Tumor-derived galectin-1 (Gal-1), a β-galactoside–binding S-type lectin, has been shown to encourage T-cell death and promote T cell–mediated tumor immune escape. In this report, we show that patients with leukemic cutaneous T-cell lymphomas, known to have limited complexity of their T-cell repertoires, have a predominant T helper type-2 (Th2) cytokine profile and significantly elevated plasma levels of Gal-1 compared with healthy controls. Circulating clonal malignant T cells were a major source of Gal-1. The conditioned supernatant of cultured malignant T cells induced a β-galactoside–dependent inhibition of normal T-cell proliferation and a Th2 skewing of cytokine production. These data implicate Gal-1 in development of the Th2 phenotype in patients with advanced-stage cutaneous T-cell lymphoma and highlight the Gal-1–Gal-1 ligand axis as a potential therapeutic target for enhancing antitumor immune responses.
Introduction
Contraction of the T-cell repertoire and Th2 cytokine skewing are 2 immunosuppressive features linked to high morbidity and mortality of patients with leukemic cutaneous T-cell lymphoma (L-CTCL).1,,,–5 Certain soluble factor(s) expressed by clonal malignant cells or dermal fibroblasts (eg, IL-18, eotaxins) could be responsible for this immunologic signature; nevertheless, mechanistic evidence on how these cells elicit their immunoregulatory function is still incomplete.6,7 Recently, the β-galactoside binding protein, galectin-1 (Gal-1), has been implicated in the induction of a Th2 signature and suppression of antitumor immune responses in patients with Hodgkin lymphoma.8,9 Gal-1 functions as a homodimer with affinity for N-acetyllactosamine–bearing glycoproteins on effector T cells that, on engagement, induces proapoptotic and/or immunosuppressive activities.10,11 Gal-1–binding interactions induce deletion of pro-inflammatory T helper (Th)–1 and Th17 cells but spare naive, regulatory, and Th2 cells, leading to a tolerogenic environment that favors tumor immune escape.12,13 Indeed, in patients with Hodgkin disease, increased Gal-1 expression is correlated with poor prognoses and implicated as a biomarker of disease progression.14
Although a prior study shows that Gal-1 expression is elevated in epidermotropic malignant T cells in patients with mycosis fungoides,15 its presence and role in more advanced leukemic forms of CTCL have not been addressed. Here we show that clonal malignant T cells in patients with stage 3 or 4 CTCL exhibited a Th2 cytokine signature and high intracellular Gal-1 expression. Moreover, L-CTCL patients exhibited higher plasma Gal-1 levels than healthy controls, and conditioned supernatants from primary L-CTCL cell cultures significantly attenuated normal T-cell proliferation and diminished Th1 responses in a β-galactoside–dependent manner. Collectively, these data suggest that elevated levels of Gal-1 in L-CTCL patients may inhibit the proliferation of nonmalignant T cells and favor Th2 skewing, leading to impaired antitumor responses and increased susceptibility to infection. These studies suggest that neutralization of Gal-1–Gal-1 ligand interactions may be an effective strategy for enhancing antitumor immune responses in L-CTCL patients.
Methods
PBMCs and plasma samples
All studies were performed in accordance with the Declaration of Helsinki and approved by the review board of the Partners Human Research Committee. PBMCs and plasma were obtained using Ficoll separation from venous blood samples of patients with stage 3 or 4 CTCL (WHO/TNM classification) or healthy controls seen at the Dana-Farber/Brigham and Women's Cancer Center (supplemental Table 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
ELISA
The 96-well plates were coated with goat anti–human Gal-1 (R&D Systems), and undiluted plasma samples and standard control, recombinant human Gal-1 (PeproTech), were incubated for 1 hour at room temperature. Bound Gal-1 was detected using rabbit anti–hGal-1-biotin/streptavidin-HRP (LS Bio), followed by spectrophotometry analysis. ELISA reagents were purchased from BD Biosciences.
Flow cytometry
PBMCs were stained with anti-CD3, CD4, CD7, CD8, CD45RO mAbs (BD Biosciences), or anti-specific T-cell receptor (TCR)–vβ clones (Beckman Coulter). Intracellular Gal-1 detection was assessed with a rabbit anti–hGal-1 Ab (LS Bio)/anti–rabbit IgG-FITC (BioLegend). Gal-1 ligand detection was performed using Gal-1hFc as previously reported.10,16,17
Cell death, cytokine expression, and proliferation assays
L-CTCL cells (> 90% purity) from advanced-stage CTCL patients were cultured for 24 hours in X-VIVO15 medium (Lonza Walkersville) at a density of 8 × 106 cells/mL. T cells from healthy donor PBMCs were activated for 48 hours with anti–human CD3 (OKT3, BioLegend) and further incubated with L-CTCL–conditioned medium or with 0.25μM Gal-1hFc (with or without 50mM lactose) for an additional 24 hours. T cells were stained with allophycocyanin–annexin V and propidium iodide or alternatively were stimulated for additional 5 hours with phorbol myristate acetate (PMA)/ionomycin/brefeldin A and analyzed for intracellular cytokines by FACS. Experiments included supernatants from 3 different L-CTCL donors (supplemental Table 1B) and were performed in triplicate. For proliferation assays, CFSE-labeled T cells were cultured in L-CTCL–conditioned medium plus IL-2 (25 U/mL, PeproTech; with or without 50mM lactose) or with 0.25μM Gal-1hFc for an additional 72 hours followed by FACS analysis.
Results and discussion
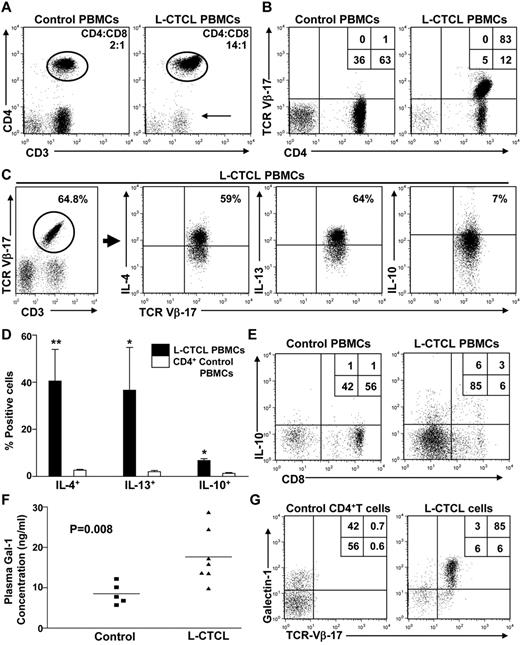
Prior reports show that L-CTCL patients have contracted T-cell repertoires, a marked Th1/Th2 cytokine imbalance, and are highly susceptible to opportunistic infections.3 Recently, these findings have been associated with increased Gal-1 levels in patients with Hodgkin lymphoma.8 To study Gal-1's role in the pathogenesis of these immunosuppressive features, we studied circulating T cells in L-CTCL patients with elevated CD4/CD8 ratios and clonal malignant T cells that could be identified by staining with anti–TCR-Vβ mAbs (Figure 1A-B). Clonal T cells in L-CTCL patients expressed a distinctive Th2 signature characterized by elevated expression of IL-4 and IL-13 (Figure 1C-D; supplemental Figure 1A). Notably, we also found increased levels of IL-10 in clonal malignant CD4+ cells and in CD8+ T cells of L-CTCL patients compared with healthy controls (Figure 1C-E).
Patients with L-CTCL exhibit a contracted T-cell repertoire and Th2 cytokine profile. (A) PBMCs from patients with stage 4 CTCL or healthy controls (n = 3/group) were analyzed by flow cytometry. Representative plots show cells analyzed for CD3 and CD4 expression. Arrow indicates contraction of the CD3+/CD4− population. (B) Th cells from patients with stage 4 CTCL show increased expression of a single malignant TCR-Vβ clone. Representative plot is shown. (C) PBMCs from healthy controls or L-CTCL patients (n = 3/group) were stimulated ex vivo with PMA/ionomycin/brefeldin A, and intracellular levels of Th2 cytokines were analyzed by flow cytometry. Representative plot of L-CTCL patients is shown. (D) Graphical representation of data from all L-CTCL and health control donors depicting mean percentage of CD4+ cells expressing IL-4, IL-13, and IL-10. Statistically significant difference compared with healthy controls using Student paired t test: *P ≤ .05, **P ≤ .001. (E) PBMCs from healthy controls or L-CTCL patients were stimulated ex vivo with PMA/ionomycin/brefeldin A, and intracellular levels of IL-10 were analyzed in CD8+ T cells by flow cytometry. A representative plot is shown. (F) Graphical representation of undiluted plasma samples from patients with advanced-stage CTCL (triangles, n = 7) and healthy controls (squares, n = 5) were analyzed for Gal-1 expression by ELISA. Experiments were performed in triplicate. (G) PBMCs from L-CTCL patients or healthy controls were gated on CD3+/CD4+ and analyzed for specific TCR-Vβ expression and intracellular Gal-1. A representative plot is shown.
Patients with L-CTCL exhibit a contracted T-cell repertoire and Th2 cytokine profile. (A) PBMCs from patients with stage 4 CTCL or healthy controls (n = 3/group) were analyzed by flow cytometry. Representative plots show cells analyzed for CD3 and CD4 expression. Arrow indicates contraction of the CD3+/CD4− population. (B) Th cells from patients with stage 4 CTCL show increased expression of a single malignant TCR-Vβ clone. Representative plot is shown. (C) PBMCs from healthy controls or L-CTCL patients (n = 3/group) were stimulated ex vivo with PMA/ionomycin/brefeldin A, and intracellular levels of Th2 cytokines were analyzed by flow cytometry. Representative plot of L-CTCL patients is shown. (D) Graphical representation of data from all L-CTCL and health control donors depicting mean percentage of CD4+ cells expressing IL-4, IL-13, and IL-10. Statistically significant difference compared with healthy controls using Student paired t test: *P ≤ .05, **P ≤ .001. (E) PBMCs from healthy controls or L-CTCL patients were stimulated ex vivo with PMA/ionomycin/brefeldin A, and intracellular levels of IL-10 were analyzed in CD8+ T cells by flow cytometry. A representative plot is shown. (F) Graphical representation of undiluted plasma samples from patients with advanced-stage CTCL (triangles, n = 7) and healthy controls (squares, n = 5) were analyzed for Gal-1 expression by ELISA. Experiments were performed in triplicate. (G) PBMCs from L-CTCL patients or healthy controls were gated on CD3+/CD4+ and analyzed for specific TCR-Vβ expression and intracellular Gal-1. A representative plot is shown.
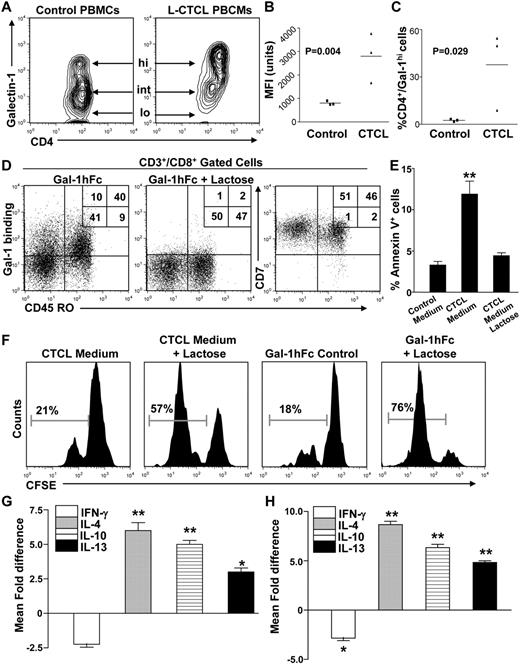
To determine whether Gal-1 could be contributing to the immunologic abnormalities observed in patients with L-CTCL, we quantified plasma Gal-1 levels in L-CTCL patients and compared them with healthy controls. As reported for other solid and hematologic malignancies,18,19 Gal-1 plasma levels were significantly elevated in patients with active L-CTCL and diminished during remission (Figure 1F; supplemental Table 1A). Moreover, analyses of intracellular Gal-1 and Gal-1 immunoblots of CTCL HUT78 cell lysates (supplemental Figure 1B) indicated that the malignant T clones were the main Gal-1 source in the PBMC compartment of L-CTCL patients (Figure 1G). In addition, we found that Gal-1 was expressed in circulating T cells at 3 distinct levels (low, intermediate, and high); and although approximately 50% of T cells in healthy volunteers express Gal-1, their expression was in the low to intermediate range, compared with clonal malignant cells, which expressed intermediate to predominantly high Gal-1 levels (Figure 2A-C; supplemental Figure 1C). Even in L-CTCL patient 10, who possessed the lowest Gal-1 expression, virtually no T cells expressed Gal-1 in the low range (supplemental Figure 1C).
Gal-1 secreted from L-CTCL cells dampens T-cell proliferation and promotes Th2 cytokine skewing. (A) Intracellular Gal-1 expression in Th cells from patients with L-CTCL or healthy controls (n = 3/group) was analyzed by flow cytometry and presented as a contour plot. Arrows indicate Gal-1 high, intermediate, and low (“lo”) populations. (B) Graphical representation of intracellular Gal-1 levels quantified by mean fluorescence intensity (MFI). Statistically significant difference compared with healthy controls using Student paired t test. (C) Graphical representation of Gal-1hi Th cells in stage 4 CTCL patients or healthy controls. Statistically significant difference compared with healthy controls using Student paired t test. (D) PBMCs from healthy donors were gated on CD8+, and expression of Gal-1 ligands (Gal-1hFc binding) on CD45RO+ memory cells was determined by flow cytometry. Coexpression of CD7 in CD45RO+/− CD8+ T cells was also analyzed. Representative plots are shown. (E) Activated T cells from healthy donors (n = 3) were incubated with conditioned medium from primary L-CTCL cultures (with or without lactose) for 24 hours and then analyzed for annexin V binding. Statistically significant difference compared with lactose-treated control using Student paired t test: **P ≤ .001. (F) Alternatively, activated T cells were labeled with CFSE, incubated with L-CTCL–conditioned medium (with or without lactose) or with 0.25μM Gal-1hFc (with or without lactose) for 3 days, and CFSE dilution was analyzed by flow cytometry. Activated T cells from healthy donors (n = 3) were incubated with conditioned medium from primary L-CTCL cell cultures (with or without lactose; G) or with 0.25μM Gal-1hFc (with or without lactose; H) for 24 hours and then stained and analyzed for CD3 and intracellular IFN-γ, IL-4, IL-10, and IL-13. All experiments were performed with L-CTCL–conditioned medium from 3 different donors and performed in triplicate. Statistically significant difference compared with lactose-treated control using Student paired t test: *P ≤ .05, **P ≤ .001.
Gal-1 secreted from L-CTCL cells dampens T-cell proliferation and promotes Th2 cytokine skewing. (A) Intracellular Gal-1 expression in Th cells from patients with L-CTCL or healthy controls (n = 3/group) was analyzed by flow cytometry and presented as a contour plot. Arrows indicate Gal-1 high, intermediate, and low (“lo”) populations. (B) Graphical representation of intracellular Gal-1 levels quantified by mean fluorescence intensity (MFI). Statistically significant difference compared with healthy controls using Student paired t test. (C) Graphical representation of Gal-1hi Th cells in stage 4 CTCL patients or healthy controls. Statistically significant difference compared with healthy controls using Student paired t test. (D) PBMCs from healthy donors were gated on CD8+, and expression of Gal-1 ligands (Gal-1hFc binding) on CD45RO+ memory cells was determined by flow cytometry. Coexpression of CD7 in CD45RO+/− CD8+ T cells was also analyzed. Representative plots are shown. (E) Activated T cells from healthy donors (n = 3) were incubated with conditioned medium from primary L-CTCL cultures (with or without lactose) for 24 hours and then analyzed for annexin V binding. Statistically significant difference compared with lactose-treated control using Student paired t test: **P ≤ .001. (F) Alternatively, activated T cells were labeled with CFSE, incubated with L-CTCL–conditioned medium (with or without lactose) or with 0.25μM Gal-1hFc (with or without lactose) for 3 days, and CFSE dilution was analyzed by flow cytometry. Activated T cells from healthy donors (n = 3) were incubated with conditioned medium from primary L-CTCL cell cultures (with or without lactose; G) or with 0.25μM Gal-1hFc (with or without lactose; H) for 24 hours and then stained and analyzed for CD3 and intracellular IFN-γ, IL-4, IL-10, and IL-13. All experiments were performed with L-CTCL–conditioned medium from 3 different donors and performed in triplicate. Statistically significant difference compared with lactose-treated control using Student paired t test: *P ≤ .05, **P ≤ .001.
To determine whether Gal-1 overexpression by lymphoma cells induces Th2 cytokine skewing in L-CTCL patients, we studied the ability of β-galactoside-binding lectins secreted from L-CTCL cells to alter the viability and cytokine production of nonmalignant T cells. Using a Gal-1-human immunoglobulin fusion protein (Gal-1hFc) that functionally mimics native Gal-1,10,17,20 we first validated Gal-1's affinity for effector T cells and found that Gal-1 predominantly binds to memory CD45RO+ T cells coexpressing CD7 in a β-galactoside–dependent manner (Figure 2D). This selective binding activity to the effector/memory T-cell compartment strengthened our hypothesis that Gal-1 secreted from clonal malignant T cells might promote deletion/immunoregulation of CD8+ T cells, promoting abnormally high CD4/CD8 ratios found in patients with advanced-stages of CTCL. Notably, L-CTCL cells have been reported to be insensitive to Gal-1–mediated apoptosis, as they lack CD7, the most remarkable Gal-1 ligand linked to cell death induction.21,22 To demonstrate that clonal malignant T cells induce apoptosis and/or regulate the proliferation of nonmalignant T cells through β-galactoside–mediated interactions, we cultured activated T cells from healthy volunteers with conditioned supernatants of clonal malignant T cells (> 90% purity) or with Gal-1hFc (with or without competitive inhibitor, lactose) for 24 hours. Our results show that, although only a minority of T cells cultured with L-CTCL–conditioned medium underwent apoptosis (annexin V+/propodium iodide+), the percentage of T cells positive by annexin V staining was significantly decreased on addition of β-galactoside–binding lectin inhibitor, lactose (P ≤ .01, Figure 2E; supplemental Figure 1D). Moreover, the addition of lactose restored the proliferative capacity of CFSE-labeled T cells incubated with L-CTCL–conditioned medium or with Gal-1hFc (Figure 2F), suggesting that a β-galactoside–binding lectin secreted by clonal malignant T cells yielded antiproliferative activity. L-CTCL cells lack CD7, a bona fide Gal-1 proapoptotic ligand (supplemental Figure 2A), allowing them to escape Gal-1–mediated cell death, and favoring their uncontrolled expansion.21,–23 Gal-1–mediated effects are not limited to apoptosis. Prior studies have shown that Gal-1 induces the production of Th2 and tolerogenic cytokines.10 To determine whether Gal-1 in L-CTCL–conditioned medium could promote Th2 skewing of nonmalignant T cells, we incubated T cells from healthy donors with L-CTCL–conditioned medium or with Gal-1hFc and assessed cytokine expression after 24 hours. Compared with lactose controls, we found that CTCL-conditioned medium significantly suppressed the production of IFN-γ+ T cells (P ≤ .05) while markedly increasing the level of IL-4+ (P ≤ .01), IL-10+ (P ≤ .01), and IL-13+ T cells (P ≤ .05). Of note, control Gal-1 incubations elicited a similar induction of Th2 cytokines in T cells (Figure 2G; supplemental Figure 2B).
In conclusion, our work suggests that L-CTCL–derived Gal-1 may impair the viability, proliferation, and Th1 responses of nonmalignant T cells, leading to a systemic Th2 bias that favors tumor survival and probably contributes to the observed susceptibility of these patients to infections. These studies highlight the pivotal role of Gal-1 in modifying adaptive immune responses and suggest that inhibition of Gal-1–Gal-1 ligand interactions may be an effective strategy for enhancing both antitumor and antipathogen responses in patients with L-CTCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute (RO1 CA118124, C.J.D) and National Institutes of Health/National Center for Complementary and Alternative Medicine (RO1 AT004268, C.J.D.), a Damon Runyon Clinical Investigator Award (R.A.C.), National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR056720; R.A.C.), National Institutes of Health/National Cancer Institute (SPORE in Skin Cancer P50 CA9368305, T.S.K.), and the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01 A1025082, T.S.K.).
National Institutes of Health
Authorship
Contribution: F.C.-L. designed the research, performed the experiments, analyzed the data, and wrote the paper; R.W. and J.E.T. performed the experiments and analyzed the data; T.S.K. and R.A.C. conceived the study, designed the research, and provided the reagents; and C.J.D. conceived the study, designed the research, provided the reagents, supervised all experimentation, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles J. Dimitroff, HIM, Rm 662, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: cdimitroff@rics.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal