Abstract
Cysteinyl leukotriene (cysLT) overproduction is a hallmark of aspirin-exacerbated respiratory disease (AERD), but its mechanism is poorly understood. Because adherent platelets can convert the leukocyte-derived precursor leukotriene (LT)A4 to LTC4, the parent cysLT, through the terminal enzyme LTC4 synthase, we investigated the contribution of platelet-dependent transcellular cysLT production in AERD. Nasal polyps from subjects with AERD contained many extravascular platelets that colocalized with leukocytes, and the percentages of circulating neutrophils, eosinophils, and monocytes with adherent platelets were markedly higher in the blood of subjects with AERD than in aspirin-tolerant controls. Platelet-adherent subsets of leukocytes had higher expression of several adhesion markers than did platelet nonadherent subsets. Adherent platelets contributed more than half of the total LTC4 synthase activity of peripheral blood granulocytes, and they accounted for the higher level of LTC4 generation by activated granulocytes from subjects with AERD compared with aspirin-tolerant controls. Urinary LTE4 levels, a measure of systemic cysLT production, correlated strongly with percentages of circulating platelet-adherent granulocytes. Because platelet adherence to leukocytes allows for both firm adhesion to endothelial cells and augmented transcellular conversion of leukotrienes, a disturbance in platelet-leukocyte interactions may be partly responsible for the respiratory tissue inflammation and the overproduction of cysLTs that characterize AERD.
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a distinctive syndrome characterized clinically by a triad of asthma, nasal polyposis, and aspirin sensitivity. It is a chronic inflammatory disease associated with eosinophilic infiltration of respiratory tissues, peripheral eosinophilia, and excessive production of cysteinyl leukotrienes (cysLTs), a class of inflammatory lipid mediators that are thought to contribute to several of the characteristic features of AERD. Individuals with this syndrome account for 4% to 11% of all adult patients with asthma, and for a disproportionate share (∼ 30%) of patients with severe asthma.1 The confirmatory diagnostic feature of AERD is an idiosyncratic respiratory reaction, including symptoms of acute bronchoconstriction, nasal congestion, and eye watering, on ingestion of aspirin or another nonselective cyclooxygenase (COX) inhibitor. Despite the strikingly consistent clinical phenotype of AERD, the pathogenesis of the disease remains unclear.
CysLTs derive from the metabolism of arachidonic acid by effector cells of the innate immune system. In inflammatory leukocytes (neutrophils, monocytes, eosinophils, mast cells, and basophils), arachidonic acid is oxidized by 5-lipoxygenase (5-LO) to form the unstable intermediate leukotriene (LT)A4.2 In neutrophils, LTA4 is preferentially hydrolyzed by LTA4 hydrolase to form LTB4, whereas in monocytes, mast cells, eosinophils, and basophils, it is conjugated to reduced glutathione by the terminal enzyme LTC4 synthase (LTC4S) to form LTC4, the parent cysLT.3 LTC4 is exported out of the cell and enzymatically converted into LTD4 and then into the stable end-metabolite LTE4. Urinary LTE4 levels, a marker of systemic cysLT production, are 3 to 5 times higher in patients with AERD than in their aspirin-tolerant counterparts at baseline, and these levels can further increase by as much as 100-fold on ingestion of aspirin.4 LTC4 and LTD4 are powerful smooth muscle constrictors,5 LTE4 potently induces the accumulation of eosinophils into the bronchial mucosa,6 and all 3 cysLTs can induce vascular leak, mucous production, edema, and fibrosis. Thus, cysLTs contribute to the chronic inflammation present in the respiratory tissue of patients with AERD. They are also critical effectors of the aspirin-induced reactions that characterize AERD, because both inhibition of 5-LO and blockade of the type 1 receptor for cysLTs (CysLT receptor) can blunt the clinical severity of symptoms occurring with aspirin challenges.7,8 However, neither the cellular source of cysLTs nor the mechanisms for their overproduction in AERD are known.
Because eosinophils, basophils, mast cells, and macrophages express both 5-LO and LTC4S, they are able to catalyze the formation of LTC4 from endogenous arachidonic acid, and they probably contribute to the production of cysLTs in AERD. However, apart from immunohistochemical studies suggesting that eosinophils in the respiratory tract mucosa express higher levels of LTC4S protein in subjects with AERD than in aspirin-tolerant controls, no abnormalities in cysLT generation have been reported in cells from individuals with AERD.9,10 Moreover, it is not understood how the 5-LO–derived substrate LTA4 is provided at sufficient quantities to permit the high basal production of cysLTs characteristic of AERD. Of the circulating cells possessing 5-LO activity, neutrophils are by far the most plentiful and can generate quantities of LTA4 that exceed the capacity of their LTA4 hydrolase to convert it into LTB4. Although neutrophils lack LTC4S activity and cannot convert LTA4 into LTC4, platelets possess abundant LTC4S activity in the absence of 5-LO.11,12 Previous ex vivo studies have shown that platelets can convert unmetabolized LTA4 from neutrophils or monocytes into LTC4 through a transcellular pathway that requires P-selectin–dependent interaction between the platelet and the leukocyte.13-15 Allergen-induced pulmonary eosinophilia and airway remodeling in mouse models of asthma also require P-selectin–dependent adherence of platelets to leukocytes and subsequent augmentation of leukocyte integrin function.16 Because AERD involves both accumulation of leukocytes, particularly eosinophils, in the respiratory tissue, and systemic overproduction of cysLTs, we hypothesized that platelet-leukocyte interactions may contribute to this disease. We determined the frequencies of platelet-adherent leukocytes in the sinus tissue and blood of subjects with AERD, and then we compared them to those found in the tissue and blood of aspirin-tolerant controls. We also investigated whether adherent platelets contributed to the activation of leukocytes and to the production of cysLTs in vivo and in vitro.
Methods
Patients, materials, and human subject characterization
Patients were recruited from the Allergy, Pulmonary, and Otolaryngology clinics at the Brigham and Women's Hospital (Boston, MA) and were classified according to their clinical characteristics. Nonasthmatic controls had no history of asthma or intolerance to aspirin or other nonsteroidal anti-inflammatory drugs. Aspirin-tolerant asthmatic (ATA) controls had physician-diagnosed persistent asthma and had taken aspirin or a nonsteroidal anti-inflammatory drug within the previous 6 months without adverse reaction. Patients were suspected of having AERD if they had asthma, nasal polyposis, and a history of respiratory reaction on ingestion of a COX inhibitor. In all subjects with a compatible clinical history, the diagnosis of AERD was confirmed with a graded oral challenge to aspirin that resulted in characteristic sinonasal symptoms and a decrease in forced expiratory volume in 1 second of at least 15%. None of the subjects smoked. All subjects with AERD were treated with the CysLT1 receptor blocker montelukast during the aspirin challenge, and none were on the 5-LO inhibitor zileuton before the challenge. Clinical data regarding the subjects including pulmonary function, presence of atopy (defined as 2 or more positive skin prick tests), and use of corticosteroids and long-acting β-agonists are summarized in Table 1. For the immunohistochemical studies, nasal polyps were collected after their surgical excision from subjects with AERD or from aspirin-tolerant controls with chronic hyperplastic sinusitis. These controls with sinusitis were judged to be aspirin-tolerant if they had taken aspirin or a nonsteroidal anti-inflammatory drug within the previous 6 months without adverse reaction; 2 of the 4 aspirin-tolerant controls also had asthma. All subjects had been treated with oral prednisone (20 mg daily) for the week leading up to their sinus surgery.
Patient characteristics
| . | AERD . | ATA . | Nonasthmatic . |
|---|---|---|---|
| No. | n = 15 | n = 13 | n = 9 |
| Sex (male:female) | 7:8 | 4:9 | 5:4 |
| Median age, y (range) | 45 (20-65) | 37 (22-76) | 34 (22-52) |
| Atopic (n) | 9/15 | 10/13 | 3/9 |
| Baseline FEV1 (mean % predicted ± SD) | 82 ± 9 | 88 ± 15 | NA |
| Receiving daily inhaled corticosteroids (n) | 15/15 | 12/13 | 0/9 |
| Receiving daily oral corticosteroids (n) | 2/15 | 1/13 | 0/9 |
| Receiving daily long-acting β-agonists (n) | 10/15 | 7/13 | 0/9 |
| Eosinophils (mean % of blood granulocytes ± SD) | 15.1 ± 9.0 | 10.4 ± 6.0 | 6.4 ± 2.7 |
| . | AERD . | ATA . | Nonasthmatic . |
|---|---|---|---|
| No. | n = 15 | n = 13 | n = 9 |
| Sex (male:female) | 7:8 | 4:9 | 5:4 |
| Median age, y (range) | 45 (20-65) | 37 (22-76) | 34 (22-52) |
| Atopic (n) | 9/15 | 10/13 | 3/9 |
| Baseline FEV1 (mean % predicted ± SD) | 82 ± 9 | 88 ± 15 | NA |
| Receiving daily inhaled corticosteroids (n) | 15/15 | 12/13 | 0/9 |
| Receiving daily oral corticosteroids (n) | 2/15 | 1/13 | 0/9 |
| Receiving daily long-acting β-agonists (n) | 10/15 | 7/13 | 0/9 |
| Eosinophils (mean % of blood granulocytes ± SD) | 15.1 ± 9.0 | 10.4 ± 6.0 | 6.4 ± 2.7 |
NA indicates not applicable.
The Brigham and Women's Hospital institutional human subjects Institutional Review Board (protocol 2003-P-002088) approved the study, and all subjects provided written consent in accordance with the Declaration of Helsinki.
Immunohistochemistry
For the nasal polyp studies, polyp tissue was excised at the time of surgery, placed in sterile normal saline, and grossly examined by the pathology department. Half of the fresh tissue was allocated for our studies and within 2 hours of surgery the specimens were fixed in 4% paraformaldehyde, embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek), and kept at −80°C until sectioning. For immunofluorescent detection of leukocytes and platelets, frozen sections were air-dried, blocked with 10% mouse serum, cut into 8-μm slices, and incubated for 1 hour at room temperature with Alexa Fluor 488–labeled anti-CD45 (4 μg/mL) and Alexa Fluor 647–labeled anti-CD61 (4 μg/mL) Abs (BioLegend). Slides were then washed with PBS and mounted with SlowFade Gold antifade reagent with 4,6 diamidino-2-phenylindole nuclear stain (Invitrogen). The dry sections were evaluated under an 80i microscope (Nikon); photographs were taken under 40× objective lens (Panfluor 40×, aperture 0.75), with pictures taken for red, green, and blue wavelengths, and overlaid into RGB pictures using ImageJ (Version 1.71) software (National Institutes of Health). At least 4 randomly selected fields from each tissue sample were photographed using a Hamatsu Orca R2 digital camera (C10600). Images were acquired with HC Image software (Version 2.0.4) and evaluated by a pathologist who was blinded as to the subject's diagnosis. Cells staining for CD45 (green) and for both CD45 and CD61 (red) were counted. Separate sections were used for H&E staining.
Flow cytometry
Whole peripheral blood was drawn into heparinized tubes, kept at room temperature, and assayed within 1 hour of collection. For subjects undergoing oral aspirin challenge, blood was collected before ingestion of aspirin. We incubated 10 μL of unstimulated blood with directly conjugated antibodies specific for CD61 and CD45, and CCR3, CD11a, CD11b, CD11c, CD16, CD18, P-selectin, and/or P-selectin glycoprotein ligand 1 (PSGL-1), or appropriate isotype controls (BD Biosciences) for 20 minutes, and then fixed the cells in 1% paraformaldehyde. At least 20 000 CD45+ cells were recorded for each sample on an FACSAria flow cytometer (BD Biosciences), and they were analyzed with FlowJo Version 7.6.4 (TreeStar). CD45+ leukocytes were classified as eosinophils, neutrophils, monocytes, or lymphocytes according to their side scatter characteristics and relative expression of CD45, CD16 (to identify neutrophils), and CCR3 (to identify eosinophils), and they were assessed for the presence of adherent platelets by relative expression of CD61. Within each leukocyte population, the mean fluorescence intensity of each activation or adhesion marker was measured separately for the platelet-adherent subset and the platelet-free subset.
Western blot analysis
To measure platelet LTC4S protein, washed platelets were separated from whole blood by centrifugation, and 10 μg of platelet protein lysate was used to generate gels and then transferred onto Immun-Blot polyvinylidene difluoride membranes (Bio-Rad Laboratories) and blocked with 5% milk in tris(hydroxymethyl)aminomethane-buffered saline. Blots were incubated with either a polyclonal anti-LTC4S antibody12 or an anti–β-actin antibody (Cell Signaling Technology), washed, and then incubated with HRP-conjugated anti–rabbit IgG (Sigma-Aldrich) and visualized by enhanced chemiluminescence (GE Healthcare).
Activation of granulocytes and analysis of 5-LO pathway products and LTC4S activity
Platelet-rich plasma was removed from whole peripheral blood by centrifugation, washed, and resuspended at a concentration of 1 × 109 platelets/mL. Leukocytes were separated by 4.5% dextran gradient, and the granulocyte fraction was obtained by Ficoll-Paque (GE Healthcare) density gradient centrifugation. Contaminating erythrocytes were lysed with a hypotonic saline wash. Granulocytes were counted and a portion was stripped of adherent platelets by incubation with 0.05% trypsin-ethylenediaminetetraacetic acid for 15 minutes at 37°C.17 Removal of adherent platelets was confirmed by cytofluorographic analysis of samples of the granulocytes before and after trypsinization. Supernatants from unstripped granulocytes and platelet-stripped granulocytes stimulated for 10 minutes with 5μM A23187 were analyzed using reverse-phase high-performance liquid chromatography (RP-HPLC; Beckman Coulter) as described previously.18 The resolved products, measured from their absorbance at 280 nm, were calculated from the ratio of the peak areas to the area of the internal standard prostaglandin B2 (PGB2) and included measurement of LTB4 (retention time, 23.9 minutes), LTC4 (retention time, 21.8 minutes), LTD4 (retention time, 23.6 minutes), (5,6)-dihydroxy-7,9-trans-11,14-cis-eicosatetraenoic acid (retention time, 26.1 minute), and 6-trans-LTB4 (retention time, 23.2 minutes).19 In some experiments, supernatants from granulocytes stimulated for 10 minutes with 2μM formyl-methionyl-leucyl-phenylalanine (fMLP) were analyzed for cysLTs using the Amersham Leukotriene C4/D4/E4 Biotrak enzyme immunoassay (GE Healthcare), because cysLT concentrations induced by fMLP are too low to detect by RP-HPLC. In some experiments, aliquots of granulocytes that had been subjected to trypsinization were stimulated with A23187 in the presence of 200 × 106 exogenously added platelets to verify that their enzyme function was not compromised by trypsinization and could be restored by platelets.
The specific activity of the terminal cysLT-generating enzyme LTC4S was measured by cellular conversion of exogenous LTA4-methyl ester (ME) to LTC4-ME as described using RP-HPLC.3 In brief, aliquots of 200 × 106 washed platelets or 6 × 106 granulocytes (with or without removal of platelets by trypsinization) were provided with 10mM glutathione and 20μM LTA4-ME. After 15 minutes at 37°C, the reaction was terminated with methanol containing PGB2. LTC4-ME was quantified from the ratio of the peak area to the area of the internal standard PGB2.
Gas chromatography–mass spectrometry
Urine samples were collected, stored at −80°C, and analyzed by gas chromatography–mass spectrometry as described previously to measure concentrations of LTE4,20 the major urinary thromboxane metabolite 11-dehydrothromboxane B2 (TXB2),21 and F2-isoprostanes,22 all normalized for creatinine. For subjects undergoing oral aspirin challenge, urine was collected before ingestion of aspirin.
Statistical analysis
The data are presented as the mean + SEM unless otherwise stated. Differences in values were analyzed with the t test, because all data presented was normally distributed; significance was defined as P < .05, and all tests were 2-tailed. Effect size was measured with Pearson correlation coefficient.
Results
Patient characteristics
The 3 groups of patients were similar in age and sex, and there were no significant differences between the 2 groups of asthmatic subjects in regard to the baseline forced expiratory volume in 1 second, the presence of atopy, or the proportion of patients receiving daily inhaled corticosteroids or long-acting β-agonists as controller therapies (Table 1). The percentage of circulating granulocytes identified as eosinophils (CCR3+ cells within the CD45+ granulocyte gate; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) did not differ significantly between the 2 groups of asthmatic subjects, although nonasthmatic control subjects had lower percentages of eosinophils than either the ATA controls (P < .05) or the subjects with AERD (P < .01).
Identification of extravasated platelets and their colocalization with leukocytes in nasal polyp tissue
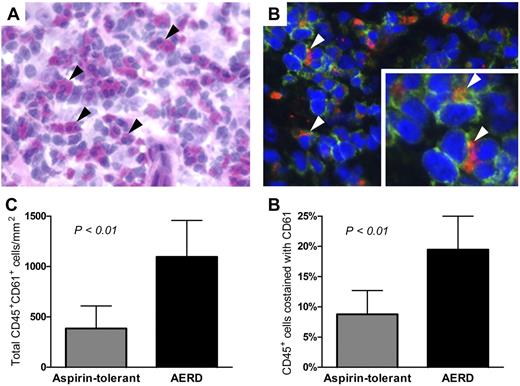
To determine whether platelet-adherent leukocytes were present in the inflamed respiratory tissue of subjects with AERD and of aspirin-tolerant controls with sinusitis, sections from surgically excised nasal polyps were double-stained for the leukocyte-specific antigen CD45 and the platelet-specific antigen CD61. Immunofluorescence was used to identify cells expressing each marker. The tissue from subjects with AERD contained abundant eosinophils, as determined by H&E staining (Figure 1A) and many extravascular CD61+ platelets (red immunofluorescent staining, Figure 1B) were present and colocalized with CD45+ leukocytes (green staining, Figure 1B). The total numbers of eosinophils were higher in subjects with AERD (196 ± 203/mm2) than in aspirin-tolerant controls (2 ± 2/mm2), although this difference did not reach statistical significance because of variation within the AERD group. The total numbers of CD45+ leukocytes in the tissue did not differ significantly between the groups (6153 ± 2108/mm2 in AERD and 4201 ± 860/mm2 in ATA). The tissue from subjects with AERD had more total platelet-associated leukocytes and a higher percentage of leukocytes that colocalized with platelets (Figure 1C-D).
Detection of platelet-leukocyte aggregates in nasal polyp tissue. (A) H&E staining of nasal polyp tissue from a subject with AERD shows many eosinophils (black arrowheads). (B) Immunofluorescent staining of the same tissue shows leukocytes (green, CD45+) with adherent platelets (red, CD61+; white arrowheads). Photographs are shown at 400× magnification. (C) Total numbers of CD45+ cells that colocalized with CD61. (D) Percentages of CD45+ cells that colocalized with CD61 in the nasal polyp tissue from aspirin-tolerant controls with sinusitis (n = 4) and subjects with AERD (n = 6). Data are expressed as mean + SD.
Detection of platelet-leukocyte aggregates in nasal polyp tissue. (A) H&E staining of nasal polyp tissue from a subject with AERD shows many eosinophils (black arrowheads). (B) Immunofluorescent staining of the same tissue shows leukocytes (green, CD45+) with adherent platelets (red, CD61+; white arrowheads). Photographs are shown at 400× magnification. (C) Total numbers of CD45+ cells that colocalized with CD61. (D) Percentages of CD45+ cells that colocalized with CD61 in the nasal polyp tissue from aspirin-tolerant controls with sinusitis (n = 4) and subjects with AERD (n = 6). Data are expressed as mean + SD.
Platelet-adherent leukocytes are detected with high frequency in AERD and exhibit altered expression of integrins
To determine whether platelet-adherent leukocytes could be identified in the peripheral blood, whole blood samples were studied by flow cytometry, using CD45 to identify leukocytes and CD61 to identify platelets. Within the CD45+ gate, eosinophils, neutrophils, monocytes, and lymphocytes were distinguished based on differential light scatter characteristics and their relative membrane expressions of CCR3 and CD16 (supplemental Figure 1). Because these cell types also could be readily distinguished from one another based solely on their light scatter characteristics and relative expression of CD45 (supplemental Figure 1), we relied on these parameters to identify the leukocyte cell types in the experiments in which we quantified adhesion receptor expression. Platelet-adherent leukocytes were identified in all patient groups (Figure 2A). There tended to be higher percentages of platelet-adherent leukocytes in the blood of ATA controls than in nonasthmatic controls, but these differences were not significant. In contrast, the percentages of platelet-adherent eosinophils, neutrophils, and monocytes in the blood of subjects with AERD were much higher than in either the ATA or the nonasthmatic controls (Figure 2B). Few platelet-adherent lymphocytes were detected in any group.
Platelet-adherent leukocytes are identifiable in peripheral blood. (A) Representative histograms of platelet-adherent eosinophils (identified as CCR3+CD45+ cells in the granulocyte side scatter [SSC] gate), neutrophils (CD16+CD45+ cells in the granulocyte SSC gate), monocytes (CD45+ in the monocyte SSC gate), and lymphocytes (CD45+ cells in lymphocyte SSC gate) in blood from an ATA control (top) and a subject with AERD (bottom). The percentages of each cell type with adherent platelets are shown. (B) Percentages of leukocytes with adherent platelets (as determined by staining with CD61) in blood of nonasthmatic controls (n = 9), ATA controls (n = 13), and subjects with AERD (n = 15). Data are expressed as mean + SD (★P < .001).
Platelet-adherent leukocytes are identifiable in peripheral blood. (A) Representative histograms of platelet-adherent eosinophils (identified as CCR3+CD45+ cells in the granulocyte side scatter [SSC] gate), neutrophils (CD16+CD45+ cells in the granulocyte SSC gate), monocytes (CD45+ in the monocyte SSC gate), and lymphocytes (CD45+ cells in lymphocyte SSC gate) in blood from an ATA control (top) and a subject with AERD (bottom). The percentages of each cell type with adherent platelets are shown. (B) Percentages of leukocytes with adherent platelets (as determined by staining with CD61) in blood of nonasthmatic controls (n = 9), ATA controls (n = 13), and subjects with AERD (n = 15). Data are expressed as mean + SD (★P < .001).
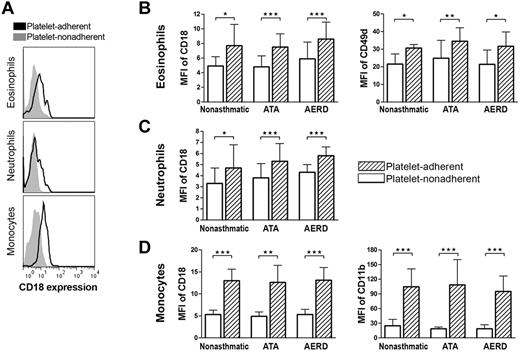
To determine the effect of adherent platelets on the expression of integrins by leukocytes, we quantified the surface expression of adhesion receptors on platelet-adherent and nonadherent subsets of leukocytes, identified as described in the previous paragraph. In all patient groups, platelet adherence was associated with modest increases in the expression of CD18, CD11a, and CD49d by eosinophils (Figure 3A-B and supplemental Figure 2A) and modest increases in the expression of CD18 by neutrophils (Figure 3A,C). Platelet-adherent monocytes displayed markedly up-regulated expression of CD18 and CD11b compared with platelet nonadherent monocytes in the same samples (Figure 3A,D), and also showed modestly increased expression of CD49d, CD11a, and CD11c (supplemental Figure 2B; data not shown). All patient groups showed similar patterns, and there were no differences between the patient groups in the expression levels of PSGL-1 on any leukocyte subsets (supplemental Figure 2C) or P-selectin by platelets (data not shown).
Expression of integrins by platelet-adherent and -nonadherent leukocyte subsets. (A) Representative histograms of relative CD18 expression by CD61− platelet-nonadherent (solid gray) and CD61+ platelet-adherent (black line) peripheral blood eosinophils (top), neutrophils (middle), and monocytes (bottom) are shown for a subject with AERD. (B-D) Relative expression of CD18 and CD49d on eosinophils (B), CD18 on neutrophils (C), and CD18 and CD11b on monocytes (D), comparing the platelet-adherent and platelet-nonadherent leukocyte subsets in nonasthmatic controls (n = 7), ATA controls (n = 10), and subjects with AERD (n = 9). MFI indicates mean fluorescence intensity. Platelet-free CD61− leukocyte subsets are shown in white columns, and CD61+ leukocyte subsets are shown in hatched columns. Data are expressed as mean + SEM (★P < .05, ★★P < .01, ★★★P < .001).
Expression of integrins by platelet-adherent and -nonadherent leukocyte subsets. (A) Representative histograms of relative CD18 expression by CD61− platelet-nonadherent (solid gray) and CD61+ platelet-adherent (black line) peripheral blood eosinophils (top), neutrophils (middle), and monocytes (bottom) are shown for a subject with AERD. (B-D) Relative expression of CD18 and CD49d on eosinophils (B), CD18 on neutrophils (C), and CD18 and CD11b on monocytes (D), comparing the platelet-adherent and platelet-nonadherent leukocyte subsets in nonasthmatic controls (n = 7), ATA controls (n = 10), and subjects with AERD (n = 9). MFI indicates mean fluorescence intensity. Platelet-free CD61− leukocyte subsets are shown in white columns, and CD61+ leukocyte subsets are shown in hatched columns. Data are expressed as mean + SEM (★P < .05, ★★P < .01, ★★★P < .001).
Adherent platelets contribute to granulocyte-associated LTC4S activity and cysLT production by activated granulocytes in vitro
To determine whether platelets could contribute to the production of cysLTs by granulocytes, and whether this contribution differed between subjects with AERD and ATA controls, we studied the expression and function of LTC4S in platelets from the blood of subjects with AERD and ATA controls. We also measured the ability of peripheral blood granulocyte fractions to generate cysLTs and to convert exogenous LTA4-ME to LTC4-ME before and after the removal of adherent platelets using trypsin. Washed platelets expressed the 18-kDa LTC4S protein (Figure 4A), with platelets from both ATA and AERD subjects displaying a range of expression levels. Trypsinization removed more than 90% of the adherent platelets from granulocytes in vitro, as determined by cytofluorographic analysis (representative histogram, Figure 4B). Platelets from both groups demonstrated specific LTC4S activity, measured by conversion of exogenous LTA4-ME to LTC4-ME (Figure 4C left), and there were no significant differences in LTC4S activity between platelets from subjects with AERD and those from controls. However, LTC4S activity was higher in the freshly isolated granulocytes of subjects with AERD, and activity decreased after trypsinization and stripping of platelets, by 54 ± 12% and 56 ± 3% in ATA and AERD subjects, respectively. The LTC4S activity of platelet-stripped granulocytes from the subjects with AERD remained higher than those from ATA controls (Figure 4C right). For the granulocyte samples used in the LTA4-ME conversion assay, the percentage of granulocytes identified as CCR3+ eosinophils was 10.4 ± 7.1% and 13.4 ± 5.2% for ATA and AERD subjects, respectively.
Contribution of platelet LTC4S to cysLT production by peripheral blood granulocytes. (A) Western blot analysis of platelets for LTC4S protein in 3 ATA controls and 4 subjects with AERD. (B) Removal of adherent platelets by trypsinization is shown cytofluorographically for a subject with AERD. Isolated granulocytes were stained for CD45 and analyzed for their expression of CD61 before trypsinization (black line) and again after trypsinization (solid gray). (C) Conversion of LTA4-ME to LTC4-ME by washed platelets (left section of panel) and by granulocytes (right section of panel) without and with trypsinization to remove adherent platelets from subjects with ATA (gray columns; n = 6) and AERD (black columns; n = 7). (D) A23187-induced production of LTC4 (top) and the sum of all 5-LO pathway products [LTB4, LTC4, LTD4, (5,6)-dihydroxy-7,9-trans-11,14-cis-eicosatetraenoic acid [5,6-DiHETE], and 6-trans-LTB4; bottom] by granulocytes without and without trypsinization to remove adherent platelets from subjects with ATA (n = 8) and AERD (n = 10). Data in panels C and D are expressed as mean +SEM (★P < .05, ★★P < .01).
Contribution of platelet LTC4S to cysLT production by peripheral blood granulocytes. (A) Western blot analysis of platelets for LTC4S protein in 3 ATA controls and 4 subjects with AERD. (B) Removal of adherent platelets by trypsinization is shown cytofluorographically for a subject with AERD. Isolated granulocytes were stained for CD45 and analyzed for their expression of CD61 before trypsinization (black line) and again after trypsinization (solid gray). (C) Conversion of LTA4-ME to LTC4-ME by washed platelets (left section of panel) and by granulocytes (right section of panel) without and with trypsinization to remove adherent platelets from subjects with ATA (gray columns; n = 6) and AERD (black columns; n = 7). (D) A23187-induced production of LTC4 (top) and the sum of all 5-LO pathway products [LTB4, LTC4, LTD4, (5,6)-dihydroxy-7,9-trans-11,14-cis-eicosatetraenoic acid [5,6-DiHETE], and 6-trans-LTB4; bottom] by granulocytes without and without trypsinization to remove adherent platelets from subjects with ATA (n = 8) and AERD (n = 10). Data in panels C and D are expressed as mean +SEM (★P < .05, ★★P < .01).
When stimulated with A23187, granulocytes generated the entire spectrum of 5-LO pathway products, including LTB4, LTC4, LTD4, and the LTA4 hydrolysis metabolites (5,6)-dihydroxy-7,9-trans-11,14-cis-eicosatetraenoic acid and 6-trans-LTB4. Freshly isolated A23187-stimulated granulocytes from subjects with AERD generated more LTC4 (Figure 4D top) and more total 5-LO pathway products (Figure 4D bottom) than did granulocytes from aspirin-tolerant controls. After removal of platelets by trypsinization, the A23187-induced production of LTC4 specifically decreased by 58 ± 10% and 68 ± 11%, and the production of total 5-LO pathway products decreased by 48 ± 8% and 41 ± 4%, in the granulocytes from ATA and AERD subjects, respectively (Figure 4D). Removal of platelets also reduced fMLP-induced generation of cysLTs by 64 ± 16% (from 623 ± 203 pg [0.99 ± 0.31 pmol] to 227 ± 129 pg [0.35 ± 0.21 pmol]/mL) in 3 separate experiments using granulocytes from subjects with AERD. The addition of 200 × 106 autologous platelets to the trypsinized granulocytes restored robust generation of all 5-LO pathway products, including LTC4, confirming that cell viability and function of the required enzymes were not intrinsically compromised by trypsinization (n = 6; supplemental Figure 3).
Platelet-adherent granulocytes correlate with systemic cysLT production in vivo
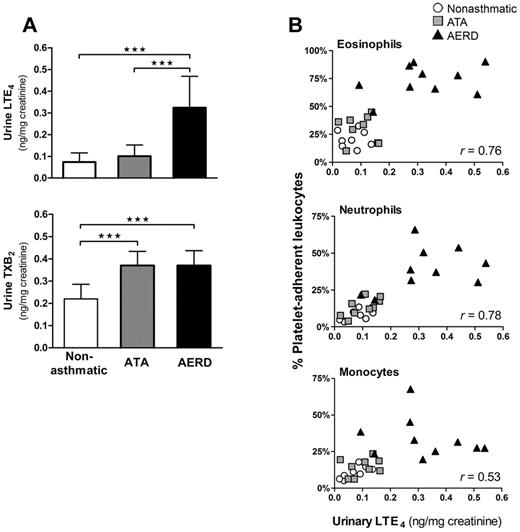
To determine whether platelet-adherent leukocytes contributed to the generation of cysLTs in vivo, we measured baseline urinary LTE4 levels from each subject. We also measured levels of TXB2, the stable metabolite of thromboxane A2, the major platelet-derived COX product, in the same samples. As expected, the urine of subjects with AERD had strikingly higher levels of LTE4 than either aspirin-tolerant control group (Figure 5A top). Urinary LTE4 levels correlated strongly with the percentages of platelet-adherent eosinophils and neutrophils and moderately with platelet-adherent monocytes in the peripheral blood (Figure 5B). Levels of urinary TXB2 levels in both groups of asthmatics were nearly double those of nonasthmatic controls (Figure 5A bottom), but they did not differ between ATA and AERD subjects and did not correlate with the percentages of circulating platelet-adherent leukocytes (data not shown). Urine from separate cohorts of 4 aspirin-tolerant controls and 9 subjects with AERD also was analyzed for baseline levels of F2-isoprostanes that can act as agonists for platelet activation and arise as a result of endogenous oxidant stress. Isoprostane levels did not differ significantly between the groups (1.2 ± 0.3 and 1.5 ± 0.4 ng/mg creatinine for aspirin-tolerant controls and AERD subjects, respectively; data not shown), and the measurements from both groups were similar to the published normal human values of 1.6 ± 0.6 ng/mg creatinine.23 Circulating total white blood cell and platelet counts were measured in 11 subjects, and neither cell count was correlated with urinary LTE4 levels (data not shown).
Platelet-adherent leukocytes correlate with systemic cysLT production. (A) Baseline urinary LTE4 (top) and TXB2 (bottom) levels analyzed by gas chromatography-mass spectrometry from nonasthmatic controls (n = 8), ATA controls (n = 9) and subjects with AERD (n = 10). Data are expressed as mean + SD (★★★P < .001). (B) Baseline urinary LTE4 levels plotted against the corresponding percentages of platelet-adherent eosinophils (top), neutrophils (middle), and monocytes (bottom) in the peripheral blood of each subject. Effect size, determined with Pearson correlation coefficient, is denoted as an r value displayed for each cell type. White circles, nonasthmatic controls; gray squares, ATA controls; black triangles, AERD subjects.
Platelet-adherent leukocytes correlate with systemic cysLT production. (A) Baseline urinary LTE4 (top) and TXB2 (bottom) levels analyzed by gas chromatography-mass spectrometry from nonasthmatic controls (n = 8), ATA controls (n = 9) and subjects with AERD (n = 10). Data are expressed as mean + SD (★★★P < .001). (B) Baseline urinary LTE4 levels plotted against the corresponding percentages of platelet-adherent eosinophils (top), neutrophils (middle), and monocytes (bottom) in the peripheral blood of each subject. Effect size, determined with Pearson correlation coefficient, is denoted as an r value displayed for each cell type. White circles, nonasthmatic controls; gray squares, ATA controls; black triangles, AERD subjects.
Discussion
Our study implicates platelets and platelet-adherent leukocytes as effectors of AERD, a distinctive variant of asthma characterized by idiosyncratic reactions to nonselective COX inhibitors, and marked bronchial and sinonasal tissue eosinophilia. Based on functional and pharmacologic studies, these features of the disease are probably causally related to dysregulated cysLT production, the basis of which has remained evasive. Platelet-leukocyte aggregates are proposed to contribute to vascular inflammation in cardiovascular disease24 and have been identified in the blood of subjects with allergic asthma during late-phase responses to inhaled allergen.25 However, no previous study provides direct evidence of a pathogenic role for platelet-leukocyte aggregates in human disease. The frequencies of platelet-adherent eosinophils, neutrophils, and monocytes in the blood of subjects with AERD are strikingly increased relative to their frequencies in the blood of aspirin-tolerant controls (Figure 2). The enhanced expression of integrin subunits on these platelet-adherent leukocyte subsets (Figure 3 and supplemental Figure 2) supports a previously hypothesized mechanism by which platelets prime leukocyte adhesion to endothelial cells,16 and suggests that platelets may amplify tissue inflammation in AERD. This conclusion is further supported by our immunohistochemical data in nasal polyp tissue (Figure 1). Moreover, the correlation between the frequencies of platelet-adherent granulocytes in the blood and baseline urinary levels of LTE4 (Figure 5), a reflection of systemic cysLT generation, and the substantial contribution from adherent platelets to total 5-LO pathway products and LTC4S activity in peripheral blood granulocytes (Figure 4), further support a causal link.
In addition to their role in hemostasis, platelets are implicated as effectors of leukocyte recruitment and as sources of bioactive mediators in cardiovascular disease and in mouse models of acid-induced lung injury, rheumatoid arthritis, and skin fibrosis.26-29 Older studies identified activated platelets in bronchial biopsies from subjects with asthma,30 and platelets are essential to the accumulation of eosinophils and the development of inflammation in lungs of allergen-sensitized and challenged mice.16,31 We initially sought direct evidence for platelet-driven leukocyte recruitment in AERD because of the dramatic tissue eosinophilia that characterizes the disease. We found eosinophilia in all 6 nasal polyps from subjects with AERD despite the fact that all subjects had been treated with systemic corticosteroids for 5 days preoperatively, but very few eosinophils in the polyps from the aspirin-tolerant controls, who also received systemic steroids. Extravasated platelets were readily detected in nasal polyps from individuals with AERD, and the numbers and percentages of CD45+ leukocytes that colocalized with CD61+ platelets in these specimens exceeded the numbers and percentages in the nasal polyps from aspirin-tolerant controls with sinusitis by 2- to 3-fold (Figure 1). Flow cytometry revealed that the frequencies not only of platelet-adherent eosinophils but also of platelet-adherent neutrophils and monocytes were markedly higher in AERD than in the controls (Figure 2). Although eosinophils dominate the inflammatory cell infiltrate typical of the bronchial and sinonasal mucosa in asthma and AERD, neutrophils and macrophages also are increased in number compared with healthy tissues.9,32 Taken together, these observations suggest that platelets may adhere to leukocytes as a prelude to their recruitment into the tissues and that AERD may involve a disturbance in the homeostasis that controls this process. Moreover, the tissue eosinophilia in AERD may be relatively resistant to corticosteroids, consistent with the refractory nature of the nasal polyposis in this syndrome.
The previously recognized platelet-dependent pathway for the development of allergen-induced pulmonary inflammation in mice requires binding of platelet-associated P-selectin to leukocyte-associated PSGL-1.16 This interaction primes leukocytes for adhesion to endothelial cells by up-regulating the expression and avidity of β1- and β2 integrins on the leukocyte membrane and has been demonstrated in eosinophils, neutrophils, and monocytes.33-35 To determine whether this pathway was operative in AERD, we studied the cell-specific effects of platelet adherence on the subsequent surface expression of leukocyte activation and adhesion receptors by separately gating on platelet-adherent and -nonadherent leukocyte subsets in the blood. We found especially strong up-regulation of CD18 expression in platelet-adherent monocytes (Figure 3), as well as significant increases in CD11a, CD11b, and CD11c (Figure 3 and supplemental Figure 2), each of which partner with CD18 to form the β2 integrins, which permit firm adhesion of leukocytes to endothelial and epithelial cells via intracellular adhesion molecule-1 (ICAM-1). In monocytes, the up-regulation of CD11b most closely paralleled the increase in CD18, suggesting that platelet adherence may potentiate the CD11b/CD18 (MAC-1)–dependent pathway for monocyte recruitment. CD18 also was modestly increased on both neutrophils and eosinophils that were platelet-adherent (Figure 3), along with CD11a in eosinophils. Compared with the platelet nonadherent fractions, platelet-adherent eosinophils and monocytes also expressed higher levels of CD49d (Figure 3 and supplemental Figure 2) that pairs with CD29 to form very late antigen-4 integrin that is particularly important for the recruitment of eosinophils, basophils, monocytes, and lymphocytes to sites of allergic inflammation.36,37 Although none of the platelet-related changes in leukocyte receptor expression were specific to cells from subjects with AERD, the substantial differences in the total percentages of circulating leukocytes to which platelets adhere in AERD implies that the platelet-dependent effects on adhesion receptor expression may be especially relevant to tissue inflammation in AERD. The mechanism for this increase in AERD remains to be determined but does not seem to reflect a difference in expression levels of either PSGL-1 on leukocytes (supplemental Figure 2) or P-selectin on platelets (data not shown).
Although cysLTs play a validated role in asthma,38,39 their pathogenic role in AERD is especially prominent because of their overproduction and to the increased function of their receptors.4,9,10 In addition to activating eosinophils, mast cells, and monocytes,40 aspirin challenges also elicit increases in LTB4 metabolites in the urine of subjects with AERD that parallel increases in LTE4,41 suggesting that dysregulation of 5-LO activity in neutrophils is another feature of AERD. More than 50% of the LTA4 synthesized by neutrophils is released unmetabolized into the extracellular milieu,42 and is only available as a substrate for reuptake by adherent cells because of its extracellular half-life of less than 5 seconds.43 Although ex vivo studies indicate that platelet conversion of neutrophil- or monocyte-derived LTA4 into LTC4 requires adherence to leukocytes via P-selectin/PSGL-1, this transcellular pathway had not been demonstrated previously in any human disease.13 Because the 3 major 5-LO–expressing cell types in the peripheral blood—neutrophils, eosinophils, and monocytes—all showed increased adherence to platelets in subjects with AERD relative to aspirin-tolerant control subjects, we suspected that adherent platelets might contribute to the increased cysLT generation that is a signature of AERD.
Platelets expressed the same 18-kDa enzyme that is expressed by eosinophils, mast cells, and monocytes (Figure 4A). By removing platelets from granulocytes using trypsinization, we determined that platelets contribute more than half of the total LTC4S activity (measured using an assay of specific enzymatic activity; Figure 4C) in freshly isolated peripheral blood granulocytes in AERD, as reflected by the “trypsin-sensitive” component of LTC4S activity. Although adherent platelets accounted for similar fractions of LTC4S activity in the granulocytes of the ATA and AERD groups, the increased frequencies of platelet-adherent granulocytes in the samples from subjects with AERD resulted in substantially greater A23187-induced production of LTC4 from these samples than from the ATA controls (Figure 4D). The higher level of trypsin-insensitive LTC4S activity in the granulocytes from subjects with AERD (Figure 4C) is likely because of slightly higher percentage of eosinophils in those samples, and because of increased LTC4S activity in eosinophils as suggested by previous immunohistochemical studies.9,10 Adherent platelets also accounted for a remarkably similar fraction (64 ± 16%) of the cysLTs produced in response to fMLP, which we chose as a physiologic agonist to activate 5-LO in granulocytes by a receptor-dependent mechanism, thereby providing LTA4 for conversion to LTC4 by platelets. We also found that adherent platelets increased the overall activity of the 5-LO pathway in granulocytes, as reflected by the net quantities of all pathway products detected in the supernatants of the A23187-stimulated samples before and after trypsinization. This priming function could be restored to the trypsinized fractions by adding platelets back (supplemental Figure 3). Activated platelets release large quantities of free arachidonic acid that can induce the translocation and activation of 5-LO in adjacent cells.44 Platelets also may prime neutrophils14 and eosinophils45 for augmented 5-LO function by their release of granulocyte macrophage–colony-stimulating factor (GM-CSF).46 Thus, platelets probably augment systemic overproduction of cysLTs in AERD by several mechanisms. Urinary concentrations of LTE4 correlate strongly with certain clinical outcomes in AERD,47 and the remarkable correlation between steady-state urinary excretion of LTE4 and the frequencies of platelet-adherent neutrophils, eosinophils, and monocytes in the peripheral blood (Figure 5), combined with the contribution of platelets to the pool of LTC4S activity in granulocytes (Figure 4), strongly supports the pathogenetic relevance of our findings. The increases in platelet–leukocyte complexes in AERD are probably not because of enhanced production of thromboxane (Figure 5A) or isoprostanes, because their respective urinary metabolites (unlike LTE4 levels) did not discriminate subjects with AERD from aspirin-tolerant controls.
Our study identifies a disturbance in the homeostasis of interactions between platelets and leukocytes that probably enhances effector cell accumulation in the tissue and augments cysLT production in AERD. Although neither priming for cysLT generation nor priming for leukocyte adhesion are unique functions intrinsic to platelets in AERD, the increased frequencies of platelet–leukocyte aggregates in the blood and nasal polyp tissue of subjects with AERD highlights the potential for a particularly important role in this disease. Given the abundance of neutrophils relative to other leukocyte subsets, the potency of their 5-LO activity, and their tendency to release unmetabolized LTA4, we suspect that the ability of platelets to convert neutrophil-derived LTA4 into LTC4 may be especially important for controlling basal cysLT levels in AERD. Moreover, the remarkable adherence of platelets to eosinophils suggests that platelets could contribute substantially to the accumulation of eosinophils in the tissue by priming them for adhesion and prolonging their survival (and perhaps reducing their sensitivity to steroid-induced apoptosis) via release of GM-CSF.48 Our study suggests that the selective increases in the proportions of eosinophils expressing LTC4S, in bronchial biopsies9 and nasal polyp mucosa10 obtained from individuals with AERD relative to ATA controls may have been due, in part, to the presence of adherent platelets on the eosinophils. Our previous studies demonstrated that exogenous cysLTs, including LTE4, do not directly cause human platelets to adhere to granulocytes.49 Nonetheless, LTE4 markedly potentiates pulmonary eosinophilia in mice by a pathway that depends on platelet-associated P2Y12 receptors,49 the target of thienopyridine drugs. Because thienopyridines also reduce the formation of platelet–leukocyte aggregates,50 we speculate that these antiplatelet therapies could be efficacious as treatments for AERD, both by blocking platelet-leukocyte adhesion and the subsequent formation of cysLTs and by blocking the actions of LTE4 by an indirect mechanism.
There is an Inside Blood commentary on this article in this issue
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Nora A. Barrett and Albert L. Sheffer for assistance with patient recruitment, to K. Frank Austen for helpful discussions and thoughtful comments on experimental designs, and to Yoshihide Kanaoka and James M. Fernandez for assistance with HPLC.
This work was supported by National Institutes of Health grants AI078908, AI095219, AT002782, AI082369, and HL36110; by an American Academy of Allergy, Asthma & Immunology 3rd & 4th year fellow-in-training research award (to T.M.L.), and by generous contributions from the Vinik family.
National Institutes of Health
Authorship
Contribution: T.M.L. phenotyped patients, performed aspirin challenges, designed the research, analyzed data, and wrote the paper; M.S.K. and H.C. performed research and analyzed data; N.B. phenotyped subjects and provided surgical specimens; W.X. and S.S. designed the histology experiments and analyzed data; G.L.M. performed the urinary leukotriene measurements and analyzed data; M.C.C. phenotyped patients and performed aspirin challenges; and J.A.B. designed the research, analyzed data, and wrote the paper with T.M.L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joshua A. Boyce, Brigham and Women's Hospital, Smith Bldg, 1 Jimmy Fund Way, Boston, MA 02115; e-mail: jboyce@rics.bwh.harvard.edu.

![Figure 2. Platelet-adherent leukocytes are identifiable in peripheral blood. (A) Representative histograms of platelet-adherent eosinophils (identified as CCR3+CD45+ cells in the granulocyte side scatter [SSC] gate), neutrophils (CD16+CD45+ cells in the granulocyte SSC gate), monocytes (CD45+ in the monocyte SSC gate), and lymphocytes (CD45+ cells in lymphocyte SSC gate) in blood from an ATA control (top) and a subject with AERD (bottom). The percentages of each cell type with adherent platelets are shown. (B) Percentages of leukocytes with adherent platelets (as determined by staining with CD61) in blood of nonasthmatic controls (n = 9), ATA controls (n = 13), and subjects with AERD (n = 15). Data are expressed as mean + SD (★P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/16/10.1182_blood-2011-10-384826/5/m_zh89991287290002.jpeg?Expires=1767714092&Signature=T2tIPm~lHqe21Scj6U70p9EbhfqcHP3ym7I8yvc25skXt36hhZrV49QteFmdvZTBBNjZlWMdOnJjBbMMkFLm50s~t-WTpb4RjlQ9q3Vohbt4uta9B~x3~eXP7HEsDk1IkJnMy~ec9VmlywxjCgJGnS6mfazMW1n41Lnt1b5tkmjS9bA3-pRtx3wXUEEyPqEA0fd8-9aLWHQ1KweX7Duvoe~NQYncTEiFpvpjlvl-GKuBl7Kw6~6DpcLAT84HntrkhMa4lDByw4k458dECecxaKGqrygEsEBiqHGhO1fyP6LPEe9vZOYDfVgt7FNj2NEoSQMn~LtKWGU3aYiMJK-4yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Contribution of platelet LTC4S to cysLT production by peripheral blood granulocytes. (A) Western blot analysis of platelets for LTC4S protein in 3 ATA controls and 4 subjects with AERD. (B) Removal of adherent platelets by trypsinization is shown cytofluorographically for a subject with AERD. Isolated granulocytes were stained for CD45 and analyzed for their expression of CD61 before trypsinization (black line) and again after trypsinization (solid gray). (C) Conversion of LTA4-ME to LTC4-ME by washed platelets (left section of panel) and by granulocytes (right section of panel) without and with trypsinization to remove adherent platelets from subjects with ATA (gray columns; n = 6) and AERD (black columns; n = 7). (D) A23187-induced production of LTC4 (top) and the sum of all 5-LO pathway products [LTB4, LTC4, LTD4, (5,6)-dihydroxy-7,9-trans-11,14-cis-eicosatetraenoic acid [5,6-DiHETE], and 6-trans-LTB4; bottom] by granulocytes without and without trypsinization to remove adherent platelets from subjects with ATA (n = 8) and AERD (n = 10). Data in panels C and D are expressed as mean +SEM (★P < .05, ★★P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/16/10.1182_blood-2011-10-384826/5/m_zh89991287290004.jpeg?Expires=1767714092&Signature=xXwrR1R~sJJYOUGry5bfC-xDhs~74bi9Fx~xL3gzeKxsPSvaf1fn9wxoZFje2bODbs5pL2D6wA13ji1g6tmgiEOQcqPYSIhYPj5PZwdrY9cTiOXYRGA5TmxTN2Jvxigi45b1mMM1n8Fhph7~U9EWQ7PAoASLdO8alEBHB~xlQSRZ8ExA6fsEUx96KTQzqA66ZTdstWNmv-46fA2BRBfj8KJUpijWKU7L0MQLX-L59~ctW3~r3K0e~Gx7lMVA53FzBEmUoGyEUcxLhuXkpVq4ClWQjQ6BeDEm7aGoB0cKEq623P9pMjJk3wJGvwSU0T1DqSOmmWOWaZdOV~3R-00Z8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal