Abstract
Mast cells play critical roles in allergic disorders and asthma. The importance of tuberous sclerosis complex 1/2-mammalian target of rapamycin (TSC1/2-mTOR) signaling in mast cells is unknown. Here, we report that TSC1 is a critical regulator for mTOR signaling in mast cells downstream of FcεRI and c-Kit, and differentially controls mast cell degranulation and cytokine production. TSC1-deficiency results in impaired mast cell degranulation, but enhanced cytokine production in vitro and in vivo after FcεRI engagement. Furthermore, TSC1 is critical for mast cell survival through multiple pathways of apoptosis including the down-regulation of p53, miR-34a, reactive oxygen species, and the up-regulation of Bcl-2. Together, these findings reveal that TSC1 is a critical regulator of mast cell activation and survival, suggesting the manipulation of the TSC1/2-mTOR pathway as a therapeutic strategy for mast cell-mediated diseases.
Introduction
Mast cells play pivotal roles in chronic allergic inflammation and acute anaphylaxis, which are largely mediated by the high-affinity immunoglobulin E (IgE) receptor (FcεRI) on their surface. Cross-linking of IgE-bound FcεRI by cognate antigen (Ag) initiates multiple signal transduction pathways that trigger the release of proinflammatory mediators, such as histamine from granules and de novo synthesis and secretion of cytokines.1-3 FcεRI engagement activates the Src-family protein tyrosine kinases (PTK) Lyn, which phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) of the β and γ-subunits of FcεRI and subsequently activates Syk.4 These events are followed by recruiting and activating downstream effector and adaptor molecules, such as linker for activated T cells (LAT),5 SH2 domain-containing leukocyte phosphoprotein of 76 kDa,6 Rac GTPase guanine nucleotide exchange factor Vav1,7 Tec family kinase Bruton tyrosine kinase,8 and phospholipase Cγ (PLCγ).9 In addition to Lyn, another Src PTK, Fyn, induces activation of Grb2-associated binder 2 without requirement of Lyn and LAT to promote phosphatidylinositol 3-kinase (PI3K) activation.10-13 In turn, these signals are transmitted to downstream signaling molecules including PKCs and MAPKs that are important for mast cell activation.14-17
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase linked with the PI3K pathway via Akt.18 mTOR senses both environmental and intracellular stimuli such as growth factors, nutrients, energy, and stress, and is capable of integrating diverse biologic processes including cell metabolism, growth, autophagy, and survival.18,19 mTOR forms 2 functionally and structurally distinct complexes: rapamycin-sensitive mTOR complex 1 (mTORC1) and rapamycin-insensitive mTORC2.19 mTORC1 phosphorylates ribosomal S6 kinases (S6Ks) and eIF4E-binding proteins (4E-BPs) to promote ribosomogenesis and cap-dependent translation.18 mTORC2 directly phosphorylates Akt at Ser473 to promote Akt activation, and is also necessary to phosphorylate PKCα at Ser657 to increase PKCα stability.20,21 In mast cells, stimulation of FcεRI, c-Kit and prostaglandin E2 receptor can induce both mTORC1 and mTORC2 activation.22-24 Although how mTOR deficiency may impact mast cell function has not been reported, studies with chemical and shRNA inhibitors targeting mTOR complex components have suggested that both mTORC1 and mTORC2 are involved in mast cell growth, survival, cytokine production, and chemotaxis.22-24 Given the importance of mTOR in mast cell function, it is critical to understand the mechanisms and the importance of mTOR regulation.
The tuberous sclerosis complex 1 (TSC1) and TSC2 tumor suppressor complex is a fundamental controller of the mTORC1 pathway. TSC2 acts as a GTPase-activating protein toward Rheb that is an upstream activator of the mTORC1 pathway,25,26 whereas TSC1 stabilizes TSC2 by inhibiting its ubiquitination.27 Loss of either TSC1 or TSC2 leads to the constitutively active status of the mTORC1 pathway. Currently, the role of TSC1/2 in mast cells is not known. In this report we explored how TSC1 controls mTOR signaling to regulate mast cell function and homeostasis using TSC1 conditional knockout mice. We demonstrate that TSC1 deficiency results in increased mTORC1 but decreased mTORC2 signaling in mast cells. TSC1-deficient mast cells display impaired degranulation in vitro and in vivo, but increased cytokine production after FcεRI engagement. In addition, TSC1-deficient mast cells manifest diminished viability, particularly after withdrawal of critical survival factors. We demonstrate further that TSC1 promotes mast cell survival through decreasing the levels of reactive oxygen species (ROS), p53, miR-34a, and by increasing Bcl-2 expression.
Methods
Mice and cell culture
TSC1f/f-ER-Cre mice were generated by mating TSC1f/f mice28 with ER-Cre mice.29 Two- to 3-month-old TSC1f/f-ER-Cre and TSC1f/f littermates were intraperitoneally injected with 2 mg of tamoxifen on days 1, 2, and 5. Mice were euthanized either on day 7 for harvesting bone marrow (BM) or on day 14 for assessing mast cell distribution. BM cells were flushed from tibias and femurs, and then cultured in IMDM–IL-3 media for 5 to 6 weeks. IMDM–IL-3 is Iscove modified Dulbecco media (IMDM; Sigma-Aldrich) supplemented with 10% FBS (Hyclone), 100 U/mL penicillin G, 100 U/mL streptomycin, and 292 μg/mL of l-glutamine, 10mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid; pH 7.4), 0.1mM nonessential amino acids (NEAAs), 1mM sodium pyruvate, and 50μM β-mercaptoethanol with 10% IL-3–conditioned medium generated from X63 cells. The experiments described in this study were reviewed and approved by the Duke University Institute Animal Care and Use Committee.
Staining and counting of mast cells
The pieces of ear, dorsal skin, spleen, and lung were quickly frozen in optimum cutting temperature medium (Tissue Tek), fixed in Carnoy solution (60% ethanol, 30% chloroform, 10% glacial acetic acid), and stained with 0.67% toluidine blue O solution (Sigma-Aldrich).
Flow cytometry
Bone marrow–derived mast cells (BMMCs) were stained directly with phycoerythrin (PE)–conjugated c-Kit or stained after 4 hours sensitization with 1 μg/mL IgE, followed by incubation with FITC-conjugated anti-IgE, and then analyzed by flow cytometry (BD FACSCanto II). For ΔΨ and ROS, the cells were incubated at room temperature (RT) with DiOC6 (50nM) for 15 minutes and dihydroethidium (DHE; 2.5μM) for 1 hour and then were analyzed using the FL1 and FL3 channel, respectively. Data were analyzed with FlowJo Version 9.2 software (Tree Star).
BMMC proliferation and apoptosis assay
BMMCs were labeled with 10μM CFSE at RT for 10 minutes and cultured in appropriate conditions for 72 hours. Cells were stained with Live/Dead Violet Dead Cell Stain (Invitrogen) to distinguish live cells, and then CFSE-dilution of live cells was analyzed by flow cytometry. For apoptosis, BMMCs (1 × 106 cells/mL) were cultured in different media for the indicated times and harvested, and then stained with 7-amino-actinomycin D (7AAD) and allophycocyanin-conjugated annexin V for 20 minutes at RT in buffer A containing 10mM HEPES (pH 7.4), 140mM NaCl, and 2.5mM CaCl2. Pri-miR-34a was generated using murine total RNA as template and 5′-gatcctcgagCTGTGCTAGCGTGCTGGCTTCC-3′ and 5′-gatcgaattcCGTGGGGTGCTAACACCAGCCTCA-3′ as primers. Amplified pri-miR-34a was cloned into pLCE lentiviral vector.30 To generate lentiviral sponge for miR-34a (SPNG-34s), 6 copies of specific sponge site for miR-34a were inserted into 3′UTR of enhanced green fluorescent protein (EGFP) in pLCE using the following oligonucleotides: 5′-CTAGACAACCAGCATTCACACTGCCAGTTTTGACAACCAGCATTCACACTGCCAGTTTTGACAACCAGCATTCACACTGCCATCTAGATTTG-3′ and 5′-AATTCAAATCTAGATGGCAGTGTGAATGCTGGTTGTCAAAACTGGCAGTGTGAATGCTGGTTGTCAAAACTGGCAGTGTGAATGCTGGTTGTCTAG-3′. Both plasmids were confirmed by DNA sequencing. Hydrogen peroxide and N-acetyl-L-cysteine were from Sigma-Aldrich, Necrostatin-1 from Biomol, and z-VAD-fmk from Calbiochem.
Lentiviral infection of BMMCs
pLCE was cotransfected with packaging plasmids (pMDL, pVSVG, and pRSV) into 293FT cells. Filtered supernatant collected 48 hours after transfection was used for spin infection of BMMCs. BMMCs (1 × 106 cells/mL) were plated in a 24-well plate with media containing virus and polybrene (4 μg/mL), and were centrifuged at 1350g for 90 minutes at RT. After 3 to 5 days, cells were used for experiments.
β-hexosaminidase and cytokine release
BMMCs (1 × 106 cells/mL) were rested overnight in IMDM–IL-3, incubated with 1 μg/mL anti-DNP IgE (clone SPE-7; Sigma-Aldrich) for at least 4 hours in IMDM media without IL-3. The cells were washed once with IMDM and then stimulated with various concentrations of anti-dinitrophenyl (DNP)–human serum albumin (DNP-HSA; Sigma-Aldrich) for 45 minutes in Tyrode buffer. Supernatants were incubated with 2mM p-nitrophenyl-N-acetylß-D-glucosamide (Sigma-Aldrich) dissolved in 0.1M citrate buffer (pH 4.5) in a final volume of 60 μL for 1 hour at 37°C, followed by terminating the enzymatic reaction by addition of 60 μL 2M NaOH. Absorbance at 405 nm was read by a plate reader. Total cellular β-hexosaminidase activity was quantified using supernatant from cells lysed with 0.5% Triton X-100. For cytokine measurements, the cells were harvested after 6 or 24 hours after stimulation and the amounts of IL-6 and tumor necrosis factor (TNF)–α from supernatants were determined by Mouse ELISA Max (BioLegend) according to the manufacturer's instruction.
Passive cutaneous and systemic anaphylaxis
In the passive systemic anaphylaxis assay (PSA), TSC1f/f-ER-Cre and wild-type (WT) mice were injected intravenously with 200 μL of 15 μg/mL anti-DNP IgE. Twenty-four hours after injection, anesthetized mice were injected intravenously with 200 μL of 0.5 mg/mL DNP-HSA. After 90 seconds, mice were killed and plasma was immediately isolated from blood. Histamine levels were determined by an EIA histamine kit (Immunotech). Alternatively, plasma was collected by bleeding the mice at 30 minutes and 180 minutes after Ag injection for analysis of mouse mast cell protease-1 (mMCP-1) and cytokines, respectively. The amounts of mMCP-1 were determined by a mouse mMCP-1 ELISA Ready set-go kit (eBioscience) according to the manufacturer's instruction. The passive cutaneous anaphylaxis assay (PCA) was conducted as previously described.16 Mice were subcutaneously injected with 25 ng IgE in 20 μL PBS in the right ear and 20 μL PBS in the left ear. Twenty-four hours later, mice were injected intravenously with 200 μL of antigen mixture (100 μg DNP IgE and 1% Evan blue in PBS). Thirty minutes after Ag injection, mice were killed and both ears were removed. The dye extracts were collected by incubation in 500 μL formamide at 55°C for 24 hours and used for measuring absorbance at 610 nm. The data were calculated as OD610 of right ear extract minus OD610 of left ear extract from the same mouse.
Immunoblot assay
BMMCs were lysed in radio immunoprecipitation assay (RIPA) buffer (0.1% sodium dodecyl sulfate[SDS], 1% Triton X-100, 0.25% sodium deoxycolate, 150mM NaCl, 50mM Tris, pH 7.4) with protease inhibitor cocktail and phosphatase inhibitors. Proteins were resolved by SDS-PAGE, transferred to Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories), and probed with appropriate antibodies. Phosphorylation of these proteins was determined by immunoblot with anti-phospho–specific antibodies for 4E-BP1 (Thr37/46), p70S6K (Thr389), Akt (Ser473), PKCδ (Thr505), Foxo3a (Ser318/321), Erk1/2 (Thr202/Tyr204), Jnk (Thr183/Tyr185), p38 (Thr180/Tyr182) and p53 (Ser15) from Cell Signaling Technology. Anti-p53 and p21 antibodies were purchased from Santa Cruz Biotechnology. For loading control, antibodies for total proteins or β-actin were used. In the stimulation assay, IgE-sensitized BMMCs were resuspended in Tyrode buffer, and then left unstimulated or stimulated with DNP-HSA (20 ng/mL) for various times.
Quantitative real-time PCR
RNAs extracted using Trizol Reagent (Invitrogen) were reverse transcribed to cDNA by Superscript III and random primers. Quantitative real-time (qRT)–PCR was performed with Mastercycler realplex and SYBR Green master mix (Eppendorf). Expressed levels of target mRNAs were normalized with β-actin or snRNA U6, calculated using 2−ΔΔCT method. Primers were described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
For statistic analysis, 2-tail student t test was performed (*P < .05; **P < .01; ***P < .001).
Results
Effects of TSC1-deficiency on FcεRI-mediated signaling
To investigate the physiologic roles of TSC1 and the importance of regulating mTOR signaling in mast cells, we bred TSC1 conditional knockout (TSC1f/f) mice with ER-Cre mice to generate TSC1f/f-ER-Cre. BMMCs were generated in vitro from TSC1f/f (WT) and TSC1f/f-ER-Cre (TSC1KO) mice after injection of tamoxifen. TSC1 protein was undetectable in TSC1KO BMMCs (Figure 1A), confirming effective deletion of the gene. Moreover, TSC2 protein in these cells was markedly reduced, which supports previous reports that the stability of the TSC2 molecule is highly dependent on the presence of TSC1.27 TSC1KO BMMCs showed similar levels of FcεRI and c-Kit on their surface (Figure 1B) and similar mRNA levels for the FcεRI subunits compared with WT BMMCs (Figure 1C). These data suggest that the absence of TSC1 does not inhibit mast cell maturation in vitro.
FcεRI-mediated signaling in TSC1-deficient mast cells. (A) Immunoblot analysis of whole-cell lysates of WT and TSC1KO BMMCs (exp indicates exposure). (B) Flow cytometry of the surface expression of FcεRI and c-Kit (solid lines). Dotted line indicates control Ig staining. (C) qRT-PCR analysis for FcεRI subunits, α, β, γ and chains. Bar graphs represent mean ± SEM (au indicates arbitrary unit). (D) mTORC1 signaling induced by FcεRI stimulation. BMMCs were sensitized for 4 hours with IgE in IL-3-free media and rested for 1 hour in Tyrode buffer before stimulation with DNA-HSA. Phosphorylation levels of mTORC1 signaling molecules were assessed by immunoblot analysis with specific anti–phospho-antibodies as indicated. (E) Akt, PKCδ, and MAPK signaling events after FcεRI-stimulation. Total protein was used as a loading control. Data are representative of 5 experiments.
FcεRI-mediated signaling in TSC1-deficient mast cells. (A) Immunoblot analysis of whole-cell lysates of WT and TSC1KO BMMCs (exp indicates exposure). (B) Flow cytometry of the surface expression of FcεRI and c-Kit (solid lines). Dotted line indicates control Ig staining. (C) qRT-PCR analysis for FcεRI subunits, α, β, γ and chains. Bar graphs represent mean ± SEM (au indicates arbitrary unit). (D) mTORC1 signaling induced by FcεRI stimulation. BMMCs were sensitized for 4 hours with IgE in IL-3-free media and rested for 1 hour in Tyrode buffer before stimulation with DNA-HSA. Phosphorylation levels of mTORC1 signaling molecules were assessed by immunoblot analysis with specific anti–phospho-antibodies as indicated. (E) Akt, PKCδ, and MAPK signaling events after FcεRI-stimulation. Total protein was used as a loading control. Data are representative of 5 experiments.
We further examined the effect of TSC1 deficiency on FcεRI signaling. WT and TSC1KO BMMCs were sensitized with (DNP IgE followed by stimulation with DNP-HSA for the indicated times. Increased phosphorylation of p70S6K at Thr389 and 4E-BP1 at Thr37/46, which are mTORC1-mediated events, was detected in TSC1KO BMMCs compared with WT BMMCs (Figure 1D), but mTORC2-dependent Akt phosphorylation at Ser473 was decreased after FcεRI stimulation (Figure 1E). Together, these data demonstrate that TSC1 inhibits FcεRI-induced mTORC1 activation, and promotes mTORC2 activation.
FcεRI engagement also activates the MAPKs and PKCδ to control mast cell function.10,15,17 As shown in Figure 1E, Jnk1/2 and p38 phosphorylation was not obviously altered in TSC1KO BMMCs compared with WT BMMCs after FcεRI stimulation. In contrast, Erk1/2 and PKCδ phosphorylation, which correlates to their activation, was significantly increased. Thus, TSC1 may act as a negative regulator for FcεRI-induced Erk1/2 and PKCδ activation. How TSC1 negatively controls Erk1/2 and PKCδ activation remains to be investigated.
Differential effects of TSC1 deficiency on mast cell degranulation and cytokine production
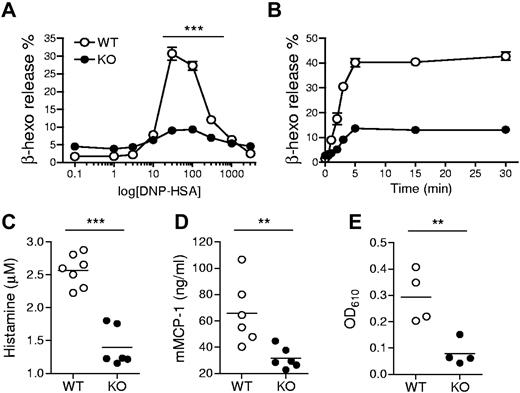
Given the ability of TSC1 to regulate multiple signaling events downstream of FcεRI, we examined whether TSC1 deficiency may affect FcεRI-mediated mast cell activation and allergic responses. We first tested BMMC degranulation by measuring β-hexosaminidase released with granular components. Anti-DNP IgE preloaded BMMCs were treated with DNP-HSA Ag to crosslink the receptor. The percentage of secreted β-hexosaminidase was increased with increasing Ag concentration, peaked at 30 ng/mL Ag, and declined with further increasing Ag concentration in WT BMMCs. However, β-hexosaminidase release from TSC1KO BMMCs at a broad range of Ag doses was substantially decreased compared with WT BMMCs (Figure 2A). The impaired degranulation in TSC1KO BMMCs was also observed in a time course experiment with an optimal Ag dose (Figure 2B). Next, we used the PSA assay to investigate whether TSC1 can regulate mast cell degranulation in vivo. To this end, TSC1f/f and TSC1f/f-ER-Cre mice were injected with tamoxifen, sensitized by intravenous injection of anti-DNP IgE, and then challenged with DNP-HSA. Blood samples were collected at 90 seconds or 30 minutes after Ag stimulation, and plasma was immediately isolated from the samples. As shown in Figure 2C-D, plasma histamine and mMCP-1 levels were notably reduced in TSC1-deficient mice compared with control mice. We further measured extravasation of Evan blue dye as an indicator for increased local blood vessel permeability using the PCA model. IgE-mediated Evan blue extravasation in ear tissue was significantly reduced in TSC1-deficient mice (Figure 2E). Thus, TSC1 plays an important role for mast cell degranulation.
Requirement of TSC1 IgE-induced mast cell degranulation. (A) Degranulation assessed by measuring release of β-hexosaminidase (β-hexo) from anti-DNP IgE-sensitized cells after stimulation with DNP-HSA at the indicated concentrations for 45 minutes. Data shown are mean ± SEM of triplicates and representative of 6 experiments. (B) Time course of mast cell degranulation using optimal Ag concentration (30 ng/mL). Data are representative of eleven experiments. (C-D) Impaired passive systemic anaphylaxis in TSC1 deficient mice. WT and TSC1KO mice were sensitized with anti-DNP IgE, after 24 hours, challenged with DNP-HSA, and the plasma histamine amounts (C) and mMCP-1 (D) were measured by ELISA (enzyme-linked immunosorbent assay) 90 seconds or 30 minutes after Ag challenge, respectively. (E) Impaired passive cutaneous anaphylaxis in TSC1 deficient mice. WT and TSC1KO mice were injected subcutaneously with IgE in ear, followed by DNP-HSA and Evan blue dye injection 24 hours later. The intensity of extracted dye from the ear collected 30 minutes after Ag challenge was measured by absorption at 610 nm (OD610). Data shown are representative of 3 (C-D) and 2 (E) experiments (**P < .01; ***P < .001).
Requirement of TSC1 IgE-induced mast cell degranulation. (A) Degranulation assessed by measuring release of β-hexosaminidase (β-hexo) from anti-DNP IgE-sensitized cells after stimulation with DNP-HSA at the indicated concentrations for 45 minutes. Data shown are mean ± SEM of triplicates and representative of 6 experiments. (B) Time course of mast cell degranulation using optimal Ag concentration (30 ng/mL). Data are representative of eleven experiments. (C-D) Impaired passive systemic anaphylaxis in TSC1 deficient mice. WT and TSC1KO mice were sensitized with anti-DNP IgE, after 24 hours, challenged with DNP-HSA, and the plasma histamine amounts (C) and mMCP-1 (D) were measured by ELISA (enzyme-linked immunosorbent assay) 90 seconds or 30 minutes after Ag challenge, respectively. (E) Impaired passive cutaneous anaphylaxis in TSC1 deficient mice. WT and TSC1KO mice were injected subcutaneously with IgE in ear, followed by DNP-HSA and Evan blue dye injection 24 hours later. The intensity of extracted dye from the ear collected 30 minutes after Ag challenge was measured by absorption at 610 nm (OD610). Data shown are representative of 3 (C-D) and 2 (E) experiments (**P < .01; ***P < .001).
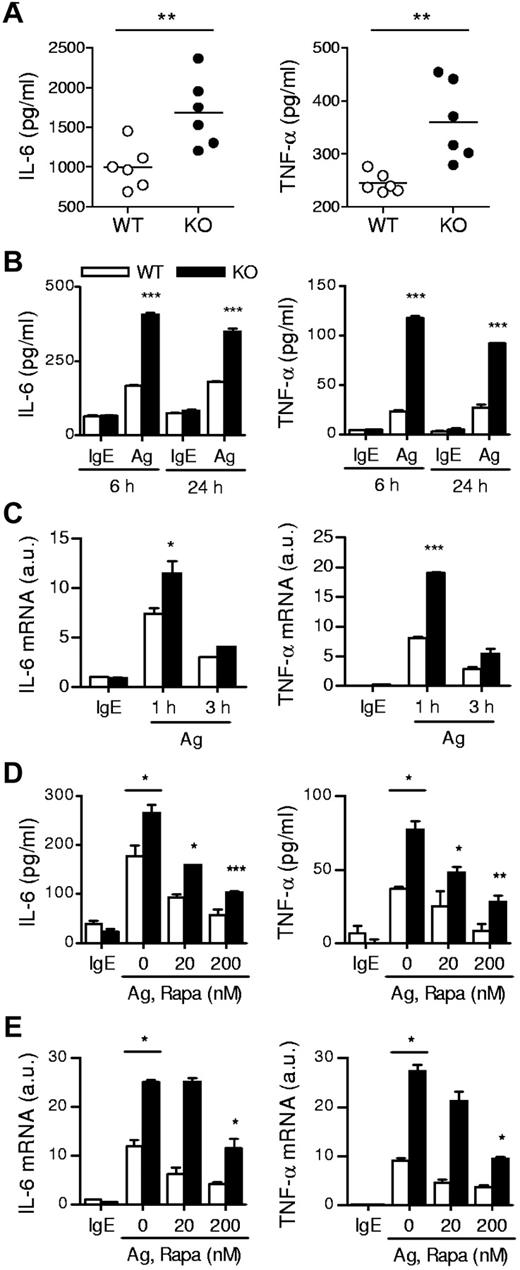
In addition to degranulation, mast cells produce and secrete proinflammatory cytokines such as IL-6 and TNF-α, which are important for mast cell mediated pathogenesis of diseases. In the PSA experiment, we also collected blood samples 180 minutes after Ag stimulation and measured plasma cytokine levels. As shown in Figure 3A, the amounts of plasma IL-6 and TNF-α were higher in TSC1-deficient mice than in WT controls. Consistent with these results, TSC1KO BMMCs produced higher levels of IL-6 and TNF-α than WT BMMCs after in vitro FcεRI stimulation (Figure 3B). Such increased cytokine production was correlated with elevated IL-6 and TNF-α mRNA levels in TSC1KO BMMCs, suggesting that TSC1 controls the production of these cytokines at least at the transcript level (Figure 3C). Thus, in contrast to its positive role for mast cell degranulation, TSC1 functions as a negative regulator of cytokine production in Ag stimulated mast cells.
IgE-mediated cytokine production in TSC1-deficient mice. (A) In vivo cytokine production on IgE-mediated PSA. Mice were sensitized and challenged as described in Figure 2C. IL-6 and TNFα in plasma 180 minutes after challenge were determined by ELISA. (B) ELISA for IL-6 and TNF-α production by BMMCs. IgE-sensitized cells were cross-linked with 30 ng/mL DNP-HSA (Ag) for 6 and 24 hours. (C) qRT-PCR analysis of mRNA encoding IL-6 and TNF-α after 1 and 3 hours of stimulation with Ag. (D) Effects of rapamycin on cytokine production. WT and TSC1KO BMMCs were stimulated with DNP-HSA for 6 hours in the presence or absence of rapamycin as indicated, and IL-6 and TNF-α in supernatants were measured by ELISA. (E) qRT-PCR of IL-6 and TNF-α transcripts after 1 hour of stimulation with DNP-HSA in the presence or absence of rapamycin. Data shown are representative of 5 experiments and bar graphs display mean ± SEM (au indicates arbitrary unit; *P < .05; **P < .01; ***P < .001).
IgE-mediated cytokine production in TSC1-deficient mice. (A) In vivo cytokine production on IgE-mediated PSA. Mice were sensitized and challenged as described in Figure 2C. IL-6 and TNFα in plasma 180 minutes after challenge were determined by ELISA. (B) ELISA for IL-6 and TNF-α production by BMMCs. IgE-sensitized cells were cross-linked with 30 ng/mL DNP-HSA (Ag) for 6 and 24 hours. (C) qRT-PCR analysis of mRNA encoding IL-6 and TNF-α after 1 and 3 hours of stimulation with Ag. (D) Effects of rapamycin on cytokine production. WT and TSC1KO BMMCs were stimulated with DNP-HSA for 6 hours in the presence or absence of rapamycin as indicated, and IL-6 and TNF-α in supernatants were measured by ELISA. (E) qRT-PCR of IL-6 and TNF-α transcripts after 1 hour of stimulation with DNP-HSA in the presence or absence of rapamycin. Data shown are representative of 5 experiments and bar graphs display mean ± SEM (au indicates arbitrary unit; *P < .05; **P < .01; ***P < .001).
TSC1 controls mast cell activation through mTORC1-dependent and independent mechanisms
To investigate the relationship between increased mTORC1 signaling by TSC1 deficiency and FcεRI-mediated mast cell responses, we treated WT and TSC1KO BMMCs with rapamycin at the indicated concentrations for 45 minutes, and then stimulated the cells by cross-linking FcεRI with DNP-HSA. mTORC1 is sensitive to rapamycin, whereas mTORC2 is relatively resistant to it. As shown in Figure 3D, administration of rapamycin reduced secretion of IL-6 and TNF-α from both WT and TSC1KO BMMCs in a dose dependent manner. In addition, mRNA levels of these cytokines were also decreased after rapamycin treatment (Figure 3E). These data suggest that mTORC1 signaling promotes mast cell cytokine production and that TSC1 regulates mast cell cytokine production by inhibiting mTORC1 activation. It is important to note that although rapamycin reduced mast cell cytokine production, significant cytokine production still occurred in TSC1KO BMMCs even when treated with 200nM of rapamycin. Furthermore, rapamycin was incapable of restoring the impaired degranulation in TSC1KO BMMCs (data not shown). Thus, these observations suggest that TSC1 regulates FcεRI-mediated mast cell activation through both mTORC1-dependent and independent mechanisms.
Increased mast cell death in the absence of TSC1
Mast cell maturation and survival are regulated by IL-3 and stem cell factor (SCF; ligand for c-Kit).31 mTOR integrates diverse stimuli and functions as a central regulator of cell growth and survival.18,19,32 These facts prompted us to investigate the effect of TSC1-deficiency on mast cell growth and survival. As shown in Figure 4A, TSC1KO BMMCs were larger than WT BMMCs, supporting the idea that TSC1 may negatively control cell growth by inhibiting mTORC1 activation. Although TSC1 deficiency increased mast cell size, we consistently observed less TSC1KO BMMCs than WT BMMCs when cultured in IL-3 sufficient medium (Figure 4B). The difference was further amplified when these cells were cultured in the absence of IL-3, as WT BMMC number gradually decreased after 2 to 3 days of culture, whereas TSC1KO BMMCs manifested an accelerated decline of cell numbers (Figure 4B). DNA contents of BMMCs cultured with IL-3 determined by propidium iodide (PI) staining showed similar percentages of WT and TSC1KO BMMCs at the S and G2/M phases (supplemental Figure 1), suggesting that TSC1 deficiency does not inhibit mast cell proliferation. However, more TSC1KO BMMCs were in the sub-G1 phase than WT BMMCs, indicating increased apoptosis of TSC1KO BMMCs. Increased apoptosis of TSC1KO BMMCs was confirmed by annexin V staining, which was further exacerbated after IL-3 withdrawal (Figure 4C). Thus, TSC1-deficient mast cells are more sensitive to apoptosis than WT mast cells, particularly in the absence of IL-3.
Loss of TSC1 increases mast cell apoptosis. (A) Increase in size of TSC1KO BMMCs determined by FACS analysis. Mean ± SEM of forward cell scatter (FCS-A) of 3 samples are shown. (B) Increased death of TSC1KO BMMCs. WT and TSC1KO BMMCs were cultured in the presence or absence of IL-3 for the indicated time. Viable cells were counted by trypan blue exclusion. Data shown are mean ± SEM from 3 paired samples. (C) Increased apoptosis of TSC1KO BMMCs. BMMCs were stained with annexin V and 7AAD after 48 hours IL-3 depletion. (D) mTORC1 signaling induced by SCF stimulation. BMMCs were rested for 1 hour in Tyrode buffer before stimulation with SCF (30 ng/mL). Phosphorylation of p70S6K, 4E-BP1, and Akt were assessed by immunoblot. Blots were stripped and probed for total protein control. (E) Increased death of TSC1KO BMMCs cultured in SCF. Cells were cultured at the indicated conditions for 30 hours and stained with annexin V. (F) Mast cell proliferation. CFSE-labeled WT and TSC1KO BMMCs were cultured in the presence of IL-3, SCF, or SCF ± IL-3 for 72 hours followed by FACS analysis. (G) Mast cell numbers in the spleen, lung, and skin. Mast cells in the spleen, lung, dorsal skin, and ears were stained with toluidine blue and were counted under a light microscope. WT and TSC1f/f-ERCre+ mice were injected with tamoxifen on days 1, 2, and 5 and were euthanized for harvesting spleen, lung, dorsal skin, and ear on day 14. Data shown are mean ± SEM of 4 to 7 mice. Top panels, representative toluidine blue staining of spleen thin sections. Bar graphs represent mean ± SEM of mast cell numbers per high power field (400×) from multiple mice. Data shown are representative of at least 3 experiments (**P < .01; ***P < .001).
Loss of TSC1 increases mast cell apoptosis. (A) Increase in size of TSC1KO BMMCs determined by FACS analysis. Mean ± SEM of forward cell scatter (FCS-A) of 3 samples are shown. (B) Increased death of TSC1KO BMMCs. WT and TSC1KO BMMCs were cultured in the presence or absence of IL-3 for the indicated time. Viable cells were counted by trypan blue exclusion. Data shown are mean ± SEM from 3 paired samples. (C) Increased apoptosis of TSC1KO BMMCs. BMMCs were stained with annexin V and 7AAD after 48 hours IL-3 depletion. (D) mTORC1 signaling induced by SCF stimulation. BMMCs were rested for 1 hour in Tyrode buffer before stimulation with SCF (30 ng/mL). Phosphorylation of p70S6K, 4E-BP1, and Akt were assessed by immunoblot. Blots were stripped and probed for total protein control. (E) Increased death of TSC1KO BMMCs cultured in SCF. Cells were cultured at the indicated conditions for 30 hours and stained with annexin V. (F) Mast cell proliferation. CFSE-labeled WT and TSC1KO BMMCs were cultured in the presence of IL-3, SCF, or SCF ± IL-3 for 72 hours followed by FACS analysis. (G) Mast cell numbers in the spleen, lung, and skin. Mast cells in the spleen, lung, dorsal skin, and ears were stained with toluidine blue and were counted under a light microscope. WT and TSC1f/f-ERCre+ mice were injected with tamoxifen on days 1, 2, and 5 and were euthanized for harvesting spleen, lung, dorsal skin, and ear on day 14. Data shown are mean ± SEM of 4 to 7 mice. Top panels, representative toluidine blue staining of spleen thin sections. Bar graphs represent mean ± SEM of mast cell numbers per high power field (400×) from multiple mice. Data shown are representative of at least 3 experiments (**P < .01; ***P < .001).
In addition to IL-3, SCF is a growth and survival factor for mast cells by activating c-Kit. We further investigated whether TSC1 regulates SCF-induced signaling. As shown in Figure 4D, SCF-induced S6K1 and 4E-BP1 phosphorylation was enhanced in TSC1KO BMMCs compared with WT BMMCs. Akt phosphorylation at S473 was slightly reduced at early time points, but was more obviously decreased at 30 minutes after SCF stimulation in TSC1KO BMMCs. Thus, TSC1 also functions as a negative regulator for mTORC1 signaling, but a positive regulator for mTORC2 signaling downstream of c-Kit. TSC1KO BMMCs displayed substantially increased death when cultured in SCF-containing medium, which was reduced in the presence of IL-3 (Figure 4E). Using a CFSE dilution assay, we observed that TSC1KO BMMCs proliferated similarly to WT BMMCs when cultured in SCF or SCF plus IL-3 containing medium for 72 hours (Figure 4F). To determine how TSC1 deficiency may affect mast cell survival in vivo, we injected tamoxifen on days 1, 2, and 5 into WT and TSC1f/f-ER-Cre mice. On day 14, we observed an approximately 50% decrease of mast cell numbers in the spleen and lung of TSC1f/f-ER-Cre mice. There was also a slight decrease of mast cells in the dorsal skin in TSC1f/f-ER-Cre mice (Figure 4G). Together, these data suggest that TSC1 is vital in mast cell survival but has minimal role in the control of mast cell proliferation.
Increased p53 accumulation but decreased mTORC2-Akt activation in TSC1KO BMMCs
To investigate the mechanisms by which TSC1 promotes mast cell survival, we evaluated mTOR signaling under IL-3–sufficient and depleted conditions. In IL-3–sufficient conditions, phospo-p70S6K and 4E-BP1 were detectable in WT BMMCs. After IL-3 withdrawal for 10 hours, the phosphorylation of 4E-BP1 and p70S6K was significantly decreased or even undetectable in WT BMMCs. However, in both conditions, the phosphorylation of these proteins was highly sustained in TSC1KO BMMCs (Figure 5A). In contrast, Akt phosphorylation at Ser473 was decreased in TSC1KO BMMCs compared with WT BMMCs (Figure 5B). Although Foxo3a phosphorylation at Ser318/321, an Akt dependent event, was not obviously decreased in TSC1KO BMMCs under IL-3–sufficient conditions, it was significantly lower than WT BMMCs under IL-3–depleted conditions. These data combined with the results of the SCF-induced signaling experiment suggest that TSC1 negatively controls mTORC1 activation but positively regulates mTORC2-Akt signaling in response to IL-3, SCF, and/or other growth factors in culture medium.
TSC1 deficiency increases p53 activity and miR-34a expression in mast cells. (A) Increased mTORC1 activation in TSC1KO BMMCs. Cell lysates from IL-3 sufficient or depleted cultures for 10 hours were analyzed by immunoblot with the indicated antibodies. (B) Decreased mTORC2 and Akt activities in TSC1KO BMMCs. Akt Ser473 and Foxo3a Ser318/321 phosphorylation was detected as in panel A. (C) Increased p53 phosphorylation and accumulation in TSC1KO BMMCs. (D) Expression of miR-34a. Total RNAs from IL-3 sufficient or depleted for 6 hours were isolated and miR-34a expression was determined by qRT-PCR after reverse transcription. (E) Overexpression of miR-34a promotes mast cell death. WT BMMCs were transduced with lentivirus encoding GFP plus primary miR-34a. After 72 hours, the cells were grown in IL-3–depleted media for 24 hours, and then stained with annexin V and 7AAD. Data shown are mean ± SEM of annexin V–positive apoptotic cells from multiple samples. (F) Effect of miR-34a neutralization on death of TSC1KO BMMCs. TSC1KO BMMCs were infected with lentivirus encoding GFP plus sponge for miR-34s (SPNG-34s). After 24 hours culture in IL-3 depleted media, the cells were stained, analyzed, and graphically presented of annexin V–positive apoptotic cells from multiple samples (mean ± SEM; *P < .05; ***P < .001).
TSC1 deficiency increases p53 activity and miR-34a expression in mast cells. (A) Increased mTORC1 activation in TSC1KO BMMCs. Cell lysates from IL-3 sufficient or depleted cultures for 10 hours were analyzed by immunoblot with the indicated antibodies. (B) Decreased mTORC2 and Akt activities in TSC1KO BMMCs. Akt Ser473 and Foxo3a Ser318/321 phosphorylation was detected as in panel A. (C) Increased p53 phosphorylation and accumulation in TSC1KO BMMCs. (D) Expression of miR-34a. Total RNAs from IL-3 sufficient or depleted for 6 hours were isolated and miR-34a expression was determined by qRT-PCR after reverse transcription. (E) Overexpression of miR-34a promotes mast cell death. WT BMMCs were transduced with lentivirus encoding GFP plus primary miR-34a. After 72 hours, the cells were grown in IL-3–depleted media for 24 hours, and then stained with annexin V and 7AAD. Data shown are mean ± SEM of annexin V–positive apoptotic cells from multiple samples. (F) Effect of miR-34a neutralization on death of TSC1KO BMMCs. TSC1KO BMMCs were infected with lentivirus encoding GFP plus sponge for miR-34s (SPNG-34s). After 24 hours culture in IL-3 depleted media, the cells were stained, analyzed, and graphically presented of annexin V–positive apoptotic cells from multiple samples (mean ± SEM; *P < .05; ***P < .001).
Tumor suppressor p53 is a transcription factor that regulates cell survival and death. Recently, it has been reported that highly activated mTOR by TSC mutations results in drastic stress-induced apoptosis in a p53-depenent manner and that p53 target genes Sestrin1/2 (Sesn1/2) inhibit mTORC1 through the activation of TSC2.33-35 We examined whether the increased apoptosis of TSC1KO BMMCs is related to p53 pathway. Phosphorylation at Ser15 and total protein level of p53 were obviously increased in TSC1KO BMMCs. Correlated with elevated p53, expression of p21WAF/Cip1, a major transcriptional target of p53, was increased in TSC1KO BMMCs in both IL-3–sufficient and depleted conditions (Figure 5C). In addition, mRNA levels of several other p53-regulated genes including Sesn1/2 and Bax were also increased in TSC1KO BMMCs (supplemental Figure 2). Together, these data indicate that accumulation and activation of p53 are enhanced in TSC1KO BMMCs, which may contribute to the decreased survival of TSC1KO BMMCs.
Increased miR-34a expression in TSC1KO BMMCs
MicroRNAs play critical roles in diverse cellular processes. miR-34a is directly transactivated by p53 and promotes cell death.36,37 Consistent with elevated p53 activity in TSC1KO BMMCs, miR-34a was also expressed at significantly higher levels in these cells than in WT BMMCs (Figure 5D). To investigate the effect of miR-34a on mast cell death, we generated GFP-coexpressing lentivirus encoding either miR-34a or sponge for miR-34a (SPNG-34s). SPNG-34s contains 6 copies of complementary seed for miR-34a to prevent miR-34a from targeting its authentic targets. Overexpression of miR-34a sensitized WT BMMCs to apoptosis in both IL-3–sufficient and depleted conditions (Figure 5E; supplemental Figure 3A) and neutralizing it with SPNG-34s significantly blocked IL-3 deprivation-induced apoptosis in TSC1KO BMMCs (Figure 5F; supplemental Figure 3B). These results suggest that TSC1 promotes mast cell survival by inhibiting the p53/miR-34a axis.
Decreased Bcl-2 expression in TSC1KO BMMCs
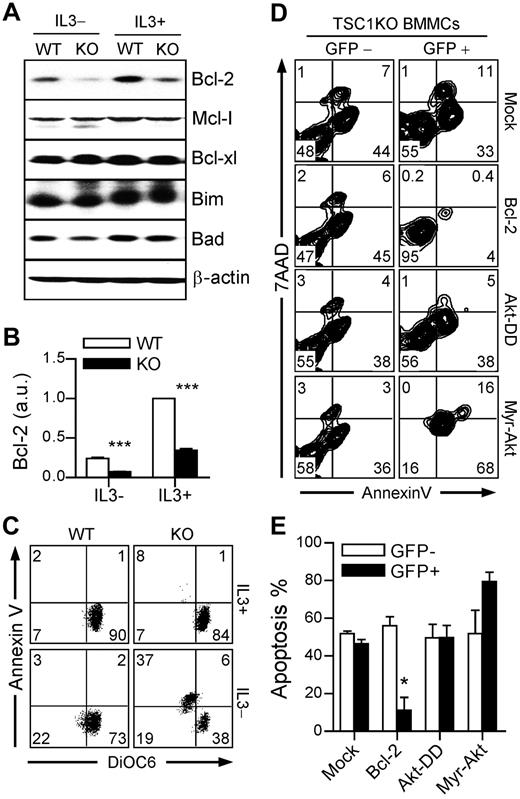
The Bcl-2 family is categorized into anti and proapoptotic molecules and the balance between these 2 subsets modulates cell life and death. The expression of the antiapoptotic molecule Bcl-2 can be negatively regulated by p53 through its transsuppressive activity,38 as well as through the induction of miR-34a to target Bcl-2 mRNA for degradation.37,39 As shown in Figure 6A-B, both Bcl-2 mRNA and protein levels were decreased in TSC1KO BMMCs compared with WT BMMCs in both IL-3–sufficient and depleted conditions. The levels of antiapoptotic Mcl-1 and Bcl-xL and proapoptotic Bim and Bad proteins were not obviously changed in TSC1KO BMMCs compared with WT BMMCs (Figure 6A). Thus, TSC1 deficiency leads to selectively down-regulation of Bcl-2 expression with up-regulation of p53/miR-34a. To examine an aspect of mitochondrial dysfunction, we measured mitochondrial membrane potential (ΔΨ) by staining cells with 3,3′-dihexyloxacarbocyanine iodide (DiOC6). Reduced staining of DiOC6 represents loss of ΔΨ. IL-3 depletion accelerated TSC1KO BMMC apoptosis, which was correlated with decreased DiOC6 (Figure 6C), indicating the hypersensitivity of TSC1KO BMMCs to mitochondrial dysfunction and apoptosis after survival factor withdrawal.
Decreased Bcl-2 expression contributes to TSC1-deficient mast cell death. (A) Bcl-2 family protein expression. Cell lysates from IL-3 sufficient or depleted cultures for 10 hours were analyzed by immunoblot with the indicated antibodies. (B) qRT-PCR to determine Bcl-2 mRNA expression. (C) Decreased mitochondrial potential in TSC1KO BMMCs. Analysis of ΔΨ by flow cytometry using DiOC6. (D-E) Rescue of TSC1KO BMMCs from death by expressing Bcl-2 but not CA-Akt. TSC1-deficient BMMCs were infected with retroviruses encoding GFP plus Bcl-2, Akt-DD, or Myr-Akt. GFP-positive populations were used to determine virus-infected cells. After 48 hours incubation in media without IL-3, cells were stained and analyzed. (D) Representative FACS plots. (E) Graphical presentation of annexin V–positive apoptotic cells. Data shown are representative of 3 experiments (*P < .05; ***P < .001).
Decreased Bcl-2 expression contributes to TSC1-deficient mast cell death. (A) Bcl-2 family protein expression. Cell lysates from IL-3 sufficient or depleted cultures for 10 hours were analyzed by immunoblot with the indicated antibodies. (B) qRT-PCR to determine Bcl-2 mRNA expression. (C) Decreased mitochondrial potential in TSC1KO BMMCs. Analysis of ΔΨ by flow cytometry using DiOC6. (D-E) Rescue of TSC1KO BMMCs from death by expressing Bcl-2 but not CA-Akt. TSC1-deficient BMMCs were infected with retroviruses encoding GFP plus Bcl-2, Akt-DD, or Myr-Akt. GFP-positive populations were used to determine virus-infected cells. After 48 hours incubation in media without IL-3, cells were stained and analyzed. (D) Representative FACS plots. (E) Graphical presentation of annexin V–positive apoptotic cells. Data shown are representative of 3 experiments (*P < .05; ***P < .001).
To determine whether decreased Bcl-2 expression and the aforementioned decrease of mTORC2-Akt signaling may contribute to the increased apoptosis of TSC1KO BMMCs, we transduced TSC1KO BMMCs with retrovirus coexpressing GFP with either Bcl-2 or constitutively active (CA) Akt. Akt is constitutively activated by N-myristoylation signal-fusion (Myr-Akt) or S473D/T308D phospho-mimic mutations (Akt-DD). After infection, these cells were cultured for 48 hours in the absence of IL-3. As shown in Figure 6D-E, all uninfected GFP– TSC1KO BMMCs had comparable apoptotic rates. Within infected GFP+ cells, apoptotic cell death was decreased only in Bcl-2 expressing, but not in vector control or CA-Akt expressing cells. Together, these data demonstrate that TSC1 plays a critical role in mast cell survival by increasing Bcl-2 expression.
Loss of TSC1 promotes mast cell apoptosis via ROS
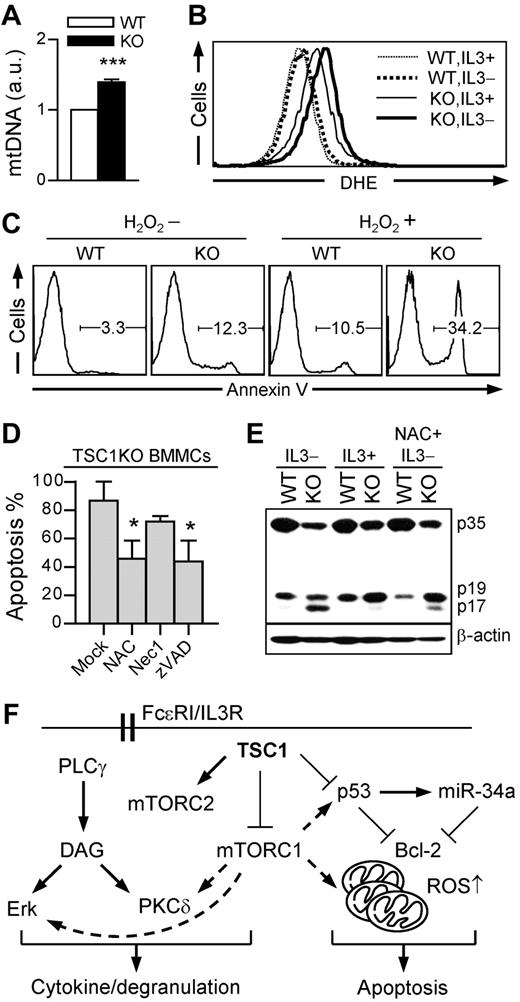
TSC1 deficiency in hematopoietic stem cells (HSCs) promotes mitochondrial biogenesis and induces the production of ROS, leading to impaired hematopoiesis because of decrease of their multipotency.40,41 Similar to TSC1-deficient HSCs, TSC1KO BMMCs also contain higher mitochondrial DNA copies than WT BMMCs (Figure 7A). Mitochondria are the major source of cellular ROS. DHE reacts with ROS and converts fluorescent ethidium to allow quantifying ROS levels by FACS (fluorescence-activated cell sorter) analysis. DHE staining revealed a higher ROS level in TSC1KO BMMCs than in WT BMMCs in the presence of IL-3, which was further increased after IL-3 depletion in TSC1KO BMMCs, but not in WT BMMCs (Figure 7B).
TSC1 deficiency promotes IL-3 depletion-induced mast cell apoptosis via ROS. (A) Increase of mitochondrial contents in TSC1KO BMMCs. Relative mitochondrial to nuclear DNA contents were analyzed by qRT-PCR using specific primers for 12S rRNA for mtDNA and 18S rRNA for nuclear DNA. (B) Increased ROS levels in TSC1KO BMMCs. ROS were analyzed by flow cytometry using DHE in the presence or absence of IL-3. (C) Hypersensitivity of TSC1KO BMMCs to H2O2-induced apoptosis. BMMCs were treated with H2O2 (100μM) in the presence of IL-3 for 24 hours, and then stained with annexin V. (D) Effect of ROS scavenger and pan-caspase inhibitor on IL-3 withdrawal-induced cell death in TSC1-deficient mast cells. TSC1KO BMMCs were cultured in IL-3–depleted media with the supplement of NAC (2mM), Nec1 (50μM), and zVAD (50μM). Annexin V–positive cells was measured by flow cytometry (mean ± SEM; *P < .05). (E) Increased caspase-3 cleavage in TSC1KO BMMCs. Cell lysates were subject to immunoblot using anti–caspase-3 antibody 24 hours after IL-3 withdrawal with or without NAC (2mM). p35 indicates procaspase-3; p19, partly processed caspase 3 fragment; and p17, fully cleaved, active form of caspase 3. Data shown are representative of 2 to 3 experiments. (F) Model for TSC1 function in mast cells. TSC1 inhibits mTORC1 as well as Erk1/2 and PKCδ but promotes mTORC2 activation in mast cells. Enhanced mTORC1, Erk1/2, and PKCδ activities in the absence of TSC1 may enhance mast cell cytokine production. However, TSC1 deficiency impairs mast cell degranulation. Furthermore, TSC1 inhibits p53/miR-34a and decreases the copy number of mitochondria, leading to increased Bcl-2 expression and decreased ROS levels to promote mast cell survival.
TSC1 deficiency promotes IL-3 depletion-induced mast cell apoptosis via ROS. (A) Increase of mitochondrial contents in TSC1KO BMMCs. Relative mitochondrial to nuclear DNA contents were analyzed by qRT-PCR using specific primers for 12S rRNA for mtDNA and 18S rRNA for nuclear DNA. (B) Increased ROS levels in TSC1KO BMMCs. ROS were analyzed by flow cytometry using DHE in the presence or absence of IL-3. (C) Hypersensitivity of TSC1KO BMMCs to H2O2-induced apoptosis. BMMCs were treated with H2O2 (100μM) in the presence of IL-3 for 24 hours, and then stained with annexin V. (D) Effect of ROS scavenger and pan-caspase inhibitor on IL-3 withdrawal-induced cell death in TSC1-deficient mast cells. TSC1KO BMMCs were cultured in IL-3–depleted media with the supplement of NAC (2mM), Nec1 (50μM), and zVAD (50μM). Annexin V–positive cells was measured by flow cytometry (mean ± SEM; *P < .05). (E) Increased caspase-3 cleavage in TSC1KO BMMCs. Cell lysates were subject to immunoblot using anti–caspase-3 antibody 24 hours after IL-3 withdrawal with or without NAC (2mM). p35 indicates procaspase-3; p19, partly processed caspase 3 fragment; and p17, fully cleaved, active form of caspase 3. Data shown are representative of 2 to 3 experiments. (F) Model for TSC1 function in mast cells. TSC1 inhibits mTORC1 as well as Erk1/2 and PKCδ but promotes mTORC2 activation in mast cells. Enhanced mTORC1, Erk1/2, and PKCδ activities in the absence of TSC1 may enhance mast cell cytokine production. However, TSC1 deficiency impairs mast cell degranulation. Furthermore, TSC1 inhibits p53/miR-34a and decreases the copy number of mitochondria, leading to increased Bcl-2 expression and decreased ROS levels to promote mast cell survival.
To determine whether the increased ROS may contribute to increased apoptosis of TSC1KO BMMCs, we treated these cells with H2O2 (a representative of ROS), N-acetyl-L-cysteine (NAC, a ROS scavenger), z-VAD-fmk (zVAD, a pan-caspase inhibitor), and necrostatin-1 (Nec1, a necroptosis inhibitor). As shown in Figure 7C, TSC1KO BMMCs were more sensitive to H2O2-induced apoptosis. Furthermore, administration of either NAC or zVAD significantly improved TSC1KO BMMC survival after IL-3 withdrawal (Figure 7D). In contrast, treatment of TSC1KO BMMCs with Nec1 could not rescue TSC1KO BMMCs from death. Correlated with increased apoptosis, the amount of cleaved active caspase-3 (p17) was increased in TSC1KO BMMCs under IL-3–sufficient conditions and further increased after IL-3 withdrawal, which was suppressed by NAC (Figure 7E). This data indicates that a caspase-dependent pathway participates in ROS-mediated mast cell death. Together, these observations suggest that increased ROS production may cause impaired mitochondria integrity of TSC1-deficient BMMCs, leading to the activation of caspases and apoptosis.
Discussion
mTOR integrates various cellular stimuli and participates in diverse biologic processes. Accumulating evidence has revealed critical roles of mTOR signaling in the immune system. In T cells, T-cell receptor stimulation induces both mTORC1 and mTORC2 activation, which is dependent on both the RasGRP1-Ras-Erk1/2 and PI3K-Akt pathways, and is inhibited by diacylglycerol kinases α and ζ.42 mTOR promotes effector T-cell differentiation, controls T-cell trafficking, inhibits generation of inducible regulatory T cells, and down-regulates memory T-cell responses.43,44 Tight control of mTOR signaling is critical for maintaining normal T-cell homeostasis.45 Both positive and negative roles of mTOR signaling have also been reported in TLR-induced responses in dendritic cells and macrophages.46 Previous studies using rapamycin have implicated mTOR signaling in regulating mast cell function and homeostasis.22-24 However, the importance of regulating mTOR signaling in mast cells is not well understood. In this report, we provide the first genetic evidence that TSC1 is a critical regulator for mast cell survival and function through multiple mechanisms.
The TSC1/2 complex has been mainly considered as a negative regulator of mTORC1 signaling by inhibiting Rheb activation. In TSC1-deficient mast cells, mTORC1 signaling is enhanced, but mTORC2 signaling is diminished. Thus, in addition to inhibiting mTORC1, TSC1 positively regulates mTORC2 in mast cells. TSC1 could promote mTORC2 signaling by inhibiting mTORC1 signaling. In HEK293 cells, p70S6K1 can phosphorylate rictor at Thr1135 to inhibit mTORC2 signaling.47 In addition, elevated mTORC1 signaling could trigger negative feedback mechanisms to inhibit upstream signaling to prevent mTORC2 activation as demonstrated in insulin receptor signaling.48 Lastly, TSC1 may promote mTORC2 signaling independent of mTORC1. It has been reported that TSC1/2 complex can directly associate with mTORC2 to promote mTORC2 signaling in cell line models.49 Further studies are needed to determine the exact mechanism by which TSC1 promotes mTORC2 activation in mast cells. Because of the drastic decrease of TSC2 protein in TSC1-deficient BMMCs, we cannot conclude that the abnormal phenotype of TSC1-deficient BMMCs is solely caused by loss of TSC1. It is possible that loss of TSC2 protein may contribute to the abnormality in TSC1-defcient BMMCs.
Our data revealed that TSC1 inhibits cytokine production but promotes degranulation by mast cells after FcεRI. Because cytokine production by TSC1 deficient mast cells can be partially suppressed by rapamycin, it supports the notion that increased mTORC1 activity contributes to the enhanced cytokine production by TSC1KO mast cells. It also suggests that other mechanisms in addition to mTORC1 may be regulated by TSC1 for proper cytokine production. Increased Erk1/2 and PKCδ activation in TSC1 deficient BMMCs may contribute to the enhanced cytokine production.14-17 At present, it is unclear how TSC1 deficiency inhibits mast cell degranulation. Enhanced PKCδ signaling and decreased Akt activity could be contributing factors. PKCδ and the PI3K/Akt pathway have been demonstrated to be able to inhibit or promote mast cell degranulation, respectively.17,23 Further studies are needed to understand how TSC1 may control PKCδ activation and mast cell degranulation.
We found that mast cell survival is dependent on TSC1. TSC1-deficient mast cells display increased p53 activities and ROS production, but decreased Akt activity and Bcl-2 expression. The decreased survival of TSC1-deficient mast cells can be partially rescued by ROS scavenger and Bcl-2 reconstitution, indicating that ROS production and decreased Bcl-2 expression contribute to the death of these cells. Bcl-2 is mainly expressed and functions in mitochondria to protect mitochondria from dysfunction. Diminished expression of Bcl-2 destroys its balance with proapoptotic Bcl-2 family members, inhibits its heterodimeric formation with proapoptotic proteins, and finally increases the susceptibility to death signals. Stress-activated p53 suppresses Bcl-2 by dual mechanisms: interfering with the activation of Bcl-2 promoter by Brn-3a transcription factor,38 and directly activating transcription of miR-34s, which can target the 3′ untranslated region of Bcl-2 mRNA.37,39 In mast cells, p53 may mainly control miR-34a, because we could not detect miR-34b/c expression (data not shown). The decreased Bcl-2 expression in TSC1KO BMMCs is correlated with increased p53 activity and up-regulation of miR-34a. Moreover, we showed that overexpression of miR-34a sensitizes mast cells to apoptosis and neutralization of it enhances TSC1KO mast cell survival. Our findings suggest that TSC1 inhibits p53/miR-34a to maintain Bcl-2 expression to promote mast cell survival.
It is worth noting that in spite of its well-known function in cell survival, constitutively active Akt could not protect TSC1KO BMMCs from growth factor withdrawal-induced apoptosis, but rather sensitized the cells to apoptosis. Akt could not inhibit ROS-mediated apoptosis, because its activation increases intracellular ROS through increasing mitochondrial activity and by inhibiting the expression of anti-oxidants.50 This indicates that increased intracellular ROS may play an important role for hypersensitivity of TSC1-deficient mast cells to growth factor depletion-induced apoptosis.
In summary, TSC1 is an important regulator in mast cells (Figure 7F). It inhibits FcεRI-induced cytokine production, but promotes degranulation, which were associated with down-regulation of mTORC1, Erk1/2, and PKCδ activities. In addition, TSC1 promotes mast cell survival through regulating ROS production, p53/miR-34a activation, and Bcl-2 expression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Weiguo Zhang, Jeff Rathmell, Eva Gottwein, and Bryan Cullen for providing reagents and Drs Michael Kulis and Tommy O'Brien for critical review of the paper.
This study is supported by funding from the National Institutes of Health (grants R01AI076357, R01AI079088, and R21AI079873), the American Cancer Society (RSG-08-186-01-LIB), the American Heart Association, and the Food Allergy and Anaphylaxis Network (to X.-P.Z).
National Institutes of Health
Authorship
Contribution: J.S. designed and conducted experiments, analyzed data, and wrote the paper; H.P. provided essential reagents; and X.-P.Z. supervised the project, designed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Ping Zhong, MD, PhD, 133 Medical Science Research Bldg, Research Dr, Dept of Pediatrics-Allergy and Immunology, Box 2644, Duke University Medical Center, Durham, NC 27710; e-mail: zhong001@mc.duke.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal