Abstract
On antigen binding by the B-cell receptor (BCR), B cells up-regulate protein expression of the key downstream signaling molecule Bruton tyrosine kinase (Btk), but the effects of Btk up-regulation on B-cell function are unknown. Here, we show that transgenic mice overexpressing Btk specifically in B cells spontaneously formed germinal centers and manifested increased plasma cell numbers, leading to antinuclear autoantibody production and systemic lupus erythematosus (SLE)–like autoimmune pathology affecting kidneys, lungs, and salivary glands. Autoimmunity was fully dependent on Btk kinase activity, because Btk inhibitor treatment (PCI-32765) could normalize B-cell activation and differentiation, and because autoantibodies were absent in Btk transgenic mice overexpressing a kinase inactive Btk mutant. B cells overexpressing wild-type Btk were selectively hyperresponsive to BCR stimulation and showed enhanced Ca2+ influx, nuclear factor (NF)–κB activation, resistance to Fas-mediated apoptosis, and defective elimination of selfreactive B cells in vivo. These findings unravel a crucial role for Btk in setting the threshold for B-cell activation and counterselection of autoreactive B cells, making Btk an attractive therapeutic target in systemic autoimmune disease such as SLE. The finding of in vivo pathology associated with Btk overexpression may have important implications for the development of gene therapy strategies for X-linked agammaglobulinemia, the immunodeficiency associated with mutations in BTK.
Introduction
B cells are of crucial importance for the host defense against pathogens. Their full activation does not solely depend on antigen recognition through the B-cell receptor (BCR), but usually requires a second signal delivered by other immune cells or by the pathogen itself.1,2 Although the integration of 2 activating signals should pose a strong barrier to the activation of autoreactive B cells, dysregulated BCR signaling alone can greatly contribute to the development of systemic autoimmune diseases.3 Genome-wide association studies point to a role of BCR-associated molecules such as BLK and BANK1 in the pathogenesis of various systemic autoimmune diseases, including systemic lupus erythematosus (SLE), systemic sclerosis, rheumatoid arthritis, and Sjögren syndrome.4 A hallmark of SLE is the loss of B-cell tolerance to nuclear self-antigens, leading to production of antinuclear autoantibodies that elicit inflammation on deposition in various organs. The crucial role of BCR signaling in development of SLE-like immune diseases is further supported by data from genetically engineered mouse models.5 In addition, defective Bcell apoptosis can induce aggressive autoimmune disease in mouse models,5 and enhanced B-cell survival has been implicated as a causative factor in Sjögren syndrome.6
Bruton tyrosine kinase (Btk) is a BCR signaling molecule that directly links BCR signals to B-cell survival, through activation of the transcription factor nuclear factor (NF)–κB.7-9 Although Btk is widely expressed in the hematopoietic system, only B cells greatly rely on Btk signaling. Btk deficiency in men or mice results in the B-cell specific immunodeficiencies X-linked agammaglobulinemia (XLA) or x-linked immunodeficiency (xid), respectively.10 Whereas XLA is characterized by an almost complete arrest of pre-B cell differentiation, in xid the pre-B cell block is limited, but differentiation and survival of mature B cells is severely impaired.11-13 The defects in murine Btk-deficient B cells range from reduced responsiveness to BCR and Toll-like receptor (TLR) stimulation to decreased signaling of chemokine receptors.14
Several lines of evidence indicate that Btk expression levels may present the rate-limiting step in BCR signaling and that tight regulation of Btk expression and kinase activity is essential for normal B-cell development and activation. Firstly, expression of subphysiologic levels of transgenic Btk only partially corrects the phenotype of Btk-deficient B cells,15 whereas physiologic levels fully rescue this phenotype.16 Secondly, on BCR stimulation, mature B cells increase Btk levels within 4 hours via a posttranscriptional mechanism.17 Thirdly, Btk uses a proteasome-dependent positive autoregulatory feedback mechanism to stimulate transcription from its own promoter via NF-κB.18 Fourthly, many factors can negatively regulate Btk, either by down-regulating Btk kinase activity, such as PKC-β,19 iBtk20 and caveolin-1,21 or by reducing Btk expression, such as Pin122 or miR185.23 Finally, detrimental effects of dysregulated Btk kinase activity are evident from the Btk-dependency of B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL). Treatment of DLBCL or CLL B cells with Btk-inhibitors markedly reduces cell survival.24,25
Given the stringent regulation of Btk expression and activation in B cells, we addressed the question whether Btk expression levels are critical for mature B-cell activation and selection. To study the effects of dysregulated Btk expression in B cells in vivo, we used mice that transgenically overexpress human Btk under the control of the MHC class II Ea locus control region16 or driven by the CD19 promoter region.26 Previous analyses of Btk transgenic mice on a mixed genetic background showed that all features of the xid phenotype were corrected by these Btk transgenes, whereas adverse affects on B-cell development were limited to elevated serum immunoglobulin (Ig)M levels and a mild increase in B-cell proliferation on BCR stimulation.26,27 In contrast to these limited effects of overexpression of wild-type (WT) Btk on B-cell functioning, expression of the constitutively active E41K-Btk mutant induced an almost complete deletion of developing immature B cells in the bone marrow (BM).26 Even low levels of E41K-Btk hampered B-cell differentiation, characterized by reduced numbers of follicular B cells and selective survival or expansion of B-1 B cells.28
Here we show that overexpression of WT Btk restricted to the B-cell lineage induced spontaneous germinal center (GC) and plasma cell formation, and eventually antinuclear autoantibody production and SLE-like autoimmune disease. This autoimmune phenotype was fully dependent on Btk kinase activity. Our findings provide evidence that expression levels of Btk set the threshold for B-cell activation and selection of autoreactive B cells.
Methods
Mice and genotyping
CD19-hBtk,26 CD19-hBtkK430R, CD19-hBtkY223F,29 and MHCII-hBtk mice27 on a mixed background (Fvb × 129/Sv × C57BL/6) were backcrossed on C57BL/6 for > 10 generations. Mice deficient for Btk were on a C57BL/6 background.12 Genotyping was performed by polymerase chain reaction (PCR), as previously described.29 Mice were analyzed at 10 to 14 weeks, or at ∼ 40 weeks to monitor autoimmune pathology. Mice were bred and kept at specified pathogen-free conditions in the Erasmus MC experimental animal facility. All experimental protocols have been reviewed and approved by the Erasmus MC Committee of animal experiments.
Flow cytometry procedures, cell cycle analysis, and measurement of Ca2+ mobilization
Preparations of single-cell suspensions, flow cytometry and Ca2+ measurements were performed using standard procedures. Monoclonal antibodies are listed in supplemental Table 3 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For intracellular isotype-specific staining of Ig components, cells were fixed in Cytofix/Cytoperm and permeabilized, and then stained in Perm/Wash buffer (BD Bioscience). For intracellular Btk staining, cells were fixed in phosphate-buffered saline (PBS)/2% paraformaldehyde (PFA), permeabilized, and then stained with phycoerythrin (PE)–anti-BTK in the presence of 0.5% saponin (Sigma-Aldrich). Flow cytometric detection of Akt and extracellular signal-regulated kinase (ERK) phosphorylation was performed essentially as described in Hoffmann et al.30 Cell-cycle analysis using propidium iodide was previously described.13 Measurement of Ca2+ mobilization was performed according to the protocol published by Middendorp et al.29 All measurements were performed on a LSRII flow cytometer (BD Bioscience), and results were analyzed using FlowJo Version 9.3.2 software (TreeStar).
MACS purification and cultures of mature splenic B cells
Splenic cell suspensions were prepared in magnetic-activated cell sorting (MACS) buffer (PBS/2mM EDTA/0.5% BSA). Non-B cells, activated B cells, B1 cells, and (pre)plasma cells were depleted by incubating splenic cell suspensions with biotinylated antibodies against CD5, CD43, CD138, CD11b, Gr-1 ad TER-119 (supplemental Table 3), followed by incubation with streptavidin-coupled magnetic beads (Miltenyi Biotec). Unlabeled cells (naive B2 lymphocytes) were collected by magnetic depletion of labeled cells (unlabeled fraction contained > 95% CD19+CD138− cells). Purified B cells were cultured at 1.2 × 106 cells/mL for 1 to 3 days in culture medium (RPMI 1640/10% FCS/50 μg/mL gentamycin/0.05mM β-mercapto-ethanol). Cells were stimulated in vitro with 10 μg/mL Ag-binding fragment [F(ab′)2]α-IgM (Jackson ImmunoResearch Laboratories), 5 μg/mL lipopolysaccharide (LPS; own production), 20 μg/mL α-CD40 (clone 3/23; BD Bioscience), 1mM CpG (Invitrogen), or 50 μg/mL Poly I:C (Invivogen).
Immunohistochemistry
Immunohistochemical analyses and periodic acid-Schiff (PAS) staining were performed according to standard procedures.31 Used antibodies are listed in supplemental Table 3. After staining, tissue sections were embedded in Kaiser glycerol gelatin (Merck). Micrographs were made using a DM LB light microscope (Leica), a DFC500 camera (Leica), and Imaging for Windows Version 1.0 software (Kodak).
ELISAs for Ig isotypes, autoantibodies, and albuminuria
Isotype-specific serum Ig concentrations were measured by sandwich enzyme-linked immunosorbent assay (ELISA).27 For IgG antinucleosome antibody levels, we used nucleosome-coated 96-well plates (Orgentec) and biotinylated anti–mouse total IgG antibodies (SouthernBiotech) as secondary reagent. IgG anti-dsDNA antibody levels were determined using a mouse anti-dsDNA IgG-specific ELISA (Gentaur). Levels of albuminuria in urine samples were determined by ELISA32 and corrected for levels of creatinin, which were measured using an autoanalyzer (Hitachi 717; Roche).
HEp2 reactivity assay
Serum samples (diluted 1/100 in PBS) or bronchoalveolar lavage fluid (BALf) samples (undiluted) were incubated for 1 hour on Kallestad HEp-2 slides (Bio-Rad Laboratories). As secondary antibody Alexa Fluor 488–conjugated donkey anti–mouse IgG (Invitrogen) was applied to the HEp2 slides, and after embedding the slides in glycerol (Sigma-Aldrich), the fluorescence intensity of HEp2 slides was evaluated using a LSM 510 META confocal fluorescence microscope (Zeiss) and LSM Image Browser Version 4.2.0.12 software (Zeiss).
Btk inhibitor treatment
Btk inhibitor PCI-3276533 or placebo was orally administered (25 mg/kg/d in water/5% mannitol/0.5% gelatin) to the mice once or for 8 consecutive days.
Influenza infection and hemagglutinin inhibition assay
Mice were infected intranasally with 105 H3N2 virus particles (X-31; Medical Research Council, London, United Kingdom) in 50 μL PBS. At 18 days postinfection (dpi), animals were killed for analysis of BALf and serum. To determine antihemagglutinin (HA) antibody titers in serum of influenza infected animals, a HA inhibition assay was performed.31
Western blotting
For analyses of c-Rel nuclear translocation, nuclear protein extracts were prepared from MACS-sorted splenic B2 cells that were stimulated in vitro for 4 hours with 10 μg/mL F(ab′)2 α-IgM (Jackson ImmunoResearch). Preparation of nuclear protein extracts and Western blotting were previously described.29 Levels of c-Rel were analyzed using ImageJ Version 1.42q software (Wayne Rasband) and normalized to nucleophosmin levels, detected with mouse-antinucleophosmin (clone B23; Santa Cruz Biotechnology) and peroxidase-conjugated rabbit-anti–mouse Ig (Dako).
Statistical analysis
For calculating the level of significance of differences between groups we used the Student t test.
Results
Btk expression levels increase on B-cell stimulation
It has been shown that Btk expression levels are up-regulated on BCR engagement in mature B cells.17,18 To test which stimuli can induce Btk up-regulation, we isolated naive splenic CD43−CD5−CD138− B lymphocytes and stimulated them in vitro through the BCR (F(ab′)2 α-IgM), CD40 (α-CD40), TLR4 (LPS), TLR9 (CpG), or TLR3 (Poly I:C) for 3 days. When we evaluated Btk expression levels by intracellular flow cytometry, we found that all stimuli tested evoked Btk up-regulation, whereby the increase was highest on BCR engagement (∼ 2.7-fold; Figure 1A, supplemental Figure 1). Modest but significant Btk up-regulation (∼ 1.2-fold) was already detected after 6 hours of α-IgM stimulation (data not shown). Therefore, various signals can increase Btk expression levels in mature B lymphocytes, indicating that Btk up-regulation by engagement of one receptor could sensitize B cells to signaling through a second pathway.
Btk overexpression in vivo enhances GC and plasma cell formation. (A) Quantification of intracellular flow cytometric detection of Btk protein in WT splenic B cells after 3 days of in vitro culture in the presence of the indicated stimuli (supplemental Figure 1). Levels were calculated relative to average Btk expression levels (MFI) in unstimulated B cells (set to 1) and background signals in unstimulated Btk−/− B cells (set to 0). Data are mean values ± SD (n = 3) from 1 of 3 representative experiments, asterisks indicate significant increases in Btk expression (P < .05). (B) Intracellular flow cytometric Btk detection in splenic CD19+ B cells, shown as histogram overlays from Btk−/− (shaded histogram), WT (black line) and CD19-hBtk (red line) mice. (C) Immunohistochemical analysis of spleen tissue sections of CD19-hBtk and WT mice. Sections were stained for CD21 (red), and metallophilic macrophage marker MOMA-1 (blue), or for GL7 (red) and CD138 (blue); arrows indicate clusters of GL7+ GC B cells (objective 10×). (D) Flow cytometric analysis of GC cells in the spleens of the indicated mice. CD19+ B cells were gated and analyzed for IgM/IgD profiles. IgDlowIgMlow cells were subsequently gated and analyzed for PNA and CD95. Data are displayed as dot plots and the percentages of cells in the indicated quadrants are given. (E-F) Flow cytometric quantification of GC B cells (PNA+CD95+IgDlowIgMlowCD19+) and IgM+CD138high or IgG+CD138high plasma cells in spleen (E) and BM (F). Horizontal lines indicate median per group; each symbol represents and individual mouse. Mice were 10 to 14 weeks old.
Btk overexpression in vivo enhances GC and plasma cell formation. (A) Quantification of intracellular flow cytometric detection of Btk protein in WT splenic B cells after 3 days of in vitro culture in the presence of the indicated stimuli (supplemental Figure 1). Levels were calculated relative to average Btk expression levels (MFI) in unstimulated B cells (set to 1) and background signals in unstimulated Btk−/− B cells (set to 0). Data are mean values ± SD (n = 3) from 1 of 3 representative experiments, asterisks indicate significant increases in Btk expression (P < .05). (B) Intracellular flow cytometric Btk detection in splenic CD19+ B cells, shown as histogram overlays from Btk−/− (shaded histogram), WT (black line) and CD19-hBtk (red line) mice. (C) Immunohistochemical analysis of spleen tissue sections of CD19-hBtk and WT mice. Sections were stained for CD21 (red), and metallophilic macrophage marker MOMA-1 (blue), or for GL7 (red) and CD138 (blue); arrows indicate clusters of GL7+ GC B cells (objective 10×). (D) Flow cytometric analysis of GC cells in the spleens of the indicated mice. CD19+ B cells were gated and analyzed for IgM/IgD profiles. IgDlowIgMlow cells were subsequently gated and analyzed for PNA and CD95. Data are displayed as dot plots and the percentages of cells in the indicated quadrants are given. (E-F) Flow cytometric quantification of GC B cells (PNA+CD95+IgDlowIgMlowCD19+) and IgM+CD138high or IgG+CD138high plasma cells in spleen (E) and BM (F). Horizontal lines indicate median per group; each symbol represents and individual mouse. Mice were 10 to 14 weeks old.
Btk overexpression in vivo promotes GC and plasma cell formation
To test the effects of Btk overexpression in vivo, we studied CD19-hBtk transgenic mice on a pure C57BL/6 background, in which the human CD19-promoter establishes overexpression of transgenic human BTK throughout B-cell differentiation.26 Using flow cytometry, we observed a rather constant level of Btk overexpression during B-cell development in the BM (∼ 1.5 to 3-fold; supplemental Figure 2A). Importantly, CD19-hBtk transgenic mature splenic B cells expressed ∼ 3-fold higher Btk levels, compared with WT B cells (Figure 1B, supplemental Figure 2A). These increased Btk levels in CD19-hBtk B cells were comparable with those found in WT B cells after 3 days of α-IgM stimulation in vitro. Btk expression could be further increased on BCR stimulation (supplemental Figure 2B), possibly by the same (post)transcriptional mechanisms acting in stimulated WT B cells.
In young CD19-hBtk mice, Btk overexpression did not affect the overall splenic microarchitecture (revealed by immunohistochemistry using CD21 for B cells and MOMA-1 for metallophilic marginal zone macrophages; Figure 1C). However, spleens of CD19-hBtk mice frequently contained GL7+ GC, which are rarely found in WT mice kept under specified pathogen-free conditions, and displayed a marked increase in extrafollicularly located CD138+ plasma cells (Figure 1C). When we used flow cytometry to quantify these differences, we found that in CD19-hBtk mice the numbers of IgDlowIgMlowCD95+PNA+ GC B cells, as well as IgM+ or IgG+ plasma cells in the spleen, were significantly increased (Figure 1D-E). In the BM an increase was only found for IgM+ plasma cells (Figure 1F), suggesting that most IgG+ plasma cells formed in the spleens of CD19-hBtk mice were short-lived.
Flow cytometric analysis of the entire B-cell compartment in CD19-hBtk mice revealed that B-cell development was largely normal, except for a small but significant increase in immature B cells and a reduction in recirculating B cells in the BM, compared with WT littermates (supplemental Table1). In the spleen, a reduction in follicular and marginal zone B-cell numbers was observed, whereas CD5+ B-1 cells were more numerous.
Taken together, these data demonstrate that B cell–restricted overexpression of Btk is sufficient to induce spontaneous GC and plasma cell formation in the spleen.
Btk overexpression selectively enhances BCR-mediated B-cell activation
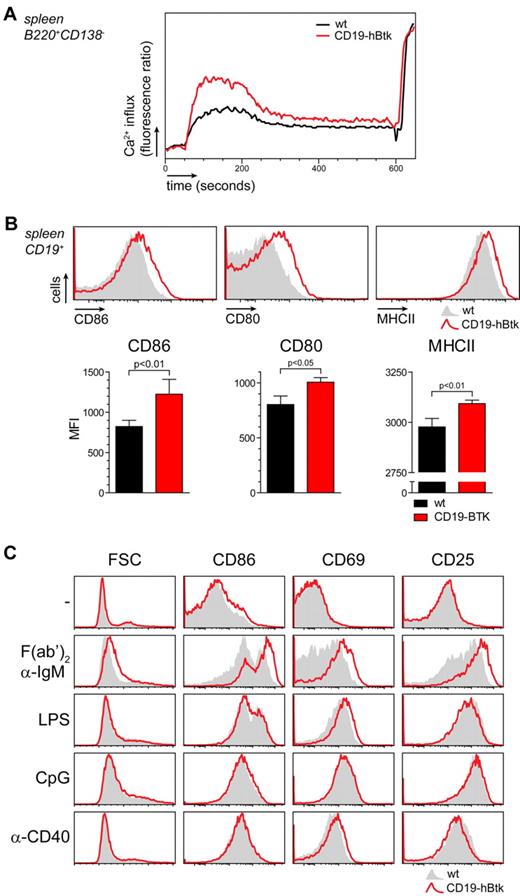
Next, we investigated whether elevated levels of Btk could amplify BCR signaling on BCR triggering with α-IgM. Splenic B cells from CD19-hBtk mice displayed a significantly stronger Ca2+ influx than WT B cells (Figure 2A). CD19-hBtk B cells also displayed in vivo higher expression of activation markers, particularly surface molecules required for T-cell costimulation including CD80, CD86, and MHCII (Figure 2B).
Btk overexpression specifically enhances BCR-mediated B-cell activation. (A) WT (black line) and CD19-hBtk (red line) B cells were stimulated with α-IgM F(ab′)2 fragments and Ca2+ mobilization was monitored and normalized for maximum influx on stimulation with ionomycin. Plots are representative for 4 mice of each genotype. (B) Flow cytometric analysis of in vivo expression of CD86, CD80, and MHCII on WT (gray) and CD19-hBtk (red line) CD19+ B cells. Graphs below summarize the MFI levels of these expression markers on WT (black bars) and CD19-hBtk cells (red bars; n = 4 per group). (C) Flow cytometric analysis of MACS-purified WT (gray) and CD19-hBtk (red line) naive B cells that were cultured in the presence of the indicated stimuli for 24 hours. Data are displayed as histogram overlays of the indicated markers; mice were 10 to 14 weeks old.
Btk overexpression specifically enhances BCR-mediated B-cell activation. (A) WT (black line) and CD19-hBtk (red line) B cells were stimulated with α-IgM F(ab′)2 fragments and Ca2+ mobilization was monitored and normalized for maximum influx on stimulation with ionomycin. Plots are representative for 4 mice of each genotype. (B) Flow cytometric analysis of in vivo expression of CD86, CD80, and MHCII on WT (gray) and CD19-hBtk (red line) CD19+ B cells. Graphs below summarize the MFI levels of these expression markers on WT (black bars) and CD19-hBtk cells (red bars; n = 4 per group). (C) Flow cytometric analysis of MACS-purified WT (gray) and CD19-hBtk (red line) naive B cells that were cultured in the presence of the indicated stimuli for 24 hours. Data are displayed as histogram overlays of the indicated markers; mice were 10 to 14 weeks old.
To dissect which Btk-mediated signaling pathways might contribute to the phenotype of enhanced B-cell activation in CD19-hBtk mice in vivo, we stimulated purified splenic B cells with α-IgM, LPS, CpG and α-CD40 in vitro. In agreement with the increased Ca2+ influx on BCR engagement, we found that after 24 hours of α-IgM stimulation CD19-hBtk B cells manifested markedly increased surface expression of CD86, CD69, and CD25, as well as a clear cell size increase compared with nontransgenic B cells (Figure 2C; for quantification see supplemental Figure 2C). Activation markers continued to be expressed at significantly higher levels throughout 3 days of in vitro stimulation (data not shown). In contrast, in the absence of stimulation or in the presence of LPS, CpG, or α-CD40, we did not detect increased up-regulation of any of the activation makers tested after 1 or 3 days of culture (Figure 2C, supplemental Figure 2C, and data not shown).
These findings indicate that Btk overexpression selectively enhances BCR-mediated B-cell activation. Although multiple activating signals are capable of inducing up-regulation of Btk protein expression (Figure 1A), this up-regulation exclusively sensitizes B cells to BCR signaling.
B-cell specific Btk overexpression in vivo results in systemic autoimmune disease
Young CD19-hBtk mice displayed increased IgM but normal IgG and IgA levels in the serum (Figure 3A), consistent with the observed increased numbers of IgM+ but not IgG+ plasma cells in the BM of these mice. Because enhanced GC and plasma cell formation in nonimmunized CD19-hBtk mice might reflect a response to self-antigens, we screened for autoantibody production in CD19-hBtk mice, using a HEp2 reactivity assay. In the serum of ∼ 25% of young CD19-hBtk mice we detected low levels of IgG autoantibodies, which were exclusively directed against nuclear epitopes (Figure 3B). However, in aging mice (> 40 weeks), high autoantibody levels were present in all animals (Figure 3B). Quantification of IgG antinucleosome and anti-dsDNA antibodies by ELISA demonstrated high levels (Figure 3C). The levels of anti-nucleosome antibodies (but not of anti-dsDNA antibodies) approximated those observed in diseased MRL/lpr mice, a classic mouse model for spontaneous development of lethal SLE.5 Antinuclear autoantibodies are a hallmark of SLE and can be detected as deposits in glomeruli in the kidney.34 Immunohistochemistry performed on kidneys from aged CD19-hBtk mice showed glomerular immunoglobulin deposition of all IgG subclasses, and periodic acid-Schiff staining revealed glomerular hypercellularity and mesangial matrix expansion (Figure 3D).
Aging CD19-hBtk mice develop systemic autoimmune disease. (A) Serum Ig concentrations of the indicated isotypes in 10- to 14-week-old CD19-hBtk mice and nontransgenic littermates, as determined by ELISA. (B) Antinuclear IgG autoantibodies, detected by HEp2 reactivity, in serum samples from CD19-hBtk mice and nontransgenic littermates, as well as serum from diseased MRL/lpr mice as reference (objective 40×). Percentages indicate proportion of animals (n = 5-8 per group) with antinuclear IgG autoantibodies. (C) Serum IgG antinucleosome and anti-dsDNA autoantibody levels in the indicated mice quantified by ELISA. Each symbol represents an individual mouse. (D) Glomerular deposition of immune complexes (red) of the indicated IgG isotypes, detected by immunohistochemistry on kidney tissue sections from old (> 40 weeks) CD19-hBtk and WT (WT) littermates. Lower panels show a PAS staining on kidneys from these mice. Dashed lines encircle individual glomeruli (objective 40×). Data are representative of 5 to 8 CD19-hBtk mice.
Aging CD19-hBtk mice develop systemic autoimmune disease. (A) Serum Ig concentrations of the indicated isotypes in 10- to 14-week-old CD19-hBtk mice and nontransgenic littermates, as determined by ELISA. (B) Antinuclear IgG autoantibodies, detected by HEp2 reactivity, in serum samples from CD19-hBtk mice and nontransgenic littermates, as well as serum from diseased MRL/lpr mice as reference (objective 40×). Percentages indicate proportion of animals (n = 5-8 per group) with antinuclear IgG autoantibodies. (C) Serum IgG antinucleosome and anti-dsDNA autoantibody levels in the indicated mice quantified by ELISA. Each symbol represents an individual mouse. (D) Glomerular deposition of immune complexes (red) of the indicated IgG isotypes, detected by immunohistochemistry on kidney tissue sections from old (> 40 weeks) CD19-hBtk and WT (WT) littermates. Lower panels show a PAS staining on kidneys from these mice. Dashed lines encircle individual glomeruli (objective 40×). Data are representative of 5 to 8 CD19-hBtk mice.
Next, we searched in kidneys of old CD19-hBtk mice for immune cell infiltrates. We were able to identify infiltrates composed of dendritic cells, T and B cells, and occasionally plasma cells in a fraction of old CD19-hBtk mice (Figure 4A, data not shown). In congruence, some mice (∼ 25%) developed detectable proteinuria (Figure 4B).
Aging CD19-hBtk mice have large perivascular infiltrates in various organs. (A) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel) or T cells (CD3+, blue; right panel) of kidney tissue sections from old CD19-hBtk mice (objective 10×). (B) Proteinuria in 2 of 8 (25%) CD19-hBtk mice; albumin levels in urine samples were measured by ELISA. (C-D) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel), T cells (CD3+, blue; middle panel), or GC B cells (GL7+, red) and plasma cells (CD138+, blue; right panel) of lungs (C) and salivary glands (D) from old CD19-hBtk mice (objective 10×). Data are representative of 5-8 CD19-hBtk mice.
Aging CD19-hBtk mice have large perivascular infiltrates in various organs. (A) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel) or T cells (CD3+, blue; right panel) of kidney tissue sections from old CD19-hBtk mice (objective 10×). (B) Proteinuria in 2 of 8 (25%) CD19-hBtk mice; albumin levels in urine samples were measured by ELISA. (C-D) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel), T cells (CD3+, blue; middle panel), or GC B cells (GL7+, red) and plasma cells (CD138+, blue; right panel) of lungs (C) and salivary glands (D) from old CD19-hBtk mice (objective 10×). Data are representative of 5-8 CD19-hBtk mice.
All CD19-hBtk mice older than 40 weeks manifested large perivascular infiltrates in the lungs, exclusively located adjacent to large veins (Figure 4C). In these infiltrates we detected dendritic cells, T and B cells, plasma cells, and frequently even GC B cells, indicative of tertiary lymphoid structure formation, although T and B cell zones were not clearly segregated (Figure 4C). Large perivascular infiltrates with similar organization were present in salivary glands of all old CD19-hBtk mice (Figure 4D).
Taken together, these data show that CD19-hBtk mice develop all hallmarks of an SLE-like autoimmune disorder, characterized by autoantibody formation including anti-dsDNA, kidney damage, and perivascular infiltration of lymphocytes.
Autoimmunity when Btk overexpression is limited to mature B cells and myeloid cells
In CD19-hBtk mice, Btk is overexpressed throughout B-cell differentiation (supplemental Figure 2A), and therefore enhanced BCR signaling in developing B cells might affect BCR selection in the BM. It is conceivable that an altered BCR repertoire, possibly enriched in autoreactive specificities, may contribute to the autoimmune disorder in these mice.
To analyze the effects of Btk overexpression in vivo, independent of possible effects on BCR repertoire selection in the BM, we took advantage of MHCII-hBtk transgenic mice. In these mice Btk overexpression is driven by the mouse MHC class II Ea gene locus control region, targeting Btk overexpression specifically to mature, peripheral B cells and myeloid cells.26,27 In these mice, only mature IgD+ B cells overexpressed Btk (∼ 7-fold compared with WT), whereas developing B cells in the BM or transitional B cells in the spleen do not express transgenic Btk (Figure 5A, supplemental Figure 3A).
Btk overexpression in mature B cells is sufficient to induce autoimmunity. (A) Intracellular flow cytometric Btk detection in splenic CD19+ B cells, shown as histogram overlays from Btk−/− (shaded histogram), WT (black line), and MHCII-hBtk (green line) mice. (B) Immunohistochemical analysis of CD21+ B cells (red), MOMA-1+ metallophilic macrophages (blue; left panel) and GL7+ GC B cells (red), and CD138+ plasma cells (blue; right panel) in the spleens of young (10-14 weeks) MHCII-hBtk mice. (C) Flow cytometric quantification of splenic IgM+ and IgG+ plasma cell numbers in young MHCII-hBtk mice and nontransgenic littermates (WT). (D) WT (black line) and MHCII-hBtk (green line) B cells were stimulated with α-IgM F(ab′)2 fragments and Ca2+ mobilization was monitored and normalized for maximum influx on stimulation with ionomycin. Plots are representative for 4 mice of each genotype. (E) HEp2 reactivity of serum IgG antibodies in aging MHCII-hBtk mice. Percentages indicate proportion of HEp2 reactive serum samples per group (objective 40×). (F) Quantification of IgG antinucleosome and anti-dsDNA autoantibodies in the indicated mice, as determined by ELISA. (G) IgG immune complex deposition in glomeruli, detected by immunohistochemistry on kidney tissue sections from old (> 40 weeks) MHCII-hBtk mice. (H) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel), GC B cells (PNA+, red) and T cells (CD3+, blue; middle panel), or GC B cells (PNA+, red) and IgG1+ plasma cells (IgG1+, blue; right panel) in lungs from old MHCII-hBtk mice (objective 10×). Data are representative of 6 MHCII-hBtk mice.
Btk overexpression in mature B cells is sufficient to induce autoimmunity. (A) Intracellular flow cytometric Btk detection in splenic CD19+ B cells, shown as histogram overlays from Btk−/− (shaded histogram), WT (black line), and MHCII-hBtk (green line) mice. (B) Immunohistochemical analysis of CD21+ B cells (red), MOMA-1+ metallophilic macrophages (blue; left panel) and GL7+ GC B cells (red), and CD138+ plasma cells (blue; right panel) in the spleens of young (10-14 weeks) MHCII-hBtk mice. (C) Flow cytometric quantification of splenic IgM+ and IgG+ plasma cell numbers in young MHCII-hBtk mice and nontransgenic littermates (WT). (D) WT (black line) and MHCII-hBtk (green line) B cells were stimulated with α-IgM F(ab′)2 fragments and Ca2+ mobilization was monitored and normalized for maximum influx on stimulation with ionomycin. Plots are representative for 4 mice of each genotype. (E) HEp2 reactivity of serum IgG antibodies in aging MHCII-hBtk mice. Percentages indicate proportion of HEp2 reactive serum samples per group (objective 40×). (F) Quantification of IgG antinucleosome and anti-dsDNA autoantibodies in the indicated mice, as determined by ELISA. (G) IgG immune complex deposition in glomeruli, detected by immunohistochemistry on kidney tissue sections from old (> 40 weeks) MHCII-hBtk mice. (H) Immunohistochemical analysis for dendritic cells (CD11c+, red) and B cells (B220+, blue; left panel), GC B cells (PNA+, red) and T cells (CD3+, blue; middle panel), or GC B cells (PNA+, red) and IgG1+ plasma cells (IgG1+, blue; right panel) in lungs from old MHCII-hBtk mice (objective 10×). Data are representative of 6 MHCII-hBtk mice.
MHCII-hBtk mice (on a C57BL/6 background) manifested a phenotype that essentially resembled that of CD19-hBtk mice. Spleens of these mice contained increased numbers of GC and IgM+ and IgG+ plasma cells (Figure 5B-C). Splenic MHCII-hBtk transgenic B cells had increased surface CD86 expression (supplemental Figure 3B), displayed enhanced Ca2+ influx (Figure 5D) and up-regulation of surface CD86, CD69, and CD25 (supplemental Figure 3C) on BCR triggering, compared with WT B cells. Using HEp2 and ELISA assays, we detected low levels of antinuclear IgG autoantibodies in the serum of a fraction of young MHCII-hBtk mice (Figure 5E). The levels of serum IgG antinucleosome and especially anti-dsDNA autoantibodies in aging MHCII-hBtk mice were comparable with levels measured in diseased MRL/lpr mice (Figure 5F). Autoantibody reactivity in MHCII-hBtk mice was highly similar to the autoreactivity observed in CD19-hBtk mice, and was mostly restricted to histones and ribonucleoproteins, as demonstrated by line immunoassay (LIA; supplemental Table 2).35 Deposits of IgG immune complexes and glomerular damage in the kidney, and perivascular inflammation in lungs and salivary glands were detected in all aging MHCII-hBtk mice (Figure 5G-H, data not shown).
In summary, Btk overexpression throughout B-cell development in CD19-hBtk mice and in mature B cells and myeloid cells in MHCII-hBtk results in a similar phenotype. We therefore conclude that overexpression of Btk selectively in mature B cells is sufficient to induce autoimmunity.
Btk kinase activity is essential for the induction of autoimmunity by Btk overexpression
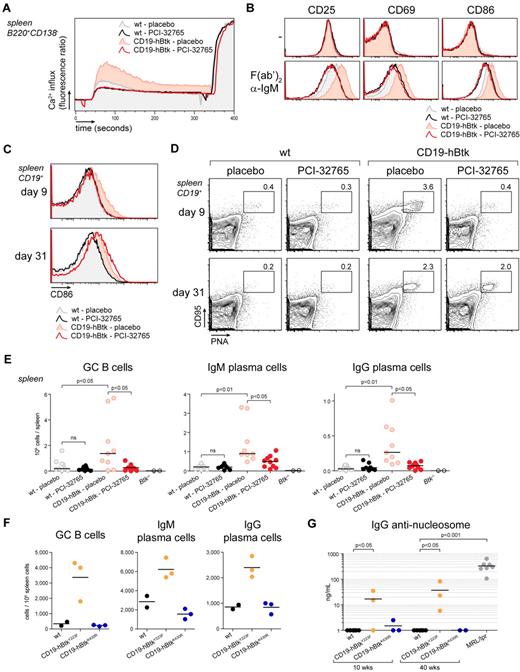
Btk can serve as a binding partner to many proteins14 and kinase-independent functions of Btk have been described.29,36,37 To determine whether the in vivo effects of Btk overexpression were dependent on Btk kinase activity, we treated CD19-hBtk mice with PCI-32765, a specific Btk-inhibitor, that irreversibly binds to C481 in the Btk kinase domain and blocks phosphorylation at Y551.33 Three hours after administering a single dose of PCI-32765 (25 μg/g) to CD19-hBtk and WT mice, splenic B cells were isolated and stimulated with α-IgM in vitro. PCI-32765 treatment strongly reduced Ca2+ influx on BCR triggering in B cells from CD19-hBtk and WT mice (Figure 6A). It also abolished hyperreactivity of CD19-hBtk transgenic B cells to BCR stimulation: α-IgM stimulated B cells from PCI-32765–treated CD19-hBtk or WT mice expressed equally low levels of CD25, CD69, and CD86 (Figure 6B).
BCR hyperresponsiveness of CD19-hBtk B cells is kinase-dependent. (A) Ca2+ influx on F(ab′)2 α-IgM stimulation of splenic naive B cells from the indicated mouse groups, 3 hours after oral administration of PCI-32765 or placebo. Signals were normalized for maximum influx on stimulation with ionomycin. Plots are representative for 2 to 4 mice of each genotype. (B) Flow cytometric analysis of expression of activation markers of MACS-purified B cells from spleens of the indicated mouse groups after culture for 24 hours with or without F(ab′)2 α-IgM. (C) Flow cytometric analysis of CD86 on splenic CD19+ B cells of the indicated mice, after 8 days of treatment with PCI-32765 or placebo. B cells were evaluated 1 or 23 days after termination of treatment (days 9 and 31, respectively). (D) Flow cytometric analysis of splenic CD95+PNA+ GC B cells, gated from CD19+ B cells. Data are shown as dot plots and the percentages GC B cells are given. (E) Numbers of IgM+ and IgG+ splenic plasma cells 9 and 31 days after start of treatment of the indicated mouse groups. Collective data from 3 independent experiments are shown. (F) Autoimmunity in Btk overexpressing mice is dependent on Btk kinase activity, but not on Btk Y223 autophosphorylation. Flow cytometric quantification of splenic GC B cells (CD95+PNA+), IgM+ and IgG+ plasma in 10- to 14-week-old mice that overexpress autophosphorylation-defective Btk (CD19-hBtkY223F) or kinase-dead Btk (CD19-hBtkK430R). (G) Quantification of serum IgG anti-nucleosome auto-antibodies, determined by ELISA, whereby sera of diseased MRL/lpr mice were used as a reference. Each symbol represents an individual mouse.
BCR hyperresponsiveness of CD19-hBtk B cells is kinase-dependent. (A) Ca2+ influx on F(ab′)2 α-IgM stimulation of splenic naive B cells from the indicated mouse groups, 3 hours after oral administration of PCI-32765 or placebo. Signals were normalized for maximum influx on stimulation with ionomycin. Plots are representative for 2 to 4 mice of each genotype. (B) Flow cytometric analysis of expression of activation markers of MACS-purified B cells from spleens of the indicated mouse groups after culture for 24 hours with or without F(ab′)2 α-IgM. (C) Flow cytometric analysis of CD86 on splenic CD19+ B cells of the indicated mice, after 8 days of treatment with PCI-32765 or placebo. B cells were evaluated 1 or 23 days after termination of treatment (days 9 and 31, respectively). (D) Flow cytometric analysis of splenic CD95+PNA+ GC B cells, gated from CD19+ B cells. Data are shown as dot plots and the percentages GC B cells are given. (E) Numbers of IgM+ and IgG+ splenic plasma cells 9 and 31 days after start of treatment of the indicated mouse groups. Collective data from 3 independent experiments are shown. (F) Autoimmunity in Btk overexpressing mice is dependent on Btk kinase activity, but not on Btk Y223 autophosphorylation. Flow cytometric quantification of splenic GC B cells (CD95+PNA+), IgM+ and IgG+ plasma in 10- to 14-week-old mice that overexpress autophosphorylation-defective Btk (CD19-hBtkY223F) or kinase-dead Btk (CD19-hBtkK430R). (G) Quantification of serum IgG anti-nucleosome auto-antibodies, determined by ELISA, whereby sera of diseased MRL/lpr mice were used as a reference. Each symbol represents an individual mouse.
To investigate whether prolonged PCI-32765 treatment could correct enhanced GC and plasma cell formation in vivo, we treated CD19-hBtk and nontransgenic mice with PCI-32765 (25 μg/g/d) for 8 consecutive days. Flow cytometric analysis revealed full normalization of CD86, CD80, and MHCII expression levels on B cells in CD19-hBtk mice at day 9 (Figure 6C, data not shown). This effect was reversible, because B cells from PCI-32765 treated CD19-hBtk mice reexpressed high levels of CD86 at day 31 (23 days after treatment termination; Figure 6C). After prolonged PCI-32765 treatment, CD19-hBtk mice lost enhanced GC formation (Figure 6D day 9), whereas termination of the treatment resulted in renewed GC formation within 23 days (Figure 6D day 31). Likewise, IgM and IgG plasma cells numbers in the spleen were reduced at day 9 (Figure 6E) and reappeared concurrently with GC in CD19-hBtk mice after termination of PCI-32765 treatment (supplemental Figure 4A). This finding demonstrates that the enhanced GC and plasma-cell formation in Btk overexpressing mice is dependent on Btk kinase function, and that most plasma cells in the spleens of CD19-hBtk mice are short-lived. The 8-day treatment with PCI-32765 did not appear to affect B-cell development, as the sizes of the B-lineage subpopulations in spleen and BM were not different between PCI-32765–treated versus placebo-treated CD19-hBtk mice or WT littermates (supplemental Figure 4B, supplemental Figure 4C).
To finally show that also autoantibody formation in Btk overexpressing mice is kinase-dependent, we analyzed CD19-hBtkK430R mice, with ∼ 15- to 20-fold increased expression of the kinase-inactive BtkK430R mutant in mature B cells,29 and CD19-hBtkY223F mice, with ∼ 3-fold overexpression of the autophosphorylation site Y223F Btk mutant,29 both analyzed on the C57BL/6 background. Flow cytometric analysis of spleens revealed that enhanced formation of GC B cells, IgM, and IgG plasma cells was not dependent on Btk autophosphorylation but did require Btk kinase activity (Figure 6F). Aging CD19-hBtkY223F but not CD19-hBtkK430R mice produced antinuclear autoantibodies (Figure 6G, supplemental Figure 4D). Consistent with these findings, old (> 40 weeks) CD19-hBtkK430R mice did not develop signs of autoimmune pathology in lungs, kidneys, or salivary glands (data not shown).
In summary, these data show that the induction of autoimmunity by Btk overexpression is not caused by an increase in Btk protein levels per se, but requires Btk kinase activity.
Btk overexpression impairs negative selection of autoreactive B cells
On activation, B cells become susceptible to Fas-mediated negative selection. As Btk is known to provide NF-κB–dependent survival signals for B cells, we studied apoptosis induction in purified Btk-overexpressing and WT B cells on in vitro stimulation of BCR, CD40 and TLRs. Whereas apoptosis rates of unstimulated, α-CD40, LPS, or CpG stimulated B cells were very similar in the 2 groups, Btk overexpression induced a clear decrease in apoptosis on α-IgM stimulation (Figure 7A, supplemental Figure 5A, and data not shown). As a control, we included Btk−/− B cells, which showed increased susceptibility to apoptosis. To test whether this apoptosis resistance could counteract T cell–derived proapoptotic signals that instruct clonal deletion of B cells, we cultured splenic CD19-hBtk and WT B cells in the presence or absence of α-IgM with increasing FasL concentrations (Figure 7B). Btk overexpression did not directly affect Fas signaling, because in the absence of BCR stimulation the apoptosis rates of FasL-stimulated CD19-hBtk cells and WT B cells were indistinguishable (Figure 7B). However, in the presence of α-IgM stimulation a dose-dependent apoptotic effect of FasL was observed in WT B cells, but not in CD19-hBtk B cells (Figure 7B).
Phenotype of Btk overexpression is associated with enhanced NF-κB signaling and B-cell survival. (A) PI DNA content analysis of MACS-sorted splenic B cells of the indicated mice after 36 hours of culture in the absence (−) or presence of F(ab′)2 α-IgM. Numbers indicate the proportions of apoptotic cells (sub-G1, left gate) or cycling cells (S/M/G2, right gate). (B) Frequencies of apoptotic cells, as identified by DNA content analysis of MACS sorted naive B cells from the indicated mice, cultured in the absence (−) or presence (+) of F(ab′)2 α-IgM and/or α-CD95/FasL antibodies. (C) MACS-purified naive splenic B cells from WT and CD19-hBtk mice were stimulated with α-Igκ (20 μg/mL), and phosphorylation of ERK and Akt (pERK and pAkt) was detected by intracellular flow cytometry. (D) Nuclear translocation of c-Rel in unstimulated (−) or F(ab′)2 α-IgM-stimulated (+) B cells from the indicated mice, analyzed by Western blotting with nucleophosmin as loading control. Data were quantified after normalization for nucleophosmin levels (n = 5 per group); c-Rel levels in unstimulated WT B cells were set to 1. (E) Using ELISA total IgG levels were determined in BALf collected from X-31 infected WT and CD19-hBtk mice at 18 dpi. Mean values ± SEM are shown (n = 8 mice/group). (F) Serum antibody titers specific for X-31 and related virus strains Eng/72 and Pch/73 in CD19-hBtk and WT mice at 18 dpi, as determined by hemagglutinin inhibition assay (HAI). (G) Autoreactive IgG antibodies, detected by HEp2 reactivity of BALf collected 18 dpi from X-31 infected or naive mice, as indicated (4 mice/group; objective 40×).
Phenotype of Btk overexpression is associated with enhanced NF-κB signaling and B-cell survival. (A) PI DNA content analysis of MACS-sorted splenic B cells of the indicated mice after 36 hours of culture in the absence (−) or presence of F(ab′)2 α-IgM. Numbers indicate the proportions of apoptotic cells (sub-G1, left gate) or cycling cells (S/M/G2, right gate). (B) Frequencies of apoptotic cells, as identified by DNA content analysis of MACS sorted naive B cells from the indicated mice, cultured in the absence (−) or presence (+) of F(ab′)2 α-IgM and/or α-CD95/FasL antibodies. (C) MACS-purified naive splenic B cells from WT and CD19-hBtk mice were stimulated with α-Igκ (20 μg/mL), and phosphorylation of ERK and Akt (pERK and pAkt) was detected by intracellular flow cytometry. (D) Nuclear translocation of c-Rel in unstimulated (−) or F(ab′)2 α-IgM-stimulated (+) B cells from the indicated mice, analyzed by Western blotting with nucleophosmin as loading control. Data were quantified after normalization for nucleophosmin levels (n = 5 per group); c-Rel levels in unstimulated WT B cells were set to 1. (E) Using ELISA total IgG levels were determined in BALf collected from X-31 infected WT and CD19-hBtk mice at 18 dpi. Mean values ± SEM are shown (n = 8 mice/group). (F) Serum antibody titers specific for X-31 and related virus strains Eng/72 and Pch/73 in CD19-hBtk and WT mice at 18 dpi, as determined by hemagglutinin inhibition assay (HAI). (G) Autoreactive IgG antibodies, detected by HEp2 reactivity of BALf collected 18 dpi from X-31 infected or naive mice, as indicated (4 mice/group; objective 40×).
Next, we analyzed signaling pathways downstream of Btk that transmit antiapoptotic BCR signals. In BCR-stimulated CD19-hBtk or MHCII-hBtk B cells phosphorylation of ERK or Akt was not increased (Figure 7C, supplemental Figure 5B). However, nuclear translocation of the NF-κB factor c-Rel, as determined by Western blotting of nuclear extracts, was more prominent in α-IgM stimulated CD19-hBtk or MHCII-hBtk transgenic than in WT B cells (Figure 7D, supplemental Figure 5C).
To study B-cell selection in CD19-hBtk mice in vivo, we studied the T-cell dependent antibody response to influenza virus. Young CD19-hBtk and WT littermates were infected intranasally with a sublethal dose of the influenza A/HKX-31 (H3N2) strain that induces robust GC formation in lungs and draining lymph nodes.31 The positive selection of X-31 specific B cells in CD19-hBtk mice proved to be normal, because the levels of X-31 specific antibodies in the serum 18 dpi were equal in CD19-hBtk and WT mice (Figure 7E). Furthermore, the production of antibodies crossreacting with X-31 related influenza strains Eng/72 and Pch/73 was similar between CD19-hBtk and WT mice (Figure 7E). Btk overexpression did not significantly affect GC B cell numbers in lungs or draining lymph nodes (data not shown), and total IgG levels in BALf of infected CD19-hBtk mice were modestly, but not significantly, increased at 18 dpi (Figure 7F). However, screening of BALf for local production of autoantibodies in the lungs revealed IgG antinuclear autoantibodies in 75% of the BALf samples obtained at 18 dpi from influenza-infected CD19-hBtk mice (Figure 7G). These autoantibodies were not detected in BALf from infected WT littermates or noninfected CD19-hBtk littermates (Figure 7G), demonstrating defective elimination of locally emerging autoreactive IgG plasma cells in lungs of X-31 infected CD19-hBtk mice.
Collectively, these data show that Btk overexpression induces apoptosis resistance and enhanced NF-κB activation in vitro on BCR stimulation, which interferes in vivo with negative selection of B cells with acquired affinity for self-antigens during GC driven antibody responses.
Discussion
In this study, we used B cell–specific transgenic overexpression of WT Btk driven by the CD19 promoter region to unravel a crucial function of this tyrosine kinase signaling protein in setting the threshold for B-cell activation and negative selection of autoreactive B cells. B lymphocytes overexpressing Btk developed normally, but showed enhanced GC and plasma cell formation. Aging Btk transgenic mice developed systemic autoimmune disease, characterized by significant levels of circulating IgG antinuclear autoantibodies and inflammation of lungs, salivary glands, and kidneys. Autoimmunity was fully dependent on Btk kinase activity, because the phenotype was corrected by Btk inhibitor treatment and was not present in mice overexpressing kinase-inactive Btk. B lymphocytes overexpressing Btk demonstrated a selective hyperresponsiveness to BCR stimulation, associated with increased Ca2+-influx and enhanced up-regulation of cell surface activation markers. Moreover, BCR stimulation of these cells induced increased NF-κB activation and resistance to Fas-mediated apoptosis. Finally, we show defective negative selection of autoreactive B cells arising in an in vivo influenza infection model.
Many previous observations, including reduced survival of Btk-deficient B cells, the identification of several inhibitory factors of Btk, and the multiple (post)transcriptional mechanisms by which Btk expression is increased on BCR stimulation11,17-23 prompted us to investigate whether the expression level of Btk is a critical factor for correct B-cell functioning. In this study, we have shown that Btk up-regulation does not only occur on BCR signaling through Btk, but seems to be inherent to B-cell activation, because CD40 or TLR stimulation also instruct Btk up-regulation. Using transgenic mice with B cell–specific overexpression of WT human Btk, we were able to demonstrate that high Btk levels exclusively enhance BCR signaling. BCR stimulation of CD19-hBtk B cells evoked stronger Ca2+ mobilization and up-regulation of activation markers, whereas increased activation was not observed on stimulation of CD40 or TLRs, even though TLR4 and TLR9 also use Btk.14 We therefore propose that Btk up-regulation in B cells on activation by BCR, CD40, or TLR signals primarily serves to sensitize the B cell for antigen recognition through the BCR. Dynamic regulation of Btk levels of activated B cells may thus aid the proper activation and selection of antigen-activated B cells.
Using CD19-hBtk mice, we demonstrated that continuous Btk overexpression induced spontaneous B-cell activation in vivo, characterized by up-regulation of surface molecules specifically involved in B-T cell interactions, leading to spontaneous differentiation into GC B cells and IgG plasma cells. Clearly, the selection of activated Btk overexpressing B cells was defective, because IgG plasma cells in aging CD19-hBtk mice produced high levels of antinuclear autoantibodies that elicited systemic autoimmune disease. From our data, it is unclear whether Btk overexpressing B cells have the capacity to breach tolerance of quiescent selfreactive T cells, which would ensure sufficient reciprocal stimulation to enable positive selection.1,38 However, it is conceivable that originally non-autoreactive B cells that enter GCs may acquire selfreactive BCRs through somatic hypermutation, whereby Btk overexpression impairs negative selection of these cells. Influenza infection in CD19-hBtk mice showed that normal antiviral antibody titers were produced with normal antiviral crossreactivity, but selfreactive B cells emerged locally in the lung, leading to autoantibody secretion in BALf. Based on the strictly antinuclear and not anticytoplasmatic specificity of these BALf autoantibodies in virus-infected CD19-hBtk mice and based on the comparable total IgG levels in BALf in CD19-hBtk and WT mice, it is less probable that these autoantibodies originate from a generally more robust antibody response in Btk transgenic mice. We propose a model for defective counterselection of autoreactive Btk-overexpressing B cells, based on recent findings showing that T cell help is the limiting factor in GC selection (supplemental Figure 6).39 In support of defective counterselection of selfreactive B cells, Btk overexpressing B cells proved to be insensitive to Fas-induced apoptosis after activated through their BCR, and this apoptosis resistance correlated to increased activation of NF-κB, a major downstream target of Btk that strongly promotes B-cell survival.7-9
To the best of our knowledge, Btk has not been directly implicated in the pathogenesis of autoimmune disease before. Although a previous study demonstrated that abrogated expression of miRNAs including miR185 in mature B cells increased Btk expression and induced systemic autoimmunity,23 many other defects in miRNA-deficient B cells may contribute to the development of autoimmunity. We previously reported that B cell–restricted expression of the constitutively active BtkE41K mutant did not evoke autoimmune disease. We proposed that strong BtkE41K mediated signaling may mimic self-antigen binding to the BCR, thereby instructing massive B-cell deletion.26-28 Nevertheless, residual B cells were hyperresponsive and spontaneously driven into GC-independent plasma cell differentiation. The critical difference between BtkE41K transgenic and Btk overexpressing mice lies in the mode of increase in Btk kinase activity, which is constitutive versus conditional, respectively. Also other mouse models with constitutively increased BCR signaling often display aberrant B-cell development without overt autoimmune pathology, for example, mice with B-cell specific expression of the latent membrane protein 2A, an Epstein-Barr virus encoded protein that substitutes for BCR-derived activation signals,40,41 or mice overexpressing the stimulatory BCR coreceptor CD19.42 In contrast, autoimmune pathology does develop in mouse models with conditionally enhanced BCR signaling, including mice deficient for the inhibitory BCR coreceptors FcγRIIB43 or CD22 and Siglec-G,44 and mice deficient for Shp1 phosphatase45,46 or the regulatory kinase Lyn.47 But, direct linking of BCR signaling aberrations to autoimmunity is complicated in these models, because these molecules are not exclusively involved in BCR signaling or have additional functions in non-B cells. However, the finding that B-cell specific Btk overexpression affects BCR but not TLR signaling and results in autoimmunity, reinforces that enhanced signaling on BCR engagement in B cells is sufficient to induce autoimmune pathology.
Although mutations leading to enhanced Btk activity have not been identified in human subjects to date, this study underlines that not hardwired mutations, but instead dysregulated expression of kinases downstream of the BCR may be the key to erroneous activation of selfreactive B cells. Moreover, the influence of TLR and CD40 signaling on Btk expression levels in mature B cells suggests that the commonly observed BCR hyperresponsiveness in SLE patients may not always directly result from intrinsic BCR signaling defects, but indirectly from aberrant signaling of other receptors that amplify the BCR signaling strength through up-regulation of Btk. Targeting Btk in systemic autoimmune disease may therefore present an attractive new therapeutic approach, and the strong in vivo effects of Btk-inhibitor treatment on B-cell activation in Btk overexpressing mice confirm the high potential of these new therapeutic agents in autoimmune disease, as well as B-cell malignancies, including CLL and DLBCL.24,25 It is reassuring that no adverse effects of Btk inhibition on B-cell development were observed, possibly because of the limited inhibitor half-life and short time window of exposure. On the other hand, the finding of in vivo pathology associated with Btk overexpression may have important implications for the development of future strategies for gene therapy for XLA.14
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Erasmus Medical Center experimental animal facility for their care for our mice and assistance at experiments, and they thank Allard Kaptein and Hans van Eenennaam for their advice with Btk-inhibitor treatment experiments, and Joyce Verburgh (Virology, Erasmu Medical Center) for experimental assistance.
This work was partly supported by the Dutch Arthritis Association (grants 08-1-103 and 09-1-302). The authors state no conflict of interests.
Authorship
Contribution: L.P.K. designed and performed research, analyzed and interpreted data, and wrote the paper; M.J.W.d.B., M.v.N., O.B.J.C., J.P.v.H., G.M.D., F.T., G.F.R., and D.E. performed research and analyzed data; P.F.v.L. and D.D. designed research; and R.W.H. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rudi W. Hendriks, Dept of Pulmonary Medicine, Erasmus Medical Center, PO Box 2040, NL 3000 CA Rotterdam, The Netherlands; e-mail: r.hendriks@erasmusmc.nl.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal