Invariant natural killer T (iNKT) cells have the ability to rapidly secret cytokines in response to diverse stimuli, and therefore influence numerous immune reactions. Although IFN-γ and IL-4 are thought to dominate iNKT cytokine production, a distinct subset of iNKT cells, expressing RORγt and producing IL-17, has now been identified in both mice and humans. Although a role in pathogen and allergic responses has been assigned to the RORγt+ iNKT subset, factors controlling their development and function remain illusive. Here, we demonstrate that RORγt+ iNKT-cell differentiation obeys transforming growth factor-β (TGF-β) signaling control, different from that described for conventional iNKT cells. We reveal that TGF-β signaling, and particularly its SMAD4-dependent pathway, is required for both the survival of RORγt+ iNKT cells during their development and IL-17 production at the periphery. Moreover, constitutive TGF-β signaling in RORγt+ iNKT cells drives higher peripheral numbers and increased tissue distribution. Finally, we found that SMAD4-dependent TGF-β signaling is mandatory for the peripheral expansion of the RORγt+ iNKT cells responding to inflammatory signals. Thus, this work demonstrates that both the development and responsiveness of the newly described IL-17–producing iNKT cell subset is under the control of a dedicated TGF-β signaling pathway.
Introduction
Natural killer T (NKT) cells compose a subset of T lymphocytes that have both innate and adaptive traits. NKT cells have RAG-dependent rearranged T-cell receptor (TCR) chains, a hallmark of the adaptive immune system, but also have the ability to rapidly respond to cytokine and pathogen derived stimuli. This early reaction and ability to produce diverse cytokine responses put NKT cells in position to greatly influence both infectious and allergic responses.1,2 A major population of NKT cells is the type 1 or invariant NKT (iNKT), so named because they express an invariant TCRα chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans) along with a restricted TCRβ chain.3,4 Invariant NKT cells are positively selected on recognition of CD1d expressed on double-positive (DP) thymocytes loaded with glycolipids. After thymic differentiation, a large proportion of iNKT cells reach the periphery concentrating in the spleen and the liver where they represent approximately 2.5% and 30% of T cells, respectively.4 Invariant NKT cells can react to CD1d loaded with α-galactosylceramide (αGalcer) as well as to glycolipids present in pathogenic bacteria such as Borrelia burgdorferi and Streptococcus pneumoniae.5,6 Heterogeneity among iNKT cells is apparent both in the surface expression of NK1.1 and CD4, and the expression of the transcription factors T-bet and Gata3, which are important for producing IFN-γ and IL-4.3
Recently, a novel subset of iNKT cells that are RORγt (retinoid-related orphan receptor γt) positive and produce IL-17 was identified in both mice and humans.7,–9 Similar to their conventional IFN-γ/IL-4 producing counterparts, IL-17 producing iNKT cells develop in the thymus and bind CD1d-tetramers loaded with glycolipid.7,10 However, RORγt positive iNKT cells have a distinct surface phenotype as they are CCR6 positive, NK1.1 negative, and CD4 negative; although thymocytes have been suggested to pass through a CD4+ step during their development.8,10,–12 Moreover, compared with conventional RORγt negative iNKT cells, these cells are only weakly observed in the liver and spleen, but are highly enriched in the peripheral lymph nodes and skin of healthy animals.12 In addition, whereas conventional iNKT cells produce IFN-γ in response to viral and bacterially induced IL-12,13,14 IL-17 production by RORγt+ iNKT cells seems to be induced by bacterially provoked IL-1β and IL-23, presumably through activation of the inflammasome.15 Furthermore, in vivo production of IL-17 by iNKT cells was observed 12 hours after S pneumoniae, which implies a role of these cells at an early time point after pathogen encounter.6 The IL-17 produced by iNKT cells has been suggested to facilitate the recruitment of neutrophils to the site of activation and thus may boost the innate arm of the immune response.7
Although the developmental stages of conventional CD4+ NK1.1+ iNKT cell differentiation and the factors controlling the progression from one stage to another have been well defined,3,4 the differentiation of RORγt+ iNKT cells has yet to be elucidated. Conventional iNKT cell precursors are first selected at the CD4+ CD8+ DP stage and then progress through a series of developmental stages: they proceed from a CD44low NK1.1− stage through a CD44high NK1.1− stage, to ultimately reach a mature CD44high NK1.1+ stage. The transition between CD4± CD44high NK1.1− to CD4+ CD44high NK1.1+ is dependent on IL-15 and CD122 (IL-15Rβ), which up-regulates Tbet expression, but the progression from one stage to another have recently been shown to be finely orchestrated by transforming growth factor-β (TGF-β) signaling pathways.16 TGF-β is widely expressed throughout mammals in an inactive form.17 This cytokine signals through 2 receptor complex composed of TGF-βRI and TGF-βRII. Signaling is initiated by the binding of activated TGF-β to its receptor which leads to autophosphorylation of TGF-βRII, which in turn phosphorylates the TGF-βRI subunit. The latter then phosphorylates the receptor associated R-SMADs (SMAD2 and/or SMAD3), which then bind to the co-SMAD, SMAD4. SMAD2/SMAD4, and SMAD3/SMAD4 complexes then migrate to the nucleus where they regulate the expression of various genes. In addition to the SMAD4-dependent pathway, other signaling branches, such as the MEK/MAP kinase pathway18 or the transcriptional intermediary factory 1 (TIF1)–γ (TRIM33) pathway,19 have been described. Clearly, the different TGF-β signaling branches play distinct functions in the thymic development of conventional iNKT cells.16 Although the SMAD4-independent pathways are crucial and control the number and differentiation of conventional iNKT cells, the role of the SMAD4 branch is minor and restricted to the maturation of the few long-term thymic-resident iNKT cells.16
The developmental stage at which RORγt+ iNKT cells and conventional iNKT cells diverge seems to be early, as RORγt is expressed in CD4+CD8+ DP iNKT precursor thymocytes and is required for their survival.20 In any case, the factors controlling RORγt+ iNKT cell thymic differentiation have not been identified. IL-6, a prominent inducer of RORγt and IL-17 expression in CD4+ T cells (Th17),21 has been hypothesized but disregarded as an inducer of RORγt+ iNKT cell differentiation as IL-6KO mice have normal numbers and responses of IL-17 producing iNKT cells.11,12
In this study, we demonstrate that TGF-β signaling is crucial for both the thymic differentiation and the peripheral function of RORγt+ iNKT cells. By using mice with selective ablation or induction of TGF-β signaling pathways, we reveal that control of RORγt+ iNKT cell differentiation obeys TGF-β signaling, in a manner different from that of the conventional iNKT cell lineage. In clear contrast to what has been described for conventional iNKT cells, we identified the SMAD4-dependent pathway as the main TGF-β signaling branch controlling both the development and the peripheral response of RORγt+ iNKT cells during inflammation. Thus, both development and peripheral responsiveness of the recently described RORγt+ iNKT subset are controlled by TGF-β signaling, in a SMAD4 pathway-dependent manner.
Methods
Mice
CD4-Cre; TgfbRIIflox/flox (TGFβRII°), CD4-Cre; Smad4flox/flox (SMAD4°), CD4-Cre; Tif1γflox/flox (TIF1γ°), and CD4-Cre; Stopflox/floxTgfbRICA (TGFβRICA), were generated as previously described.16,22,23 CD4-Cre; Rosa26YFP mice were generated by crossing CD4-Cre and Rosa26YFP mouse strains.24 All strains were backcrossed to C57BL/6 mice 8 to 10 generations and Cre negative littermates were used as wild-type (WT) controls. C57BL/6 and RAG2° mice were purchased from Charles River Laboratories or bred in-house. Mice were maintained in a specific pathogen-free animal facility, AniCan, at the Cancer Research Center-Lyon and handled in accordance with the institutional guidelines and protocols approved by the animal care and use committee (Comité d'Evaluation Commun au Centre Léon Bérard, ă l'Animalerie de transit de l'ENS, au PBES et au laboratorie P4).
Lymphocyte isolation
Cell suspensions were prepared from the thymus, spleen, and peripheral lymph nodes (pLNs; pool of inguinal, axillary, brachial, and cervical lymph nodes) by manual disruption using glass slides. Liver and lung were cut into small pieces, digested with collagenase IV (20 mg/mL; Sigma-Aldrich) and DNAse I (10 mg/mL; Roche) for 90 minutes at 37°C, and separated on a 30%/70% Percoll gradient (Sigma-Aldrich). For skin analysis, ear sheets were separated and digested with liberase (0.3 mg/mL; Roche) and collagenase D (3 mg/mL; Roche) for 45 minutes at 37°C.
Antibodies and flow-cytometry
Antibodies were purchased from BD Bioscience, EBioscience, Biolegend, R&D Systems, and Santa Cruz Biotechnologies: CD4 (L3T4), CD8 (53-6.7), B220 (RA3-6B2), TCRβ (H57-597), CD122 (TM-β1), Thy1.1 (H1S51), Thy1.2 (53-2.1), CCR6 (140706), CCR7 (4B12), RORγt (2B2), Ki67 (B56), IL-17α (ebio17B7), TGF-βRII (goat IgG), p-STAT-5 (pY694; clone 47), p-SMAD2/3 (ser423/425; polyclonal goat IgG), donkey anti–goat IgG biotin, bovine anti–goat IgG fluorescein isothiocyanate (FITC) and PBS57/CD1d tetramers National Institutes of Health (NIH) tetramer core facility.
For intra-cellular cytokine staining, cells were stimulated with 500 ng/mL PMA (Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) for 3 hours and then tetramer and surface stained. For intranuclear staining, cells were fixed and permeablized using the Foxp3 staining kit (EBioscience). For P-STAT5 staining, freshly isolated cells were stimulated at 37°C for 10 minutes with or without 10 ng/mL recombinant human IL-15 (AbCys). Cells were then stained for tetramer and surface markers for 10 minutes on ice, washed, and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 minutes at room temperature. Next, cells were treated with ice-cold methanol (Sigma-Aldrich) for 10 minutes on ice, washed, and stained with anti–p-signal transducer and activator of transcription (STAT5) and anti-RORγt antibodies for 20 minutes at 4°C. The annexin V staining kit was used in agreement with the protocol from BD Bioscience. Flow cytometry was performed using a 4-laser Fortessa cytometer (BD Bioscience) and analyzed using FlowJo Version 8.8.6 software (TreeStar).
Bone marrow chimera
Bone marrow from Thy1.1 C57BL/6 WT and Thy1.2 CD4-Cre; TgfbRIIflox/flox mice was isolated and depleted of CD3+ cells using Miltenyi-Biotec anti-CD3 beads. One million cells of each genotype were mixed (1:1) and transferred intravenously into irradiated Rag2° recipient mice (10 gray). Mice were used 4 to 5 weeks after transfer.
Mouse immunization
Equal volumes of complete Freund adjuvent (CFA; 1 mg/mL; Sigma-Aldrich) and PBS (Gibco-Invitrogen) were emulsified by syringe mixing. Then 2 × 50μl of the CFA emulsion was injected subcutaneously at the tail base 7 days before mouse sacrifice.
Statistics
Paired and unpaired, 2-tailed Student t test were used to assess the significance of differences observed between 2 groups. The 1-way ANOVA was used to assess the significance of differences observed between 3 or more groups. P values were calculated using Bonferroni multiple comparison test.
Results
RORγt+ iNKT cells show a greater sensitivity to TGF-β
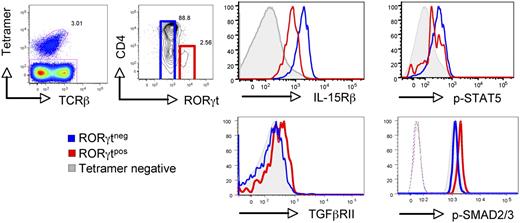
The development and homeostasis of conventional iNKT cells is highly influenced by IL-15/CD122 signaling because mice deficient in either IL-15 or CD122 (IL-15Rβ) fail to develop conventional iNKT cells.25,–27 Similar to conventional iNKT cells, we found the RORγt+ iNKT cells appear in the thymus approximately day 3 after birth (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, in contrast to conventional iNKT cells, we observed that RORγt+ iNKT cells had low surface expression of IL-15Rβ and exhibited weak STAT-5 phosphorylation in response to IL-15 (Figure 1). The low sensitivity of RORγt+ iNKT cells to IL-15 suggests that factors other than IL-15 signaling are important for their differentiation and homeostasis. TGF-β has recently been described as a key actor of conventional iNKT cell development,16 and is a well-known contributor to Th17 differentiation.28,29 Thus, we next examined whether RORγt+ iNKT cells and conventional iNKT cells exhibit the same sensitivity to TGF-β. Strikingly, we found that RORγt+ iNKT cells from the thymus expressed higher levels of TGF-βRII at their surface compared with conventional iNKT cells or CD4 T cells (Figure 1). Moreover, in agreement with the higher level of TGF-βRII expression, freshly isolated RORγt+ iNKT cells showed a stronger phosphorylation of SMAD2/3 proteins indicative of TGF-β signaling (Figure 1). Thus, these experiments reveal that as opposed to conventional iNKT cells, RORγt+ iNKT cells are less responsive to IL-15 and more sensitive to TGF-β.
RORγt+ iNKT cells respond weakly to IL-15 but are highly sensitive to TGFβ. Flow cytometry analysis to compare IL-15Rβ (CD122) and TGFβRII surface expression, and intracellular phospho-STAT5 (p-STAT5) and phospho-SMAD2/3 (p-SMAD2/3) levels in RORγt+ iNKT, RORγt− iNKT, and non-iNKT cells in the thymus of 6- to 8-week-old C57BL/6 WT mice. Dashed lines represent staining of the secondary antibody without the primary antibody. For p-STAT5 staining, cells were incubated with IL-15. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. Data are representative of at least 3 experiments with 1 to 2 individual mice per experiment.
RORγt+ iNKT cells respond weakly to IL-15 but are highly sensitive to TGFβ. Flow cytometry analysis to compare IL-15Rβ (CD122) and TGFβRII surface expression, and intracellular phospho-STAT5 (p-STAT5) and phospho-SMAD2/3 (p-SMAD2/3) levels in RORγt+ iNKT, RORγt− iNKT, and non-iNKT cells in the thymus of 6- to 8-week-old C57BL/6 WT mice. Dashed lines represent staining of the secondary antibody without the primary antibody. For p-STAT5 staining, cells were incubated with IL-15. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. Data are representative of at least 3 experiments with 1 to 2 individual mice per experiment.
Thymic differentiation of RORγt+ iNKT cells requires TGF-β signaling
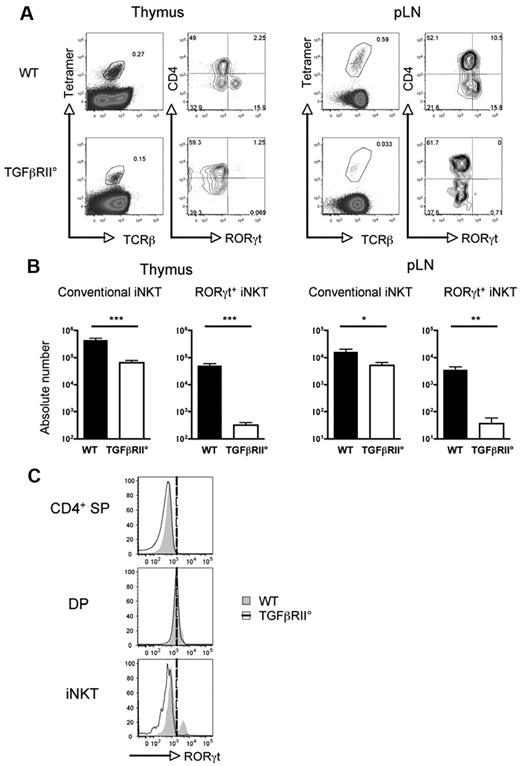
Given the sensitivity of RORγt+ iNKT cells to TGF-β, we hypothesized that TGF-β signaling could play a major role in their ontogeny. Therefore, we next investigated the effect of removing TGF-β signaling from RORγt+ iNKT cells on their differentiation and homeostasis. For this, we took advantage of the observation that RORγt+ iNKT cells express CD4 during their thymic development (supplemental Figure 2) and used conditional knockout CD4-Cre; TgfbRIIflox:flox, (TGFβRII°) mice.22 In contrast to conventional iNKT cells, for which the lack of TGF-β signaling reduced their number by 6- to 10-fold in the thymus and pLNs, but remain observable; RORγt+ iNKT cells were barely detectable in both thymus and pLNs of TGFβRII° mice (Figure 2A-B). Interestingly, this effect on RORγt expression seems to be specific to iNKT cells as DP thymocytes, known to express high levels of RORγt, expressed similar levels of this transcription factor irrespective of TGFβRII genotype (Figure 2C). In addition, the action of TGF-β signaling on RORγt+ iNKT cells is intrinsic, because mixed WT/TGFβRII° bone marrow chimeras showed that more than 95% of the RORγt+ iNKT cells developed from WT bone marrow (supplemental Figure 3). Together, these results show that TGF-β signaling is crucial for the thymic differentiation of RORγt+ iNKT cells.
TGFβ signaling is required for RORγt+ iNKT development. (A) Flow cytometry analysis of the presence of iNKT cell subsets in the thymus and pLNs of CD4-Cre; TgfbRIIflox/flox mice (TGFβRII°) and WT littermate control. Mice were analyzed at 2 weeks old, before the onset of T cell–mediated autoimmunity.22,41 (B) Mean absolute numbers ± SEM of CD4− RORγt+ and conventional RORγt− iNKT cells from the thymus and pLNs. (C) Flow cytometry analysis of the expression of RORγt in CD4 single-positive (CD4 SP), DP, and total iNKT cells (iNKT) in the thymus. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. Representative plots and combined data of 6 experiments totaling 8 to 10 mice in each group. P values were calculated using an unpaired Student t test (*P < .05; **P < .01; ***P < .001).
TGFβ signaling is required for RORγt+ iNKT development. (A) Flow cytometry analysis of the presence of iNKT cell subsets in the thymus and pLNs of CD4-Cre; TgfbRIIflox/flox mice (TGFβRII°) and WT littermate control. Mice were analyzed at 2 weeks old, before the onset of T cell–mediated autoimmunity.22,41 (B) Mean absolute numbers ± SEM of CD4− RORγt+ and conventional RORγt− iNKT cells from the thymus and pLNs. (C) Flow cytometry analysis of the expression of RORγt in CD4 single-positive (CD4 SP), DP, and total iNKT cells (iNKT) in the thymus. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. Representative plots and combined data of 6 experiments totaling 8 to 10 mice in each group. P values were calculated using an unpaired Student t test (*P < .05; **P < .01; ***P < .001).
SMAD4-dependent TGF-β signaling pathway prevents the apoptosis RORγt+ iNKT thymocytes
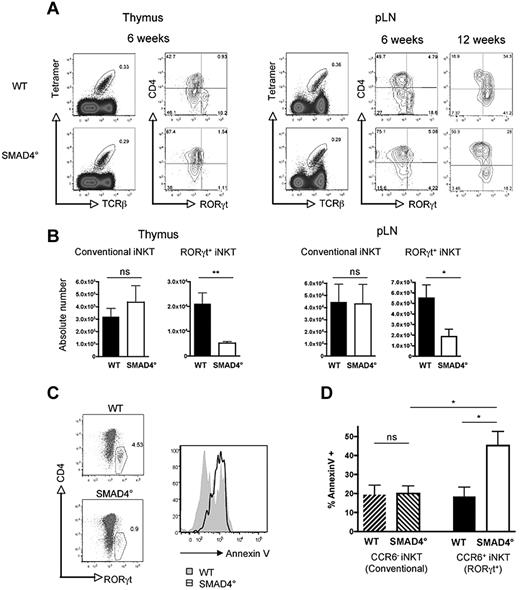
The phosphorylation of SMAD2/3 in RORγt+ iNKT cells suggests that the SMAD-dependent pathway could be important for RORγt+ iNKT cell generation. Moreover, in clear contrast with that reported for total iNKT cells,16 CD4-Cre; Tif-1γflox/flox mice, deficient for TIF-1γ (TRIM33) in their T cells, have similar numbers of RORγt+ iNKT cells to their WT littermate controls (supplemental Figure 4A). This rules out a major role for the Tif-1γ branch of TGF-β signaling in RORγt+ iNKT cell ontogeny, reinforcing the idea that the SMAD4 branch could be a key actor. To definitively address the role of the SMAD4-dependent pathway, we used CD4-Cre; Smad4flox/flox (SMAD4°) mice. In comparison with conventional iNKT cells for which the absence of SMAD4 had no effect on their numbers, the ablation of the SMAD4 branch led to a significant decrease of RORγt+ iNKT cells, both in the thymus and in the pLNs, demonstrating a selective effect of the SMAD4-dependent pathway on RORγt+ iNKT cell ontogeny (Figure 3 A-B). The loss of RORγt+ iNKT cells in the absence of SMAD4 dependent signaling was associated with a greater susceptibility of RORγt+ iNKT thymocytes to apoptosis based on annexin V staining of fixed iNKT cells stained for RORγt (Figure 3C). We confirmed this finding using live iNKT thymocytes, by counterstaining annexin V and CCR6 (Figure 3D), a chemokine receptor, shown to be expressed on CD1d tetramer+ RORγt+ cells but not on CD1d tetramer+ RORγt− cells.12 Furthermore, in agreement with a selective antiapoptotic effect of the SMAD4-dependent pathway on RORγt+ iNKT cell thymic differentiation, no difference in annexin V staining was observed between conventional iNKT cell from the thymus of SMAD4° mice and their WT littermate controls and we failed to detect any difference in annexin V staining between mature RORγt+ iNKT cells from the pLNs of SMAD4° mice and WT littermate control mice as well between RORγt+ iNKT cells from the pLNs of TGF-βRICA mice and their littermate controls (supplemental Figure 4B and data not shown). Interestingly, as in WT mice, the few differentiated SMAD4° RORγt+ iNKT cells gradually accumulate in the pLNs as the animals age (Figure 3A). Together, our results point to the SMAD4-dependent pathway as the crucial branch of TGF-β signaling necessary for the thymic differentiation of RORγt+ iNKT cells. They strongly suggest that SMAD4-dependent signaling prevent selectively RORγt+ iNKT cells from apoptosis during their thymic differentiation. Thus, this set of experiments reveals that TGF-β signaling controls the differentiation of RORγt+ iNKT cells and conventional iNKT cells using distinct signaling branches.
SMAD4-dependent signaling pathway controls survival of RORγt+ iNKT thymocytes. (A) Flow cytometry analysis of the presence of iNKT cell subsets in the thymus and pLNs of CD4-Cre; Smad4flox/flox (SMAD4°) mice and WT littermate controls. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (B) Mean absolute numbers ± SEM of RORγt+ and conventional RORγt− iNKT cells from the thymus and peripheral lymph nodes (pLNs) of 6- to 8-week-old mice. Representative plots and combined data from at least 6 experiments totaling 8 to 10 mice in each group. P values were calculated using unpaired Student t test (*P < .05; **P < .01; NS indicates not significant). (C) RORγt+ iNKT cells costained for annexin V and RORγt in the thymus after 36 hours incubation. (D) Annexin V staining on CD4− CCR6+ iNKT thymocytes (RORγt+ iNKT) and CD4−CCR6− iNKT thymocytes (conventional iNKT). Annexin V data includes 4 experiments totaling 8 mice in each group. The 1-way ANOVA was used to determine overall significance between groups and P values were calculated using Bonferroni multiple comparison test. (*P < .05; NS indicates not significant).
SMAD4-dependent signaling pathway controls survival of RORγt+ iNKT thymocytes. (A) Flow cytometry analysis of the presence of iNKT cell subsets in the thymus and pLNs of CD4-Cre; Smad4flox/flox (SMAD4°) mice and WT littermate controls. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (B) Mean absolute numbers ± SEM of RORγt+ and conventional RORγt− iNKT cells from the thymus and peripheral lymph nodes (pLNs) of 6- to 8-week-old mice. Representative plots and combined data from at least 6 experiments totaling 8 to 10 mice in each group. P values were calculated using unpaired Student t test (*P < .05; **P < .01; NS indicates not significant). (C) RORγt+ iNKT cells costained for annexin V and RORγt in the thymus after 36 hours incubation. (D) Annexin V staining on CD4− CCR6+ iNKT thymocytes (RORγt+ iNKT) and CD4−CCR6− iNKT thymocytes (conventional iNKT). Annexin V data includes 4 experiments totaling 8 mice in each group. The 1-way ANOVA was used to determine overall significance between groups and P values were calculated using Bonferroni multiple comparison test. (*P < .05; NS indicates not significant).
TGF-β signaling leads to accumulation of RORγt+ iNKT cells in both immune and nonimmune peripheral tissues
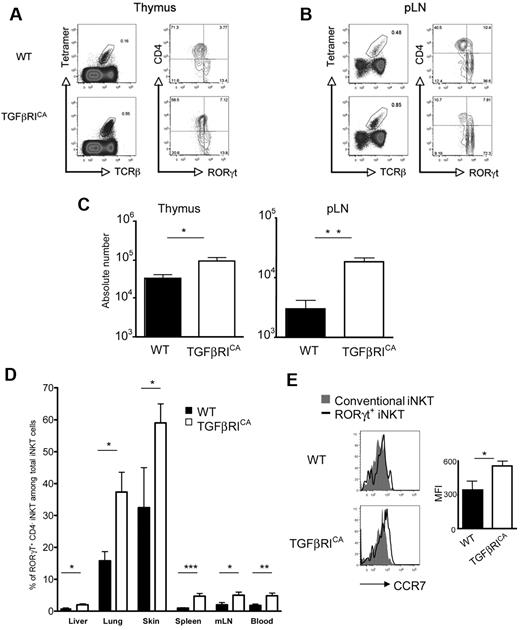
IL-15/CD122 signaling is known to regulate peripheral homeostasis of conventional iNKT cells, particularly those which localize in the spleen and liver.25,–27 The weak sensitivity of RORγt+ iNKT cells to IL-15 (Figure 1), along with their distinct peripheral distribution, mainly in the pLNs, but also the skin and lungs,7,12 strongly suggest that homeostasis of RORγt+ iNKT obeys different mechanisms. TGF-β has been proposed as a key cytokine for the homeostasis and IL-17 production of peripheral CD4+ Th17 cells.29,–31 To clearly address the role of this cytokine on RORγt+ iNKT cell homeostasis, we used CD4-Cre; Stopflox/floxTGF-RICA (TGFβRICA) mice, in which only CD4+ T cells and their progeny were constitutively subjected to TGF-β signaling because of conditional expression of a mutated form of the TGF-βRI.23 Interestingly, the constitutive activation of TGF-β signaling was not associated with an increase in the proportion of RORγt+ iNKT cells in the thymus, suggesting that iNKT thymocytes that have entered into the RORγt negative differentiation pathway cannot be converted to RORγt+ iNKT cell precursors by TGF-β signaling (Figure 4A). However, in the pLNs of TGFβRICA mice the proportion of RORγt+ iNKT cells was dramatically increased, representing more than 70% of the total iNKT cells in adult mice, as opposed to 30%-40% of the iNKT cell population in WT mice (Figure 4B). Moreover, as reported for conventional iNKT cells,16 the constitutive activation of TGF-β signaling led to substantial increases in the number of RORγt+ iNKT cells both in the thymus and the periphery (Figure 4C). The increased presence of RORγt+ iNKT cells was not because of greater proliferation or survival in response to TGF-β because no significant differences between TGFβRICA mice and their littermate controls were observed using Ki67 and annexin V staining (data not shown). It is notable that the enrichment of RORγt+ iNKT cells was not limited to the pLNs. Spleen, liver, mesenteric lymph nodes, skin, and lung, were found to contain higher percentages of RORγt+ iNKT cells in TGFβRICA mice compared with their littermate controls (Figure 4D). The factors controlling the homing and retention of iNKT cells in the peripheral lymph nodes are unknown; however, it seems probable that RORγt+ iNKT cells are more responsive to such homing factors. In line with their substantial enrichment in lymphoid and nonlymphoid tissue of TGFβRICA mice, a sizable proportion of RORγt+ iNKT cells expressed high levels of CCR7 (Figure 4E), a chemokine receptor not only important for the migration of T cells to lymph nodes and T cell/dendritic cell interactions, but also in peripheral tissues, such as the lungs and skin.32 Interestingly, blood analysis of TGFβRICA mice showed a significant increase of RORγt+ iNKT cells compared with WT mice, which reinforces the idea of a role for TGF-β signaling on the tissue distribution of RORγt+ iNKT cells through the blood. Thus, RORγt+ iNKT cells naturally accumulate in the pLNs, independent of a proliferation or survival advantage over conventional iNKT cells, but probably through a higher sensitivity to homing chemokines, which is intensified by TGF-β signaling permitting greater access to other immune and peripheral tissues from the blood.
TGFβ signaling enhances enrichment of RORγt+ iNKT cells in immune and peripheral tissues. Flow cytometry analysis of the presence of iNKT cell subsets in the (A) thymus and (B) pLNs of CD4-Cre; Stopflox/floxTgfbRICA (TGFβRICA) mice and WT littermate control 6- to 8-week-old mice. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (C) Mean absolute numbers ± SEM of RORγt+ iNKT cells from the thymus and pLNs. Representative plots and combined data from 7 experiments totaling 10 to 12 mice in each group. P values were calculated using unpaired Student t test (*P < .05; **P < .01; NS indicates not significant). (D) Percentage ± SEM of RORγt+ iNKT cells among all iNKT cells in the spleen, mesenteric lymph nodes (mLNs), liver, lung, blood, and skin of TGFβRICA mice and WT littermate control mice. P values were calculated using unpaired Student t test (*P < .05; **P < .01; ***P < .001). (E) Flow cytometry analysis of CCR7 on RORγt+ and conventional RORγt− iNKT cells from the pLNs. Graph illustates the mean of fluorescence intensity (MFI) of CCR7 staining on RORγt+ iNKT cells ± SEM. Representative plots and combined data from at least 6 experiments totaling 10 to 12 mice in each group (A-B). Representative plots and combined data from 3 to 6 experiments totaling 4 to 8 mice in each group (D-E).
TGFβ signaling enhances enrichment of RORγt+ iNKT cells in immune and peripheral tissues. Flow cytometry analysis of the presence of iNKT cell subsets in the (A) thymus and (B) pLNs of CD4-Cre; Stopflox/floxTgfbRICA (TGFβRICA) mice and WT littermate control 6- to 8-week-old mice. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (C) Mean absolute numbers ± SEM of RORγt+ iNKT cells from the thymus and pLNs. Representative plots and combined data from 7 experiments totaling 10 to 12 mice in each group. P values were calculated using unpaired Student t test (*P < .05; **P < .01; NS indicates not significant). (D) Percentage ± SEM of RORγt+ iNKT cells among all iNKT cells in the spleen, mesenteric lymph nodes (mLNs), liver, lung, blood, and skin of TGFβRICA mice and WT littermate control mice. P values were calculated using unpaired Student t test (*P < .05; **P < .01; ***P < .001). (E) Flow cytometry analysis of CCR7 on RORγt+ and conventional RORγt− iNKT cells from the pLNs. Graph illustates the mean of fluorescence intensity (MFI) of CCR7 staining on RORγt+ iNKT cells ± SEM. Representative plots and combined data from at least 6 experiments totaling 10 to 12 mice in each group (A-B). Representative plots and combined data from 3 to 6 experiments totaling 4 to 8 mice in each group (D-E).
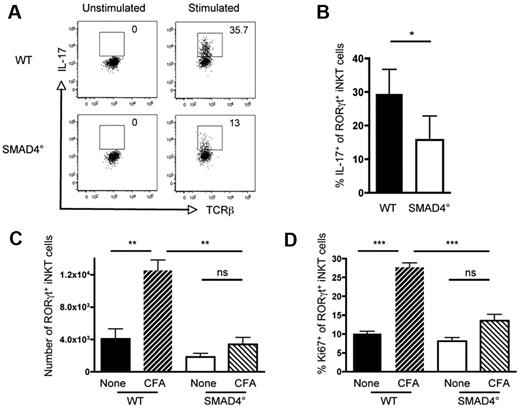
SMAD4-dependent signaling is required for IL-17 production and proliferation of RORγt+ iNKT cells during an inflammatory response
Because of their tissue distribution and rapid response to cytokines and pathogen derived stimuli, iNKT cells are regarded as a powerful T-cell subset, which contributes to the first line of defense of the organism.2,6,15 In contrast to conventional iNKT cells that mainly produce IFN-γ and IL-4, RORγt+ iNKT cells rapidly produce high amounts of IL-17. TGF-β signaling is known to allow the production of IL-17 by CD4+ T cells, in a SMAD4-independent manner (data not shown).33 In agreement with their low number in the thymus, few RORγt+ iNKT cells were observed in the pLNs of SMAD4° mice (Figure 3B). Surprisingly, whereas peripheral RORγt+ iNKT cells from SMAD4° and WT mice expressed similar levels of RORγt (Figure 3A), ex vivo analysis showed that fewer RORγt+ iNKT cells from the pLNs of SMAD4° mice were capable of producing IL-17 (Figure 5A-B). Thus, contrary to CD4+ Th17 cells, IL-17 production by RORγt+ iNKT cells is dependent on the SMAD4 branch of the TGF-β signaling pathway.
SMAD4-dependent signaling is required for IL-17 production and proliferation of RORγt+ iNKT cells during an inflammatory response. Ex vivo intracellular flow cytometry analysis of IL-17 producing RORγt+ iNKT cells in the pLNs of CD4-Cre; Smad4flox/flox (SMAD4°) and WT littermate control mice 10 to 16 weeks old. (A) Representive flow cytometry plots of the percentage of IL-17+ RORγt+ iNKT cells in SMAD4° and WT mice with and without PMA/ionomycin stimulation. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (B) The mean percentage ± SEM of RORγt+ iNKT cells that produce IL-17. Combined data of 3 experiments. P values were calculated using a paired Student t test (*P > .05). (C) Number of RORγt+ and (D) percentage of Ki67+ RORγt+ iNKT cells in the draining inguinal lymph nodes with and without subcutaneous injection of CFA at the base of the tail. Invariant NKT cells were identified as previously described. One experiment representive of 3 to 5 unimmunized mice and 6 to 8 immunized mice of each genotype in total, 10 to 16 weeks old. Mean ± SEM are shown. The 1-way ANOVA was used to determine overall significance between groups and P values were calculated using Bonferroni multiple comparison test. (P < .01; ***P < .001; NS indicates not significant).
SMAD4-dependent signaling is required for IL-17 production and proliferation of RORγt+ iNKT cells during an inflammatory response. Ex vivo intracellular flow cytometry analysis of IL-17 producing RORγt+ iNKT cells in the pLNs of CD4-Cre; Smad4flox/flox (SMAD4°) and WT littermate control mice 10 to 16 weeks old. (A) Representive flow cytometry plots of the percentage of IL-17+ RORγt+ iNKT cells in SMAD4° and WT mice with and without PMA/ionomycin stimulation. Invariant NKT cells were identified by excluding B220+ and CD8+ cells and gating on CD1d tetramer+ TCRβ+ cells. (B) The mean percentage ± SEM of RORγt+ iNKT cells that produce IL-17. Combined data of 3 experiments. P values were calculated using a paired Student t test (*P > .05). (C) Number of RORγt+ and (D) percentage of Ki67+ RORγt+ iNKT cells in the draining inguinal lymph nodes with and without subcutaneous injection of CFA at the base of the tail. Invariant NKT cells were identified as previously described. One experiment representive of 3 to 5 unimmunized mice and 6 to 8 immunized mice of each genotype in total, 10 to 16 weeks old. Mean ± SEM are shown. The 1-way ANOVA was used to determine overall significance between groups and P values were calculated using Bonferroni multiple comparison test. (P < .01; ***P < .001; NS indicates not significant).
Given that in addition to producing IL-17, RORγt+ iNKT cells are known to rapidly proliferate in response to inflammatory conditions,9,15 we analyzed the contribution of TGF-β signaling, particularly the SMAD4 branch, on RORγt+ iNKT cell expansion in vivo. Strikingly, whereas RORγt+ iNKT cells from WT mice immunized with CFA expanded early after immunization as assessed by Ki67 staining, little expansion of RORγt+ iNKT cells was observed in SMAD4° mice even later after CFA injection (Figure 5C-D, data not shown). Thus, TGF-β signaling, through its SMAD4 signaling branch, allows the proliferation and expansion of RORγt+ iNKT cells in response to inflammatory signals, as well as the production of IL-17, pointing out a crucial role for TGF-β signaling for fully functional RORγt+ iNKT cells.
Discussion
The recently described RORγt+ iNKT cells constitute, in both mice and humans, a distinct subset of iNKT cells characterized by their unique capacity to produce large amounts of IL-17.9,15,34 This massive and rapid production of IL-17 in response to inflammatory cytokines and bacterial infection, along with their distribution in the peripheral tissues, suggests that these cells are sentinel cells, which are important for rapidly recruiting other innate-immunity actors, such as neutrophils to the local tissue site.7,14,15,35 Thus, identifying factors controlling both the differentiation and function of the RORγt+ iNKT cell subset is critical for a better understanding of the immune response on encounter with pathogens or inflammatory agents.
Similar to conventional iNKT cells, RORγt+ iNKT cells develop in the thymus in a CD1d-dependent manner. However, our work demonstrates that their thymic differentiation probably follows a different developmental sequence in response to alternative factors. Our findings reveal that the thymic differentiation of RORγt+ iNKT cells is under TGF-β signaling control, and that the TGF-β signaling branches implicated in their differentiation are different from those involved in conventional iNKT differentiation. For conventional iNKT cell thymic development, it has been established that the main TGF-β signal is SMAD4-independent.16 The Tif1-γ–dependent pathway has been shown to prevent apoptosis of CD44low iNKT cells, whereas SMAD4-Tif1γ independent pathways control the acquisition of T-bet and CD122 during the transition from CD44high NK1.1− cells to CD44high NK1.1+ cells. In clear contrast with what is known for conventional iNKT cells, our data reveal the crucial role of the SMAD4-dependent pathway in the control of RORγt+ iNKT differentiation by TGF-β signaling. The SMAD4 protein is necessary to assure the survival of the RORγt+ iNKT thymocytes, as well as IL-17 production and expansion of peripheral RORγt+ iNKT cells in response to inflammation. Thus, through distinct signaling pathways, TGF-β controls the development of iNKT cells, and assures the dichotomy between conventional and RORγt+ iNKT cell subsets.
The role of TGF-β signaling in controlling the expression of the transcription factor RORγt has been largely reported in CD4+ T lymphocytes.28,29 However, contrary to CD4+ T cells that require both TGF-β and IL-6 signaling to express RORγt and become Th17 cells, RORγt expression in iNKT cells is independent of IL-6 signaling because RORγt+ iNKT cells develop normally in IL-6KO mice.11,12 Unlike Th17 cells, RORγt+ iNKT cells do not differentiate under inflammatory conditions, which possibly explains why they do not require IL-6 signals. Interestingly, IL-17–producing γδ T cells have also been shown to develop in the thymus in a TGF-β–dependent manner and in the absence of IL-6.36 Together, these findings point to the idea that IL-17 producing innate-like T lymphocytes are programmed in the thymus by TGF-β, and then exported to peripheral lymphoid and barrier sites to act as sentinel cells for infection and inflammation. However, whether TGF-β signaling alone is sufficient to induce RORγt expression during iNKT cell thymic development remains to be determined, although the observation that TGFβRCA mice did not skew a greater percentage of precursor cells into the RORγt+ iNKT developmental pathway in the thymus goes against this hypothesis. Interestingly, TGF-β has been reported to induce Foxp3 expression in iNKT cells in vitro and in vivo when cells are activated in the presence of high levels of TGF-β.37 In the constitutively active TGF-β signaling mice, we failed to identify Foxp3+ iNKT cells (data not shown) leading us to conclude that contrary to RORγt expression, iNKT activation seems necessary for the induction of Foxp3+ iNKT cells by TGF-β.
Although the differentiation steps of conventional iNKT cells are well defined, much less is known about the developmental sequence of the RORγt+ iNKT cells. Given their TCR restriction and their CD1d-dependent development, it has been suggested that these cells could be derived from CD24+ CD4+ CD8± precursors, similar to conventional iNKT cells.3,10 Using CD4-expression reporter mice, we definitively confirmed that the RORγt+ iNKT cell developmental sequence involves a CD4+ step. However, whether these cells differentiated from CD24+ CD4+ CD8± precursors or earlier from DP thymocytes remains to be clarified. RORγt is highly and uniformly expressed at the DP stage. Notably, lack of TGF-β signaling from the DP stage in TGF-βRIIKO mice does not affect the level of RORγt expression in DP thymocytes, suggesting that the RORγt+ iNKT lineage differentiation branches after the DP stage and from selected cells that have lost CD8 and CD4 expression. Moreover, RORγt+ iNKT thymocytes express higher levels of RORγt than the DP cells. Compared with conventional iNKT cells, we found that RORγt+ iNKT thymocytes are more sensitive to TGF-β and less responsive to IL-15. We propose that the sensitivity to TGF-β of RORγt+ iNKT cells could singularly condition their differentiation away from that of conventional iNKT cells. After their selection, some CD4+ CD8± precursor cells express higher levels of TGF-βR allowing them to acquire a strong level of RORγt and maintain its expression. These RORγt+ iNKT cells continue to mature, going from CD44low to CD44high and losing CD4 and CD8 expression. However, unlike conventional iNKT cells for which the down-regulation of their TGF-βR allows them to express T-bet and become CD122high (IL-15Rβ) and NK1.1+, the enhanced sensitivity of RORγt+ iNKT cells to TGF-β gives rise to NK1.1− and CD122low cells.16
A recent report identified IL-17–producing iNKT cells as continuously supplied in the periphery by thymic export as recent thymic emigrants.38 Our work demonstrates that thymic production reaches a plateau, approximately 3 weeks of age and that homeostasis of peripheral RORγt+ iNKT cells is highly influenced by TGF-β signaling. RORγt+ iNKT cells receiving constitutive TGF-β signals are over-represented in both lymphoid and nonlymphoid tissues without affecting their relative production by the thymus. The increased peripheral distribution of RORγt+ iNKT cells by TGF-β signaling in the absence of inflammation seems in part because of an increased sensitivity to chemokines, such as those binding to CCR7. This idea is supported by findings that show exacerbated chemotaxis of NK1.1− iNKT cells to CCL21, a ligand of CCR7 expressed in lymphatic vessels of various nonlymphoid organs, such as lungs and skin, in both mice and humans.32,39 Interestingly, CCL21 has been shown to increase the speed of T lymphocytes,32 hence it is tempting to propose that by enhancing the sensitivity of RORγt+ iNKT cells to CCL21, TGF-β signaling increases their speed and their localization to pathogen barrier tissues, such as lungs and skin, and thus their potential role as sentinels. In response to activation signals or inflammation, we assigned novel functions to TGF-β, in that it contributes to the expansion of iNKT cells and their production of the proinflammatory cytokine IL-17. Whether this lack of functionality in the periphery in SMAD4° mice is because of disturbed development in the thymus or a bona fide need for TGF-β signals during the peripheral response is not possible to address in TGF-βRII° mice, as there are very few RORγt+ iNKT cells in the periphery of these mice. However, the few RORγt+ iNKT cells found in the periphery of SMAD4° mice are partially functional in that they do proliferate at a similar rate to those found in WT mice in steady state conditions (Figure 5D) and express similar levels of RORγt. Thus, at the steady state TGF-β signaling supports the normal tissue distribution of RORγt+ iNKT cells, matching with the wide tissue distribution of this cytokine and a sentinel role for RORγt+ iNKT cells. Interestingly, numerous infections are associated with high levels of the active form of TGF-β.40–41 Hence, we can propose that in the case of infection, inflammatory stimuli associated with high levels of TGF-β allow expansion of RORγt+ iNKT cells in the tissues and peripheral lymph nodes, establishing a rapid line of defense against the infectious agent. At first glance these results may seem contradictory to the immuno-supressive function usually associated with this cytokine, as TGF-β controls conventional T cells and prevents their autoreactivity, which leads to over activation and tissue damage.22,41 Based on our results, we propose that TGF-β also contributes to the innate immune response and the early defense of the organism by regulating the functionality of RORγt+ iNKT cells. Both functions of TGF-β lead to the protection of tissue integrity, from either pathogens or autoreactive T cells, and are compatible with the wide tissue distribution of this cytokine in the organism.
In summary, our results propose TGF-β as a key actor in the biology of the recently described RORγt+ iNKT cell subset, by controlling both thymic differentiation and peripheral function. We have revealed that TGF-β, through its SMAD4 signaling pathway, selectively affects the thymic development of RORγt+ iNKT cells, their capacity to produce IL-17, and expansion under the inflammatory conditions in the periphery. Thus TGF-β, known as an immunosuppressive cytokine of the T-lymphocyte response, contributes to the innate arm of the immune system, assuring the development of IL-17 producing iNKT cells and their response to inflammatory signals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mc Carron for expert technical assistance and editing of the manuscript (supported by FRM); and C. Maisin and C. Faure (supported by InCa and ANR grants, respectively) for technical assistance. They thank S. Karlsson, C. B. Wilson, F. Constantini, and R. Losson for providing TgfbRIIfl/fl, Smad4fl/fl, CD4-Cre, R26RYFP, and Tif1γfl/fl mice. PBS57/CD1d Tetramers were kindly provided by the NIH Tetramer facility. They also thank members of the laboratory for advice and helpful discussion.
This work was supported by grants from ANR-08-JCJC-0005-01 (J.M.), InCa Atip Avenir program (J.M.), la fondation pour la recherche médicale INE20091217951 (J.M.), le comité Rhone ligue (J.M.), ANR investissement d'avenir ANR-10-LABX-61 (J.M.) and ANR-R07119KS (K.B.), and the foundation Bettencourt-Schueller (J.M.). C.H.-D. was supported by ANR and InCa grants, and S.L. by the institut universitaire d'hématologie, Hopital St Louis.
Authorship
Contribution: C.H.-D. designed, performed, and analyzed the experiments, and wrote the paper; S.L. performed experiments; K.B. designed the experiments and provided essential knowledge; and J.C.M. designed and analyzed the experiments, wrote the paper, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julien C. Marie, Cancer Research Center of Lyon, UMR Inserm 1052, CNRS 5286, Bat. Cheney A, Centre Léon Bérard, 69373 Cedex 08, Lyon, France; e-mail: julien.marie@inserm.fr; and Kamel Benlagha, Inserm U940, Hôpital St Louis, 1 av Claude Vellefaux, 75010, Paris, France; e-mail: kamel.benlagha@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal