Genetic engineering has substantially improved antibodies used in the treatment of cancer and related diseases, initially by providing more compatible reagents (chimeric, humanized, human) for patients but more recently by improving their clinical efficacy.1 In this issue of Blood, Gupta et al have used a novel genetic engineering method, termed “dock and lock,”2-4 to construct a HexAb bispecific antibody directed against 2 B-cell epitopes (CD20, CD74). By so doing, they have tapped into a distinctive B-cell homeostatic mechanism that controls B-cell growth and proliferation.5
Earlier it was observed that the agglutination of malignant B cells by certain antibodies induced the movement of bound antigens into cell surface lipid rafts located sporadically on B cells to give a speckled membrane pattern of antibody binding by immunofluorescence microscopy.6 For example, it is now known that anti-CD20 antibodies fall into 2 groups: type I and type II.7,8 Type I antibodies represented by Rituxan and Veltuzumab used in this study produce a ring pattern on lymphoma cells after binding and by themselves do not produce significant apoptosis. By comparison, type II anti-CD20 antibodies represented by tositumomab produce a speckled membrane pattern and are very effective in inducing cell death by a caspase-independent mechanism. Type I antibodies can be induced to produce this type of apoptosis after cross-linking by antibodies that bind Rituxan or using multivalent antibody polymers to simulate this cross-linking phenomenon.9
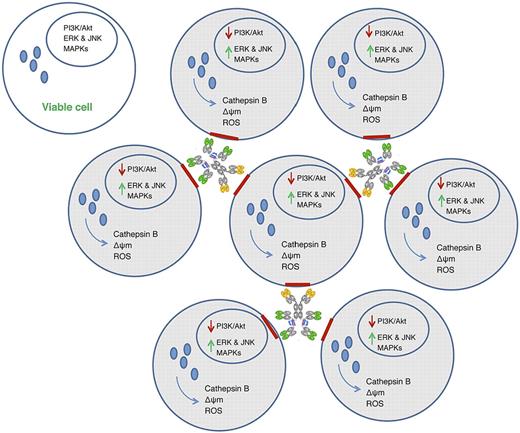
In the paper by Gupta et al, the authors have found another method of inducing B lymphoma cell death using a bispecific antibody that cross-links 2 different B-cell antigens, CD20 and CD74.2 By testing different compositions of HexAbs, they determined that 2 combinations are clinically active, necessitating additional studies to determine which is most efficacious in patients. As shown in the figure, the cross- linking of CD74, which is directed against the invariant chain of the HLA-Dr molecule,10 and CD20, which is highly expressed on B-cell neoplasms but whose function is still not fully understood, produced cell clustering that is associated with actin reorganization, antigen migration into lipid rafts, swelling of mitochondria and lysosomes to cause influx of calcium and release of capthepsin B, respectively, down-regulation of Bcl-xL and pAkt, and the rapid and sustained phosphorylation of ERK and JNK second signals culminating in cell death.
Biologic effect of cross-linking multivalent anti-CD20/CD74 bispecific antibodies in B-cell lymphomas. Aggregation of lymphoma cells by bispecific antibodies causes reorganization of actin and migration of antigen (red) into lipid rafts that in turn induces the release of lysosomal cathepsin B and reactive oxygen species (ROS) and influx of calcium ions into mitochondria. Swollen lysosomes and mitochrondria are accompanied by the deactivation of the PI3K/Akt signaling pathway and the rapid and sustained activation of ERK, JNK, and MAPKs associated with cell death. Nonaggregated cells remain viable in the absence of antibody binding.
Biologic effect of cross-linking multivalent anti-CD20/CD74 bispecific antibodies in B-cell lymphomas. Aggregation of lymphoma cells by bispecific antibodies causes reorganization of actin and migration of antigen (red) into lipid rafts that in turn induces the release of lysosomal cathepsin B and reactive oxygen species (ROS) and influx of calcium ions into mitochondria. Swollen lysosomes and mitochrondria are accompanied by the deactivation of the PI3K/Akt signaling pathway and the rapid and sustained activation of ERK, JNK, and MAPKs associated with cell death. Nonaggregated cells remain viable in the absence of antibody binding.
Because Veltuzumab is a type I anti-CD20 and by itself is not apoptotic, it would be of interest to determine whether substituting a type II anti-CD20 in the HexAb might be more effective. CD74 is a good choice for the HexAb because it binds a critical molecule for B-cell survival and proliferation.10 Nonetheless, the emergence of this novel genetically engineered bispecific antibody should be a welcome new product for the treatment of mantle zone lymphoma, chronic lymphocytic leukemia, and other difficult-to-treat B-cell tumors expressing both CD20 and CD74 antigens. The ingenuity and creativity displayed in this study demonstrates further the ever-increasing role of genetic engineering in the development of promising new antibody reagents for cancer immunotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal