Abstract
CD33 is expressed on the majority of acute myeloid leukemia (AML) leukemic blasts and is the target for gemtuzumab ozogamicin (GO), a toxin-conjugated anti-CD33 mAb. In the present study, we quantified the CD33 mean fluorescent intensity of leukemic blasts prospectively in 619 de novo pediatric AML patients enrolled in Children's Oncology Group GO-containing clinical trials and determined its correlation with disease characteristics and clinical outcome. CD33 expression varied more than 2-log fold; a median mean fluorescent intensity of 129 (range, 3-1550.07) was observed. Patients were divided into 4 quartiles, quartiles 1-4 (Q1-4) based on expression and disease characteristics and clinical response defined across quartiles. High CD33 expression was associated with high-risk FLT3/ITD mutations (P < .001) and was inversely associated with low-risk disease (P < .001). Complete remission (CR) rates were similar, but patients in Q4 had significantly lower overall survival (57% ± 16% vs 77% ± 7%, P = .002) and disease-free survival from CR (44% ± 16% vs 62% ± 8%, P = .022). In a multivariate model, high CD33 expression remained a significant predictor of overall survival (P = .011) and disease-free survival (P = .038) from CR. Our findings suggest that CD33 expression is heterogeneous within de novo pediatric AML. High expression is associated with adverse disease features and is an independent predictor of inferior outcome. The correlation between CD33 expression and GO response is under investigation. These studies are registered at www.clinicaltrials.gov as NCT00070174 and NCT00372593.
Introduction
CD33 is a myeloid antigen (Ag) expressed on malignant blasts of most patients with acute myeloid leukemia (AML) and is a target of the toxin-conjugated humanized IgG4 anti-CD33 mAb gemtuzumab ozogamicin (GO; Mylotarg). The clinical efficacy of GO was initially demonstrated in relapsed AML patients with CD33+ AML.1-4 Although in vitro studies revealed a direct relationship between CD33 expression and GO-induced cytotoxicity,5 conflicting data were obtained from correlative studies conducted within the context of GO clinical trials for adult relapsed AML, suggesting that CD33 expression may be associated with other AML prognostic factors.2,6-8 With recent data from the Medical Research Council AML 15 clinical trial suggesting that GO may have preferential efficacy in select AML populations,9 there is increased interest in determining which subset of patients may most benefit from this targeted agent. Because GO targets surface CD33, our aim in the present study was to determine the variability of CD33 expression and disease characteristics within pediatric AML. We quantified CD33 expression on the surface of leukemic blasts prospectively and determined the correlation of expression levels of this Ag with disease characteristics and clinical outcome within the context of 2 consecutive Children's Oncology Group (COG) trials of GO.
Methods
Patients and treatment
Pediatric patients with de novo AML who were enrolled in COG trials AAML03P1 and AAML0531 were eligible for the present study. COG AAML03P1 was a pilot study in which patients with de novo AML received GO in combination with conventional chemotherapy. Details of the eligibility criteria and the treatment regimen have been described previously.10-12 The dosing and schedule of chemotherapy used in AAML0531 was identical to that of AAML03P1, with the exception that only half of the patients enrolled in AAML0531 received GO because of the randomized nature of that trial.13 There were, however, slight differences in hematopoietic cell transplantation (HCT) allocation for the 2 trials. In brief, for AAML03P1 HCT was limited to those patients with a matched family donor (MFD) and was independent of disease-risk classification. In contrast, for AAML0531, risk classification, defined by cytogenetic and molecular characteristics, dictated the use of HCT. Specifically, low-risk disease precluded HCT and standard-risk patients went to HCT only if a MFD was identified. MFD or a suitably defined unrelated donor was used in the context of high-risk disease. The criterion for off-protocol therapy also differed slightly for the 2 protocols. Within the context of AAML03P1, a patient would be considered off-protocol if > 20% disease was present after induction I or > 5% of disease was present after induction II. For the subsequent AAML0531, patients only received off-protocol therapy if they retained > 5% blasts after induction II. Eligibility criteria were similar for each study, although AAML03P1 had more stringent criteria for baseline performance status, cardiac function, and renal function and restricted enrollment to children < 21 years of age. In contrast, AAML0531 expanded the upper age limit for enrollment to < 30 years and permitted enrollment of Down syndrome patients > 4 years of age. For the purposes of our analysis, the latter population was excluded. Neither study mandated a threshold of CD33 expression for enrollment.
All AAML03P1 patient samples were eligible for our correlative study if consent for biology studies was obtained. Any patient enrolled in AAML0531 before September 20, 2008 who consented to optional biology studies was also eligible. The institutional review boards of all participating institutions approved the clinical protocol and the COG Myeloid Disease Biology Committee approved this research.
Risk stratification
Cytogenetic and molecular abnormalities were used to stratify the study population into risk groups. The low-risk group included patients with core-binding factor (CBF) AML [t(8;21) or inv(16)/t(16;16)] and/or those with nucleophosmin-1 (NPM1) or CEBPA mutations without FLT3/ITD mutations. The high-risk group included patients with disease having a high allelic ratio (> 0.4) FLT3/ITD+ mutation and/or monosomy 5, del(5q), or monosomy 7. The remaining patients were designated as having standard-risk (ie, intermediate-risk) disease.
Assessment of CD33 expression
CD33 mean fluorescent intensity (MFI) of myeloid progenitor cells, as defined by CD45 low and side scatter, was determined by flow cytometry. BM cells were incubated with CD14-FITC (clone ΜΦP9, CD33-PE (clone p67.6), CD45-peridinin chlorophyll protein (clone 2D1), and CD34-allophycocyanin (clone HPCA-2; BD Biosciences) at saturating concentrations for 20 minutes in the dark, followed by lysis of erythroid cells with NH4Cl (pH 7.2 at 37°C) for 5 minutes. Cells were then washed in buffered saline and fixed with 1% paraformaldehyde. Flow cytometric data were collected in list mode using a FACSCalibur (BD Biosciences) as described previously.10 The instruments used in this study were standardized before each analysis using 2 sets of fluorescent beads (RFP-30-5a and RCP-30; Spherotech). The linear MFI14 was determined using WinList (Verity Software House) for each patient without knowledge of other clinical characteristics.
Statistical analyses
Clinical outcome data for patients enrolled in COG AAML03P1 were analyzed until March 31, 2011. The median follow-up for eligible de novo AML patients from AAML03P1 who were alive at last contact and included in our analysis was 1942 days (range 10-2521 days). AAML0531 was closed to accrual on June 15, 2010. Data summarizing study entry characteristics were analyzed as of March 31, 2011. At the time of analysis, AAML0531 remained under the purview of the COG Data Monitoring Study Committee; therefore, analyses of study entry characteristics are summarized for patients enrolled in AAML0531, but induction response and outcome have not been approved for release. For AAML03P1, patients were defined as being in complete remission (CR) if they had 5% or fewer blasts and absence of extramedullary disease after 1 course of induction chemotherapy. Overall survival (OS) was determined both from time of study entry and from end of course I. Disease-free survival (DFS) was defined as the time from the end of course 1 for patients in CR until relapse or death, and relapse-free survival was defined as the time from the end of course 1 for patients in CR until relapse or death due to progressive disease where deaths from nonprogressive disease were censored. Relapse risk was defined as the time from end of course 1 for patients in CR to relapse or death because of progressive disease, where deaths from nonprogressive disease were considered competing events.15 The significance of predictor variables was tested with the log-rank statistic for OS, EFS, and DFS and with the Gray statistic for relapse risk. Patients lost to follow-up were censored at their date of last known contact or at a cutoff of 6 months before the frozen date of study data to compensate for the tendency of deaths and relapses to be reported sooner than ongoing follow-up. The significance of the observed difference in proportions was tested by the χ2 test comparing groups of patients. The Wilcoxon rank-sum test was used to determine the significance between differences in medians of groups. The Cox proportional hazards model was used to estimate hazard ratios (HRs) for univariate and multivariate analyses.
Results
CD33 expression levels and correlation with disease characteristics
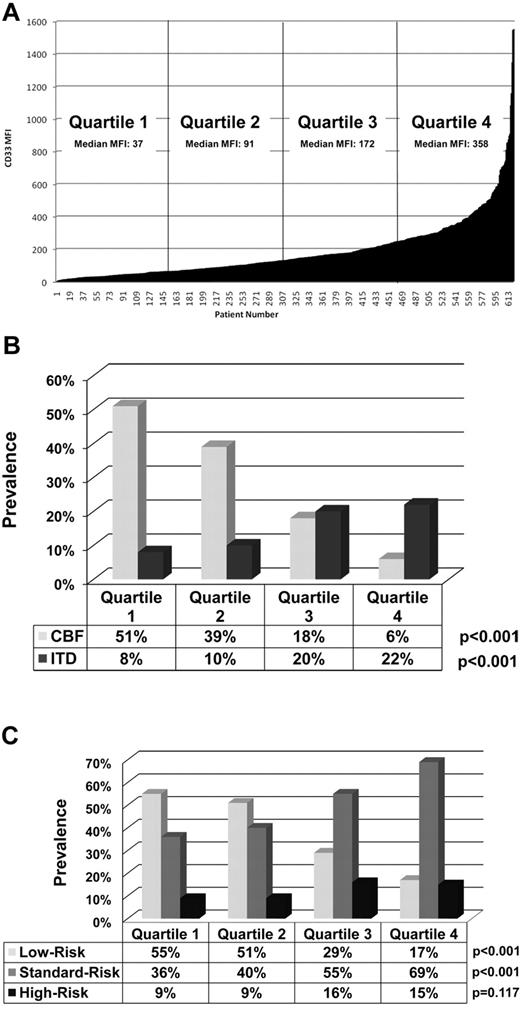
We evaluated CD33 expression in 619 de novo pediatric AML patients prospectively with the goal of correlating expression levels with disease characteristics and clinical outcome. The CD33 MFI of the blast population varied more than 2 log fold, with a median MFI of 129 (range, 3-1550). For the purposes of clinical correlation, the study population was divided into quartiles on the basis of CD33 expression. The median MFI for Q1-4 was as follows: Q1 (n = 155), 37 (range, 3-61.29); Q2 (n = 155), 91 (range, 62-129); Q3 (n = 155), 172 (range, 130-248.57); and Q4 (n = 154), 358 (range, 249-1550.07; Figure 1A). CD33 expression levels were correlated with demographics, pretreatment laboratory findings, and disease characteristics across the 4 quartiles of CD33 expression (Table 1). CD33 expression was also correlated with molecular prognostic factors.16-19 FLT3/ITD, NPM1, and CEBPA mutations were detected in 15%, 6%, and 6% of evaluable samples, respectively. There was a statistically significant increase in FLT3/ITD prevalence with increasing CD33 expression when analyzed by quartiles (8% for Q1, 10% for Q2, 20% for Q3, and 22% for Q4; P < .001, Figure 1B) and for NPM1 mutations (1% for Q1, 5% for Q2, 7% for Q3, and 10% for Q4; P = .001), but there was no definitive trend in CEBPA prevalence among quartiles (Table 1).
Distribution of CD33 expression. (A) Distribution of CD33 expression for the 619 participants in our study cohort. (B) Correlation of CD33 expression level with specific cytogenetic/molecular disease characteristics by quartile. (C) CD33 expression and association with disease risk-group classification by quartile.
Distribution of CD33 expression. (A) Distribution of CD33 expression for the 619 participants in our study cohort. (B) Correlation of CD33 expression level with specific cytogenetic/molecular disease characteristics by quartile. (C) CD33 expression and association with disease risk-group classification by quartile.
Disease characteristics and induction response by CD33 expression quartile
| . | Q1 (n = 155) . | Q2 (n = 155) . | Q3 (n = 155) . | Q4 (n = 154) . | P . |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 83 (54%) | 83 (54%) | 79 (51%) | 86 (56%) | .864 |
| Female | 72 (46%) | 72 (46%) | 76 (49%) | 68 (44%) | |
| Median age, y (range) | 9.9 (0.02-23.9) | 11.4 (0.06-20.8) | 9.4 (0.2-22.8) | 11.1 (0.09-23.8) | .166 |
| Race, n (%) | |||||
| American Indian or Alaska Native | 3 (2%) | 0 (0%) | 1 (1%) | 0 (0%) | .085 |
| Asian | 7 (5%) | 8 (6%) | 6 (4%) | 12 (8%) | .549 |
| Hawaiian/Pacific Islander | 2 (2%) | 1 (1%) | 1 (1%) | 1 (1%) | .857 |
| Black | 13 (10%) | 18 (13%) | 22 (16%) | 21 (14%) | .555 |
| White | 106 (81%) | 112 (81%) | 111 (79%) | 113 (77%) | .826 |
| Unknown | 24 | 16 | 14 | 7 | |
| Ethnicity | |||||
| Hispanic or Latino | 36 (24%) | 28 (19%) | 22 (15%) | 17 (11%) | .016 |
| Not Hispanic or Latino | 111 (76%) | 117 (81%) | 129 (85%) | 134 (89%) | |
| Unknown | 8 | 10 | 4 | 3 | |
| Cytogenetics, n (%) | |||||
| Normal | 19 (13%) | 24 (17%) | 31 (21%) | 37 (25%) | .057 |
| t(8;21) | 46 (32%) | 29 (20%) | 12 (8%) | 4 (3%) | < .001 |
| inv(16) | 28 (19%) | 27 (19%) | 15 (10%) | 5 (3%) | < .001 |
| Abnormal 11 | 18 (12%) | 25 (18%) | 36 (24%) | 47 (32%) | < .001 |
| t(6;9)(p23;q34) | 2 (1%) | 1 (1%) | 8 (5%) | 5 (3%) | .058 |
| Monosomy 7 | 1 (1%) | 3 (2%) | 3 (2%) | 1 (1%) | .549 |
| Del(7q) | 5 (3%) | 3 (2%) | 3 (2%) | 3 (2%) | .83 |
| −5/5q− | 2 (1%) | 3 (2%) | 2 (1%) | 0 (0%) | .411 |
| 8 | 9 (6%) | 5 (4%) | 15 (10%) | 18 (12%) | .032 |
| Other | 16 (11%) | 22 (15%) | 22 (15%) | 29 (19%) | .246 |
| Unknown | 9 | 13 | 8 | 5 | |
| Mutations, n (%) | |||||
| FLT3/ITD | 13 (8%) | 15 (10%) | 30 (20%) | 33 (22%) | < .001 |
| CEBPA mutant | 9 (6%) | 11 (7%) | 10 (7%) | 5 (3%) | .499 |
| NPM mutant | 2 (1%) | 7 (5%) | 10 (7%) | 14 (10%) | .011 |
| Risk group, n (%) | |||||
| Standard | 53 (36%) | 57 (40%) | 82 (55%) | 104 (69%) | < .001 |
| Low | 82 (55%) | 74 (51%) | 43 (29%) | 25 (17%) | < .001 |
| High | 13 (9%) | 13 (9%) | 24 (16%) | 22 (15%) | .117 |
| Unknown | 7 | 11 | 6 | 3 | |
| Median WBCs, × 103 μL (range) | 21.9 (1.1-415.7) | 34.5 (1.3-526) | 36.2 (0.8-409) | 18.4 (0.8-447.3) | .003 |
| BM blasts, %, n (range) | 65.5 (2-100) | 70 (3-99) | 70 (5-100) | 71 (13-99) | .118 |
| Median platelets, 1000/μL (range) | 40.5 (2-468) | 41 (4-571) | 55.5 (4-578) | 63.5 (1-524) | .007 |
| Median hemoglobin, g/dL (range) | 8.1 (2.8-15.2) | 8 (3.3-17) | 8.2 (2.3-14.9) | 8.4 (3.1-15.3) | .289 |
| . | Q1 (n = 155) . | Q2 (n = 155) . | Q3 (n = 155) . | Q4 (n = 154) . | P . |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 83 (54%) | 83 (54%) | 79 (51%) | 86 (56%) | .864 |
| Female | 72 (46%) | 72 (46%) | 76 (49%) | 68 (44%) | |
| Median age, y (range) | 9.9 (0.02-23.9) | 11.4 (0.06-20.8) | 9.4 (0.2-22.8) | 11.1 (0.09-23.8) | .166 |
| Race, n (%) | |||||
| American Indian or Alaska Native | 3 (2%) | 0 (0%) | 1 (1%) | 0 (0%) | .085 |
| Asian | 7 (5%) | 8 (6%) | 6 (4%) | 12 (8%) | .549 |
| Hawaiian/Pacific Islander | 2 (2%) | 1 (1%) | 1 (1%) | 1 (1%) | .857 |
| Black | 13 (10%) | 18 (13%) | 22 (16%) | 21 (14%) | .555 |
| White | 106 (81%) | 112 (81%) | 111 (79%) | 113 (77%) | .826 |
| Unknown | 24 | 16 | 14 | 7 | |
| Ethnicity | |||||
| Hispanic or Latino | 36 (24%) | 28 (19%) | 22 (15%) | 17 (11%) | .016 |
| Not Hispanic or Latino | 111 (76%) | 117 (81%) | 129 (85%) | 134 (89%) | |
| Unknown | 8 | 10 | 4 | 3 | |
| Cytogenetics, n (%) | |||||
| Normal | 19 (13%) | 24 (17%) | 31 (21%) | 37 (25%) | .057 |
| t(8;21) | 46 (32%) | 29 (20%) | 12 (8%) | 4 (3%) | < .001 |
| inv(16) | 28 (19%) | 27 (19%) | 15 (10%) | 5 (3%) | < .001 |
| Abnormal 11 | 18 (12%) | 25 (18%) | 36 (24%) | 47 (32%) | < .001 |
| t(6;9)(p23;q34) | 2 (1%) | 1 (1%) | 8 (5%) | 5 (3%) | .058 |
| Monosomy 7 | 1 (1%) | 3 (2%) | 3 (2%) | 1 (1%) | .549 |
| Del(7q) | 5 (3%) | 3 (2%) | 3 (2%) | 3 (2%) | .83 |
| −5/5q− | 2 (1%) | 3 (2%) | 2 (1%) | 0 (0%) | .411 |
| 8 | 9 (6%) | 5 (4%) | 15 (10%) | 18 (12%) | .032 |
| Other | 16 (11%) | 22 (15%) | 22 (15%) | 29 (19%) | .246 |
| Unknown | 9 | 13 | 8 | 5 | |
| Mutations, n (%) | |||||
| FLT3/ITD | 13 (8%) | 15 (10%) | 30 (20%) | 33 (22%) | < .001 |
| CEBPA mutant | 9 (6%) | 11 (7%) | 10 (7%) | 5 (3%) | .499 |
| NPM mutant | 2 (1%) | 7 (5%) | 10 (7%) | 14 (10%) | .011 |
| Risk group, n (%) | |||||
| Standard | 53 (36%) | 57 (40%) | 82 (55%) | 104 (69%) | < .001 |
| Low | 82 (55%) | 74 (51%) | 43 (29%) | 25 (17%) | < .001 |
| High | 13 (9%) | 13 (9%) | 24 (16%) | 22 (15%) | .117 |
| Unknown | 7 | 11 | 6 | 3 | |
| Median WBCs, × 103 μL (range) | 21.9 (1.1-415.7) | 34.5 (1.3-526) | 36.2 (0.8-409) | 18.4 (0.8-447.3) | .003 |
| BM blasts, %, n (range) | 65.5 (2-100) | 70 (3-99) | 70 (5-100) | 71 (13-99) | .118 |
| Median platelets, 1000/μL (range) | 40.5 (2-468) | 41 (4-571) | 55.5 (4-578) | 63.5 (1-524) | .007 |
| Median hemoglobin, g/dL (range) | 8.1 (2.8-15.2) | 8 (3.3-17) | 8.2 (2.3-14.9) | 8.4 (3.1-15.3) | .289 |
Cytogenetic data were available for 584 of 619 patient samples (94%). Prevalence of CBF AML was inversely associated with CD33 expression across the 4 quartiles, with a prevalence of 51% in patients with the lowest and 6% in those with the highest CD33 expression (P < .001; Figure 1B and Table 1). A similar decline was observed for t(8;21) and inv(16) cytogenetic abnormalities when analyzed individually (Table 1). The prevalence of trisomy 8 and abnormalities of chromosome 11 increased with increasing quartile (P = .032 and P < .001, respectively; Table 1). There was no association between CD33 expression and high-risk cytogenetics; however, analysis was limited by the small number of patients with such alterations (15 of 584, 2.6%).
For risk-group classification, complete cytogenetic and molecular data were available for 592 of 619 (96%) samples; 224 of 592 (38%) were classified in the low-risk group, 296 of 592 (50%) in the standard-risk group, and 74 of 592 (12%) in the high-risk group. There was an inverse association between CD33 expression and prevalence of low-risk AML (P < .001; Figure 1C and Table 1). In contrast, the prevalence of standard-risk disease increased significantly with increasing quartile (P < .001, Figure 1C). There was no statistically significant trend in prevalence by quartile for high-risk disease (P = .117; Figure 1C and Table 1); however, there was a significantly higher median CD33 MFI with high-risk disease (median MFI, 191.985; range, 12-1160) than with low-risk disease (median MFI, 82.905; range, 5-1550.07; P < .001).
CD33 expression levels and correlation with clinical outcome
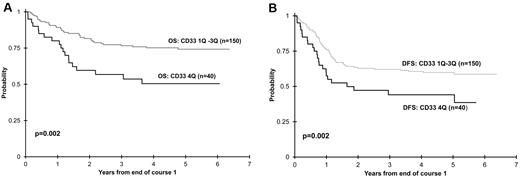
Induction response and outcome data were available for patients treated in AAML03P1 (n = 238), in which all patients received GO during courses I and IV of therapy. The remission induction rate at the end of induction I was similar across all quartiles (P = .522). Importantly, CR rates for patients in Q4 were similar to those with lower CD33 expression (data not shown). Clinical outcomes of patients with the highest CD33 expression (Q4) were compared with those of the remaining patients (Q1-3). OS from CR for patients in Q4 was 57% ± 16% versus 77% ± 7% for those in Q1-3 (P = .002; Figure 2A). The corresponding DFS from CR for Q4 was 44% ± 16% for patients in Q4 versus 62% ± 8% for Q1-3 (P = .022; Figure 2B).
Correlation of clinical outcome with CD33 expression quartile. (A) OS from CR for Q1-3 versus Q4 for all AAML03P1 patients. (B) DFS from CR for Q1-3 versus Q4 for all patients enrolled in AAML03P1.
Correlation of clinical outcome with CD33 expression quartile. (A) OS from CR for Q1-3 versus Q4 for all AAML03P1 patients. (B) DFS from CR for Q1-3 versus Q4 for all patients enrolled in AAML03P1.
We evaluated the clinical impact of CD33 expression in specific clinical risk groups. In low-risk patients, those with high CD33 expression (Q4) had an OS from CR of 67% ± 39% versus 90% ± 8% for those with low CD33 expression (Q1-3; P = .009; Figure 3A), with a corresponding DFS of 50% ± 41% for Q4 versus 73% ± 11% for Q1-3 (P = .286). In high-risk patients, those in Q4 had an OS from CR of 29% ± 34% versus 58% ± 28% for those in Q1-3 (P = .163; Figure 3B), with a corresponding DFS of 29% ± 34% versus 50% ± 29% (P = .173). In patients with standard-risk disease, OS from CR for those in Q4 was 61% ± 21% versus 72% ± 12% for those in Q1-3 (P = .222; Figure 3C). The corresponding DFS from CR was 45% ± 21% for patients in Q4 versus 58% ± 13% for those in Q1-3 (P = .171).
Correlation of clinical outcome with CD33 expression by cytogenetic/molecular risk classification. Shown is the OS from CR based on CD33 expression for patients with low-risk (A), high-risk (B), and standard-risk disease (C).
Correlation of clinical outcome with CD33 expression by cytogenetic/molecular risk classification. Shown is the OS from CR based on CD33 expression for patients with low-risk (A), high-risk (B), and standard-risk disease (C).
Given the significant association between CD33 expression and cytogenetic and molecular risk groups, we performed Cox regression analyses to evaluate the impact of CD33 expression level as a predicator of clinical outcome in the context of established prognostic features. Established cytogenetic/molecular risk groups were used as a covariate in both univariate (Table 2) and multivariate models (Table 3). In the univariate model, high CD33 expression (Q4) was a significant prognostic factor for inferior OS (HR = 2.35; P = .002) and DFS from CR (HR = 1.74; P = .022). There was a trend toward decreased OS from study entry (HR = 1.55; P = .075) for patients in Q4. In separate univariate models, patients with low-risk cytogenetic and molecular features had improved OS from study entry (HR = 0.37; P = .001) and improved OS (HR = 0.44; P = .020) and relapse-free survival from CR (HR = 0.54; P = .035). The DFS from CR showed a trend toward significance (HR = 0.65; P = .094; Table 2). In a multivariate model that included the aforementioned prognostic features, high CD33 expression retained prognostic significance for OS (HR = 2.18; P = .011) and DFS from CR (HR = 1.73; P = .038; Table 3). High-risk cytogenetic and molecular features were not an independent predictor of outcome. In contrast, low-risk cytomolecular features remained an independent prognostic factor for OS from study entry (HR = 0.39; P = .002).
Cox regression analysis of CD33 expression (Q1-3 vs Q4) and other prognostic factors
| . | Univariate . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry . | OS from CR . | DFS from CR . | |||||||||
| n . | HR . | 95% CI . | P . | n . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CD33 expression | |||||||||||
| Q1-3 | 188 | 1 | 150 | ||||||||
| Q4 | 50 | 1.55 | 0.96-2.50 | .075 | 40 | 2.35 | 1.35-4.09 | .002 | 1.74 | 1.08-2.82 | .022 |
| Risk group (cyto/mutation) | |||||||||||
| Standard | 116 | 1 | 87 | 1 | 1 | ||||||
| Low | 79 | 0.37 | 0.21-0.68 | .001 | 69 | 0.44 | 0.22-0.88 | .02 | 0.65 | 0.39-1.08 | .094 |
| High | 26 | 1.67 | 0.94-2.99 | .083 | 19 | 1.97 | 0.95-4.05 | .067 | 1.5 | 0.77-2.93 | .232 |
| . | Univariate . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry . | OS from CR . | DFS from CR . | |||||||||
| n . | HR . | 95% CI . | P . | n . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CD33 expression | |||||||||||
| Q1-3 | 188 | 1 | 150 | ||||||||
| Q4 | 50 | 1.55 | 0.96-2.50 | .075 | 40 | 2.35 | 1.35-4.09 | .002 | 1.74 | 1.08-2.82 | .022 |
| Risk group (cyto/mutation) | |||||||||||
| Standard | 116 | 1 | 87 | 1 | 1 | ||||||
| Low | 79 | 0.37 | 0.21-0.68 | .001 | 69 | 0.44 | 0.22-0.88 | .02 | 0.65 | 0.39-1.08 | .094 |
| High | 26 | 1.67 | 0.94-2.99 | .083 | 19 | 1.97 | 0.95-4.05 | .067 | 1.5 | 0.77-2.93 | .232 |
Cox regression analysis of CD33 expression (Q1-3 vs Q4) and other prognostic factors
| . | Multivariate . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry . | OS from CR . | DFS from CR . | |||||||||
| n . | HR . | 95% CI . | P . | n . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CD33 expression | |||||||||||
| Q1-3 | 174 | 1 | 138 | ||||||||
| Q4 | 47 | 1.38 | 0.84-2.28 | .209 | 37 | 2.18 | 1.19-3.99 | .011 | 1.73 | 1.03-2.89 | .038 |
| Risk group (cyto/mutation) | |||||||||||
| Standard | 116 | 1 | 87 | 1 | 1 | ||||||
| Low | 79 | 0.39 | 0.22-0.72 | .002 | 69 | 0.53 | 0.26-1.08 | .082 | 0.72 | 0.43-1.23 | .228 |
| High | 26 | 1.65 | 0.92-2.96 | .091 | 19 | 1.93 | 0.94-3.99 | .074 | 1.48 | 0.76-2.89 | .25 |
| . | Multivariate . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry . | OS from CR . | DFS from CR . | |||||||||
| n . | HR . | 95% CI . | P . | n . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| CD33 expression | |||||||||||
| Q1-3 | 174 | 1 | 138 | ||||||||
| Q4 | 47 | 1.38 | 0.84-2.28 | .209 | 37 | 2.18 | 1.19-3.99 | .011 | 1.73 | 1.03-2.89 | .038 |
| Risk group (cyto/mutation) | |||||||||||
| Standard | 116 | 1 | 87 | 1 | 1 | ||||||
| Low | 79 | 0.39 | 0.22-0.72 | .002 | 69 | 0.53 | 0.26-1.08 | .082 | 0.72 | 0.43-1.23 | .228 |
| High | 26 | 1.65 | 0.92-2.96 | .091 | 19 | 1.93 | 0.94-3.99 | .074 | 1.48 | 0.76-2.89 | .25 |
Discussion
In this large, prospective study, we demonstrated significant variability of surface CD33 expression on leukemic blasts obtained from pediatric de novo AML patients enrolled in serial GO-containing COG clinical trials. Increased CD33 expression was directly associated with adverse disease features and inversely associated with low-risk disease. For patients enrolled in AAML03P1, in which GO was given in both induction and postremission courses, remission induction rates were similar across different levels of CD33 expression. Low or absent CD33 expression, an exclusion criterion in previous AML clinical trials of GO, was not associated with inferior clinical outcome in our study; on the contrary, lower CD33 expression was associated with superior response. These findings reflect in part the inherent association of CD33 expression with clinically relevant cytogenetic and molecular risk factors. Specifically, there was a higher prevalence of low-risk disease features (eg, CBF AML) in patients with low CD33 expression, whereas patients with high CD33 expression were more likely to have high-risk disease (eg, FLT3/ITD+ disease) and inferior outcome. However, although CD33 expression was correlated with clinically relevant cytogenetic and molecular features, multivariate analysis that included these characteristics also showed that high CD33 expression remained an independent prognostic factor for inferior OS and DFS from CR. Further, in low-risk patients, high CD33 expression was associated with significantly inferior outcome. A similar trend was observed in patients with high- and standard-risk disease, suggesting that high CD33 expression may provide prognostic information independently of historically defined cytogenetic and molecular features of clinical importance.
In the present study, we have demonstrated that there is significant overlap between CD33 expression and disease-risk classification, which may confound interpretation of our outcome analysis. However, when outcome measures for the highest CD33 expression group (Q4) were analyzed within the context of a Cox regression analysis, the HRs observed were similar in both the univariate and multivariate models. This suggests that the confounding impact of other variables (eg, high-risk disease) is minimal and supports the notion that high CD33 expression is an independent predictor of outcome, at least within the context of OS and DFS from CR. Further, analysis of outcome by risk group (Figure 3) demonstrates similar trends in outcome for patients based on CD33 expression, with inferior outcome observed for patients with the highest CD33 expression. This finding, which was statistically significant for low-risk patients, may reach statistical significance for intermediate- and high-risk patients when conducted within the larger AAML0531 series. Although the prognostic impact of high-risk disease did not reach statistical significance in the Cox regression model, this may reflect the small number of high-risk samples analyzed (n = 26) within the context of AAML03P1. The HRs observed for OS from study entry and OS or DFS from CR for high-risk patients were in the expected direction for high-risk disease compared with standard- and low-risk disease and may reach statistical significance when a similar analysis is done within the context of the significantly larger phase 3 study, AAML0531.
CD33, a sialic acid–binding, Ig-like lectin (Siglec) located on chromosome 19q13.3, is absent on pluripotent hematopoietic stem cells but expressed on early multilineage hematopoietic progenitors.20 As multipotent precursors differentiate into committed cells of either the myeloid or monocytic lineage, the concentration of CD33 expression decreases, with the most mature myeloid and monocytic progenitors expressing markedly lower levels of CD33 compared with their less mature, multipotent counterparts.21 Almost 2 decades ago, Dinndorf et al reported that, among 98 AML patients treated on the Children's Cancer Group 251 protocol (CCG-251), those with “bright” blast CD33 expression had inferior OS and EFS compared with patients with “dim” CD33 expression.21 These earlier findings are consistent with data from our present study, supporting the notion that high blast CD33 expression may be a predictor of poor outcome. CD33-targeted therapy was not used as part of the CCG-251 treatment regimen, indicating that the clinical impact of CD33 expression is, at least in part, unrelated to the use of CD33-targeted agents such as GO. It is conceivable that favorable-risk patients with low blast CD33 expression reflect a subset of patients with leukemic disease that is derived from a more mature leukemic progenitor, one that has already lost multipotent potential as well as the ability to express significant amounts of CD33 on the blast population. Previous in vitro work suggested that the maturational stage at which leukemic transformation occurs may dictate clinical response, with more favorable outcomes seen when disease is derived from a more committed myeloid progenitor.22-25 If this is the case, then those patients with CD33 low/absent blast expression may be more sensitive to conventional chemotherapy and potentially GO, assuming that CD33 expression, if present, is sufficient for GO targeting.
Because patients in the AAML03P1 trial uniformly received GO, our data do not allow us to draw conclusions regarding the relationship between CD33 expression levels and response to GO. Fundamentally, if patients with high CD33 expression have an inherently worse prognosis, then the potential benefit of adding GO to a conventional chemotherapy backbone may be masked in the context of AAML03P1, a single-arm study. Moreover, although in vitro studies show a striking, quantitative dependence of GO efficacy on CD33 expression levels,5 several other factors such as drug efflux activity and antiapoptotic proteins26 may affect the anti-AML activity of GO and possibly the relationship between CD33 expression and GO efficacy, particularly in the context of high Ag burden. Because a high CD33 Ag load on the blast population may also restrict the access of GO to relevant AML cells in the BM,27 high expression levels of CD33 may even have adverse effects on GO efficacy. As clinical data from the randomized trial COG AAML0531 matures, correlative studies can further address these concerns. If high CD33 expression negatively affects GO response because of excessive Ag burden, additional clinical trials may be warranted to explore whether dose escalation of GO or alternative CD33 targeted agents can overcome this limitation and be tolerated in the setting of combination chemotherapy and possibly HCT. Conversely, recent results from Medical Research Council AML15 suggest that GO significantly improves outcome for low- and potentially standard-risk disease.9 Outcome analyses of patients enrolled in AAML0531 will also help to determine whether GO can further augment the superior clinical response traditionally observed for low-risk patients when low, but clinically relevant, CD33 expression is present. This subset of patients with largely favorable disease characteristics was historically excluded from adult relapsed AML GO clinical trials because of their absent or limited CD33 expression.28
It is conceivable that AAML0531 will fail to demonstrate a survival benefit with GO irrespective of disease-risk classification. In that case, one could argue that evaluation of CD33 expression may still facilitate clinical prognostication within the context of pediatric AML. For example, whereas the majority of low-risk patients have favorable outcome, we know that this population is heterogeneous and that approximately 25% of low-risk patients will still succumb to their disease. Analysis of CD33 expression by risk group may delineate a subset of low-risk AML patients with higher CD33 expression and corresponding higher risk disease. Such patients may benefit from additional chemotherapy and possibly HCT. Conversely, if a subset of low-risk patients with low CD33 expression show clear survival benefit irrespective of GO, one could also consider the benefit of conventional chemotherapy de-escalation for this cohort of patients, similar to that seen for acute promyelocytic leukemia. A similar paradigm could also be considered within the context of intermediate- and high-risk disease, assuming that CD33 expression predicts for response within these subgroups in the context of AAML0531.
In summary, the data from the present study indicate that high CD33 expression is associated with adverse disease features in pediatric AML and suggest that high CD33 expression is an independent predictor of inferior outcome, particularly DFS, in pediatric AML patients. These findings add to our understanding of the significance of CD33 in pediatric AML and have important implications for the planning and interpretation of studies using CD33-targeting therapeutics such as GO.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vani J. Shanker, PhD, for scientific editing; the Children's Oncology Group AML Reference Laboratory, particularly Sommer Castro, for providing diagnostic specimens; and the patients and families who consented to the use of biologic specimens in these trials.
This research was supported by the National Cancer Institute (5K12CA076930-08 to J.P., R21 CA10262 and R01 CA114563 to S.M., and Children's Oncology Group Chair's grant NIH U10 CA98543), a St Baldrick's Foundation Career Development Award (to J.P.), and a CureSearch Research Fellowship Award (to J.P.).
National Institutes of Health
Authorship
Contribution: J.A.P. performed the research, collected, analyzed, and interpreted the data, and wrote the manuscript; T.A.A. designed and performed the research, collected, analyzed, and interpreted the data, performed the statistical analysis, and wrote the manuscript; M.L. designed and performed research, collected, analyzed, and interpreted the data, and wrote the manuscript; R.B.G. collected, analyzed, and interpreted the data, performed the statistical analysis, and wrote the manuscript; P.A.H. performed the research, collected and interpreted the data, and wrote the manuscript; I.D.B. designed the research, analyzed and interpreted the data, and wrote the manuscript; S.C.R., B.H., and R.B.W. analyzed and interpreted the data and wrote the manuscript; J.F. designed the research; A.G. designed and performed the research and wrote the manuscript; and S.M. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.P. received research funding from CureSearch that helped support this project. M.L. is the president, laboratory director, and owner of Hematologics Inc. The remaining authors declare no competing financial interests.
Correspondence: Jessica Pollard, MD, Seattle Children's Hospital, 4800 Sandpoint Way NE, Division of Heme/Onc, MS-B-6553, Seattle WA 98105; e-mail: jessica.pollard@seattlechildrens.org.
References
Author notes
J.A.P. and T.A.A. contributed equally to this work.