-cell trait protects against progression of blood-stage infection to symptomatic malaria
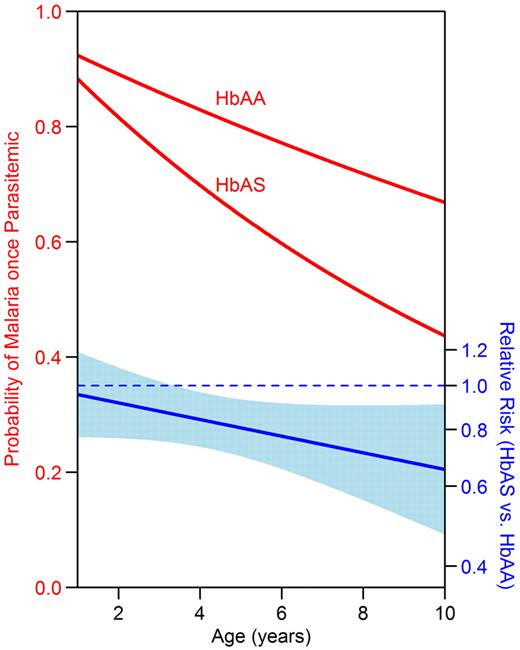
We have previously shown that the incidence of malaria was lower in HbAS children in this cohort (relative risk [RR] = 0.70; 95% CI, 0.52-0.95; P = .007).11 To determine whether children with HbAS were protected against the prevalence of parasitemia or progression to symptomatic malaria, the probability of being parasitemic and having symptomatic malaria was assessed for each month of follow-up. HbAA and HbAS children had the same probability of being parasitemic in a given month, but HbAS children were 19% less likely to develop symptomatic malaria once parasitemic (Table 3). The monthly risk of developing malaria once parasitemic decreased with advancing age in both HbAA children (RR = 0.96 per 1-year increase in age; 95% CI, 0.95-0.98; P < .001) and HbAS children (RR = 0.92 per 1-year increase in age; 95% CI, 0.87-0.97; P = .002). However, the risk of malaria decreased more rapidly with advancing age in HbAS children (Figure 4, P value for interaction = .11). As a result, there was no significant protection against progression to symptomatic malaria conferred by HbAS in younger children (at 2 years of age: RR = 0.92; 95% CI, 0.77-1.10; P = .3), whereas children more than 4 years of age with HbAS were significantly protected against progression to symptomatic malaria (at 9 years of age: RR = 0.68; 95% CI, 0.51-0.91; P = .008).
Risk of parasitemia and progression to symptomatic malaria in HbAS versus HbAA children
| Outcome . | HbAA . | HbAS . | RR (95% CI) . | P . |
|---|---|---|---|---|
| Risk of being parasitemic in a month | ||||
| Person-months evaluated | 7340 | 1455 | ||
| Person-months with parasitemia | 984 | 193 | ||
| Proportion of months with parasitemia | 0.13 | 0.13 | 1.00 (0.78-1.29) | .98 |
| Risk of malaria once parasitemic | ||||
| Person-months with parasitemia | 984 | 193 | ||
| Person-months with malaria | 684 | 105 | ||
| Proportion of parasitemic months with malaria | 0.70 | 0.54 | 0.81 (0.69-0.94) | .006 |
| Outcome . | HbAA . | HbAS . | RR (95% CI) . | P . |
|---|---|---|---|---|
| Risk of being parasitemic in a month | ||||
| Person-months evaluated | 7340 | 1455 | ||
| Person-months with parasitemia | 984 | 193 | ||
| Proportion of months with parasitemia | 0.13 | 0.13 | 1.00 (0.78-1.29) | .98 |
| Risk of malaria once parasitemic | ||||
| Person-months with parasitemia | 984 | 193 | ||
| Person-months with malaria | 684 | 105 | ||
| Proportion of parasitemic months with malaria | 0.70 | 0.54 | 0.81 (0.69-0.94) | .006 |
HbAS children had a lower risk of progression to symptomatic malaria once parasitemic than HbAA children at older ages. The y-axis on the left denotes the probability of developing symptomatic malaria in a given month if parasitemic. The y-axis on the right denotes the RR of symptomatic malaria once parasitemic. The light blue band indicates the 95% CI for the RR; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using generalized estimating equations with robust inference.
HbAS children had a lower risk of progression to symptomatic malaria once parasitemic than HbAA children at older ages. The y-axis on the left denotes the probability of developing symptomatic malaria in a given month if parasitemic. The y-axis on the right denotes the RR of symptomatic malaria once parasitemic. The light blue band indicates the 95% CI for the RR; protection is significant at the P = .05 level for ages where the band does not cross the dotted blue line. Data were analyzed using generalized estimating equations with robust inference.
To determine how long an individual parasite strain persisted in the blood, the duration of infection was evaluated. On average, parasite strains persisted approximately 11 days longer in HbAS compared with HbAA children (95% CI, 2.7-19.3; P = .006). The relationship between hemoglobin type and duration of infection did not change significantly with age. Consistent with the longer duration of infection, children with HbAS were almost twice as likely to have gametocytes detected by microscopy (RR = 1.84; 95% CI, 1.26-2.71; P = .002).
Discussion
In the present study, we provide detailed evidence on how HbAS alters the course of infection with P falciparum. For the first time to our knowledge, we have shown that HbAS children acquire fewer parasite strains in the blood than do HbAA children, demonstrating that they are protected against the establishment of patent bloodstream infections. Once an infection is established, HbAS children have lower parasite densities and a lower risk of progression to symptomatic malaria. Finally, we showed that whereas HbAS-associated protection against high parasite densities was strongest in the youngest children, protection against acquisition of bloodstream infections and progression to malaria was not present in the youngest children but developed over the first 10 years of life. These results suggest that HbAS-associated protection against high-density parasitemia is mediated by an innate mechanism, whereas HbAS-associated protection against acquisition of infection and development of symptomatic malaria is mediated by acquired mechanisms.
Protection against the establishment of blood-stage P falciparum infection may in part explain protection against symptomatic malaria in HbAS children. As a result of the lower force of infection, there was a lower multiplicity of infection in HbAS children, with fewer parasite strains present during each parasitemic episode. To our knowledge, no other studies have investigated the association between hemoglobin type and the force of infection. A few studies have investigated the effect of HbAS on multiplicity of infection, but results have been conflicting.6,37-40 One study suggested an increased multiplicity of infection with HbAS,38 leading investigators to theorize that children with HbAS have greater exposure to blood-stage P falciparum infections, inducing a more robust immune response. On the contrary, in our study, children with HbAS acquired fewer patent P falciparum infections and harbored lower multiplicities of infection, in agreement with other recent studies.6,40 An alternative explanation for the increased multiplicity of infection in HbAS children observed in one prior study is that decreased symptomatic malaria may have resulted in decreased treatment for malaria and the accumulation of more infections despite these children having a lower force of infection.
Once patent infection is established, we found that HbAS children were better able than HbAA children to control parasitemia, resulting in lower parasite densities. These data are consistent with previous studies consistently finding lower parasite densities during symptomatic disease in HbAS children compared with HbAA children.4,6,9,10,18-20 In addition, for the first time to our knowledge, we showed that parasitemia is less likely to progress to symptomatic malaria in HbAS children. This may in part be explained by either the lower number of distinct parasites acquired and/or concurrently present in the blood of HbAS children when parasitemic. With a lower likelihood of becoming symptomatic, HbAS children were less likely to be treated with antimalarials, allowing strains to persist longer in the bloodstream and explaining an equivalent prevalence of parasitemia in HbAA and HbAS children despite a reduced force of infection in HbAS children. The longer duration of infection may also explain the higher prevalence of gametocytemia that we observed in HbAS children.
Investigators have proposed multiple innate mechanisms of protection for HbAS that may in part explain the difference in infection dynamics observed in HbAS and HbAA children in our study. First, parasite viability may be diminished in altered erythrocytes; in vitro studies found decreased erythrocyte invasion,22 reduced parasite growth,21,22 and increased sickling on parasitization of HbAS compared with HbAA RBCs.23,41 Second, phagocytosis by splenic macrophages may be enhanced by morphologic changes or the increased presence of opsonins in infected HbAS erythrocytes.42 Third, altered expression of PfEMP-1 may reduce cytoadherence in infected HbAS erythrocytes,43 inhibiting the ability of these cells to sequester in the vasculature and improving clearance by the spleen.44-48 Finally, a recent study in a mouse model of severe malaria suggested that sickle hemoglobin confers tolerance to Plasmodium infection by inducing the expression of heme-oxygenase-1 (HO-1).49 These innate mechanisms could in theory inhibit the ability of strains to establish patent bloodstream infections in HbAS individuals or, once they do, the ability to reach high parasite densities and/or lead to symptoms. However, our data suggest that whereas HbAS children exhibit enhanced protection against high parasite densities at early ages, enhanced protection against both the establishment of patent bloodstream infections and progression to symptomatic malaria are not present initially but develop with advancing age. Based on these results, we posit that innate mechanisms mediate protection against high parasite densities and possibly severe disease in young HbAS children, but that enhanced development of acquired immunity results in additional protection against the establishment of patent parasitemia and progression to symptomatic malaria as HbAS children are subject to multiple infections over time.
Consistent with the hypothesis that HbAS accelerates the development of acquired immunity to P falciparum, a cross-sectional study of children 9 months to 6 years of age found that HbAS was most protective in children between 2 and 6 years of age,25 and a more recent study found a trend toward increased protection with advancing age, from 20% at 2 years of age to 56% at 10 years of age.24 Enhanced acquisition of acquired immunity in HbAS individuals may be a consequence of innate mechanisms of protection, with increased phagocytosis of infected RBCs in the spleen resulting in improved Ag presentation. Consistent with this theory, improved humoral responses to P falciparum proteins present on the surface of infected RBCs were seen in HbAS subjects.19,26,27 In addition, it has been hypothesized that high-density parasitemia may interfere with the development of an effective immune response,50 and therefore innate protection against high parasite densities afforded by HbAS may also enhance the development of acquired immunity.
In the present study, we used molecular genotyping to estimate the force of infection. A limitation inherent with any genotyping method is the inability to detect alleles below the threshold of sensitivity for the given assay. Given that parasite densities were lower in HbAS than in HbAA subjects, it is possible that we underestimated the force of infection more in HbAS than in HbAA subjects. However, this potential bias was unlikely to have affected our conclusions for several reasons. First, we have shown that nondetection of alleles is a function of relative allele proportions more than absolute parasite density.51 Second, our assay is sensitive to minority alleles, reliably detecting those present at ≥ 2%. Third, genotyping of a random subset of smear-negative samples demonstrated that subpatent parasites had a negligible effect on the results. Finally, and most importantly, parasite densities were lower in young HbAS than in HbAA subjects but differences between these groups narrowed with increasing age (Figure 3), whereas the molecular force of infection showed the opposite relationship (Figure 2). Therefore, any systematic bias in our finding of increasing protection against the establishment of patent parasitemia with age in HbAS individuals would have likely been toward the null hypothesis.
In conclusion, we have provided evidence that HbAS protects against high parasite density, likely through innate mechanisms, and against the establishment of patent parasitemia and progression to symptomatic malaria, likely as a result of enhanced development of acquired immunity. Our results suggest that several different mechanisms, both innate and acquired, are involved in the protection against malaria afforded by the single point mutation present in HbAS subjects. Further dissection of these protective mechanisms may enable us to learn valuable lessons from evolution regarding our attempts to develop interventions aimed at improving the host response against this deadly disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the parents and guardians for kindly giving their consent, the study participants for their cooperation, the members of the study team in Uganda, and Teun Bousema and Federica Verra for helpful comments on the manuscript.
This work was supported in part by a grant the National Institutes of Immunology, Allergy, and Infectious Disease (grant K23-AI076614). P.J.R. is a Doris Duke Charitable Foundation Distinguished Clinical Scientist. G.D. and B.G. are recipients of the Doris Duke Charitable Foundation Clinical Scientist Development award.
National Institutes of Health
Authorship
Contribution: L.G. performed the research, analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript; C.M.-S. collected the data; P.J.R. and C.J.D. interpreted the data and assisted with manuscript preparation; A.E.H. performed the statistical analysis; G.D. collected, analyzed, and interpreted the data and assisted with manuscript preparation; and B.G. designed the research, analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bryan Greenhouse, University of California, San Francisco, Box 0811, San Francisco, CA 94110; e-mail: bgreenhouse@medsfgh.ucsf.edu.