Abstract
This was a 24-week, multicenter phase- 2 study designed to assess safety, tolerability, and pharmacodynamics of FBS0701, a novel oral chelator, in adults with transfusional iron overload. Fifty-one patients, stratified by transfusional iron intake, were randomized to FBS0701 at either 14.5 or 29 mg/kg/d (16 and 32 mg/kg/d salt form). FBS0701 was generally well tolerated at both doses. Forty-nine patients (96%) completed the study. There were no drug-related serious adverse events. No adverse events (AEs) showed dose-dependency in frequency or severity. Treatment-related nausea, vomiting, abdominal pain, and diarrhea were each noted in < 5% of patients. Mean serum creatinine did not change significantly from Baseline or between dose groups. Transaminases wer increased in 8 (16%), three of whom acquired HCV on-study from a single blood bank while five had an abnormal baseline ALT. The 24 week mean change in liver iron concentration (ΔLIC) at 14.5 mg/kg/d was +3.1 mg/g (dw); 29% achieved a decrease in LIC. Mean ΔLIC at 29 mg/kg/d was −0.3 mg/g (dw); 44% achieved a decrease in LIC (P < .03 for ΔLIC between doses). The safety and tolerability profile at therapeutic doses compare favorably to other oral chelators. This trial was registered at www.clinicaltrials.gov as NCT01186419.
Introduction
Despite major advances in chelation therapy, morbidity and excess mortality remain a major problem for transfusion-dependent patients. The iron chelators currently in clinical use (deferoxamine, deferasirox, and deferiprone) are effective for many patients, but dosage form convenience, palatability, adverse events (AEs), and frank toxicity limit both full compliance and fully effective dosing.1 In addition, safety remains a concern for oral iron chelators.
FBS0701 is a novel, orally available member of the desazadesferrithiocin class of siderophore-related tridentate chelators currently in clinical development.2 In preclinical studies, FBS0701 bound Fe(III) with very high affinity and selectivity and demonstrated a more than or equal to 4-fold higher no-observable-adverse-effect level compared with deferasirox (Exjade), a tridentate chelator with similar formation constant with Fe(III), suggesting a favorable clinical safety profile, especially with respect to gastrointestinal and renal toxicity.3,4 Multidose safety and pharmacokinetic studies in transfusionally iron-overloaded patients established the acute safety of FBS0701 and the feasibility of once-a-day dosing.5
The objective of this study was to assess the safety, tolerability, and pharmacodynamics, including dose-response, of FBS0701 in adults with transfusion-dependent anemias.
Methods
Study design
This was a phase 2, multicenter, randomized, open-label study of adult patients with documented transfusional iron overload in need of chelation therapy. Patients were enrolled from 9 centers in the United States, Italy, United Kingdom, Turkey, and Thailand between September 2010 (first patient-first visit) and February 2011 (last patient-first visit). Patients received study treatment, FBS0701, in capsules, for 24 weeks.
This study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and International Clinical Harmonization guidelines and all applicable regulations governing human subject protections. The study protocol was approved by the institutional review board or ethics committees at each participating center before any patients were enrolled. Written informed consent was obtained from each patient before the patient underwent any protocol-mandated testing or procedures in accordance with the Declaration of Helsinki. All authors had access to clinical data.
Patients
Men and women 18 to 60 years of age with a diagnosis of transfusional iron overload were eligible. Transfusional iron overload was defined as liver iron concentration (LIC) by R2 (FerriScan) magnetic resonance imaging (MRI; ≥ 3.5 mg/g liver dry weight or serum ferritin > 500 ng/mL). Patients were excluded if they had at screening an LIC > 30 mg/g liver (dry weight), cardiac T2* MRI of less than 10 milliseconds (ms), left ventricular ejection fraction by MRI of less than 50%, platelet count at screening of less than 100 000/mL, alanine aminotransferase (ALT) of 200 IU/L at screening, or an ALT of more than 5 times the upper limit of normal on 2 occasions in the last 12 months or evidence of severe renal impairment (eg, > 1 g/day proteinuria or calculated creatinine clearance < 40 mL/min). All patients underwent a screening evaluation within 45 days of the start of dosing (day 1).
Treatment
FBS0701 (FerroKin BioSciences Inc) was provided as capsules in strengths of 50, 100, 250, and 375 mg of the salt form (MW440, active form MW400). Patients were instructed to orally administer capsules of FBS0701 in a fasted state. Patients were first stratified by transfusional iron intake to either “high” or “low” transfusion burden groups according to their calculated mean daily iron intake during the previous 6 transfusion sessions. Stratification by transfusion iron intake was necessary to account for the strong influence of iron intake on iron balance.6 Transfusion burden was defined as “high” if the average daily transfusional iron intake (TII) was more than 0.4 mg/kg per day and “low” if the mean daily TII was less than or equal to 0.4 mg/kg per day. Patients within each transfusion burden group were then randomized using an interactive web response system to one of 2 treatment arms: 14.5 or 29 mg/kg per day (active API). Patients were required to wash out from their previous chelation therapy for 1 to 5 days before dosing on day 1 depending on the chelator. Beginning day 1, patients received FBS0701 orally once daily for 24 weeks. At the conclusion of the first 24-week dosing period, patients who qualified and chose to continue treatment were allowed to do so for up to an additional 72 weeks. The “end of study” visit for the purposes of this phase 2 study report was at week 28; the majority of patients (82%) continued on FBS0701. For those patients not participating in the extension portion of the amended protocol, FBS0701 was discontinued at week 24, the previous chelation therapy was restarted after a 1-week washout of FBS0701, and an end-of-study evaluation was conducted in the clinic at week 28.
Safety and pharmacodynamic assessments
Safety and tolerability of FBS0701 were primary objectives of the study and were assessed in the clinic at day 1 and then weekly for weeks 1 to 4 of therapy, biweekly for weeks 6 to 8, then every 4 weeks during weeks 12 to 24. Safety assessments consisted of AE recording, physical examinations, vital signs measurements, clinical laboratory measures including biochemistry, hematology, coagulation, and urinalysis with microscopy, and ECGs. All clinical pathology determinations were performed at BARC Laboratories in Ghent, Belgium or Sydney, Australia. ECGs were performed at baseline, week 12, and week 24 and reviewed by an independent cardiologist (J.C.W.).
Pharmacodynamic response by LIC was assessed by FerriScan (Resonance Health) abdominal R2 MRI and reported by Resonance Health. MRIs were performed at baseline (1-2 weeks before dosing), approximately week 12 (± 10 days), and approximately week 24 (−10 to −5 days).
AEs
AEs were defined as any unexpected, unfavorable, harmful, or pathologic change in a patient as indicated by physical signs or symptoms, including intercurrent illnesses or injuries and/or clinically significant laboratory abnormalities occurring over the course of the 24-week treatment and 4-week posttreatment period. A serious AE (SAE) was defined as an event that was fatal or life-threatening, requiring hospitalization, surgery, or resulting in a persistent disability. All AEs and SAEs were assessed by each site investigator for their possible relationship to the study drug.
AEs were captured electronically on a standard case report form. Each investigator graded the severity of the AEs for their patients, including elevations in their transaminases. All AEs reported in this study were coded using the Medical Dictionary for Regulatory Activities. A safety review committee composed of the principal investigator, the sponsor's medical monitor, and a cardiologist reviewed safety and tolerability every 4 weeks.
Statistical analysis
Safety and tolerability.
Demographics, medical and surgical history data at screening, physical examination data including height and weight, vital signs, and ECG parameters were tabulated and summarized. Treatment-emergent AEs were listed and summarized at each visit. Clinical significance of abnormal laboratory values was assessed by each investigator. All patients who had taken at least one dose were included in the safety analysis consistent with an intention-to-treat analysis.
Pharmacodynamics, including dose-response.
A second primary objective was pharmacodynamics assessed as a difference in the proportion of patients in the 2 treatment arms with a net negative change in LIC over the 24-week treatment period and a difference in the mean change in LIC in the 2 treatment arms.
Statistical methods.
A sample size over 21 per arm was needed to provide the study with a statistical power of 0.80 to detect, within 6 months, an LIC difference of 3 mg/g liver (dry weight) between the 2 dose groups as determined by liver R2 MRI, or a difference between the 2 treatments arms in the proportion of patients with a net negative hepatic loss. Baseline characteristics within dose groups were compared using Fisher exact test. All transformed continuous parameters were subject to analysis of covariance with a critical value of 0.05 (95% CI). For 2 group comparisons, Student t and the Wilcoxon rank-sum test were used. Linear regression was applied to the data with determination of the correlation coefficient (R2). All tests were performed with SAS Version 9.1 software or higher. For safety and tolerability, all patients who had at least 1 dose were included in the analysis; missing data were the last value carried forward. Only patients who completed the 24-week dosing period and had at least baseline and week 24 MRI studies were included in the dose-response analysis.
Results
Patient disposition
Seventy-three patients consented; 51 passed screening and were randomized. All screening failures were the result of laboratory or clinical parameters outside the permissible range listed in the protocol; the most common causes were LVEF, T2* and LIC outside the permitted range. All 51 patients received at least 1 dose of FBS0701. Forty-nine patients completed the study. Two patients withdrew for reasons unrelated to FBS0701.
Patient demographics and clinical characteristics
All patients enrolled in this study were diagnosed with a hemoglobinopathy. Table 1 shows the primary hematologic diagnoses in the 51 randomized patients. The 2 treatment arms were generally balanced with respect to demographics and baseline disease characteristics (Table 2). The mean and median LIC, transfusion iron intake, and serum ferritin for the 2 groups were not significantly different. The average transfusion burden was between 0.35 and 0.38 mg iron/kg per day, and the average patient weight was 57 kg. Thus, the average patient received approximately 3387 mg of iron over the 168-day course of the study. In the absence of iron chelation therapy, the expected change in LIC would be approximately 6 mg/g liver (dry weight). The average daily TII in the 14.5-mg/kg per day dose cohort was positively correlated with increasing LIC at 24 weeks, whereas there was no significant influence of TII on changes in LIC in the 29-mg/kg per day dose cohort (data not shown). The median age of the patients was 23 years (range, 18-48 years).
Primary hematologic diagnoses transfusion burden of study population
| Diagnosis . | N (% of total) . | Mean transfusion burden, mg iron/kg per d (mean ± SD) . |
|---|---|---|
| β-thalassemia major | 38 (75) | 0.389 ± 0.106 |
| α-thalassemia major | 2 (4) | 0.278 ± 0.055* |
| Non-thalassemia major disorders | 11 (21) | 0.283 ± 0.083† |
| β-thalassemia intermedia‡ (transfused > 7/year) | 2 (4) | 0.203 ± 0.024* |
| HbE-β0 thalassemia | 6 (12) | 0.304 ± 0.098 |
| Sickle cell disease | 3 (6) | 0.293 ± 0.077 |
| Diagnosis . | N (% of total) . | Mean transfusion burden, mg iron/kg per d (mean ± SD) . |
|---|---|---|
| β-thalassemia major | 38 (75) | 0.389 ± 0.106 |
| α-thalassemia major | 2 (4) | 0.278 ± 0.055* |
| Non-thalassemia major disorders | 11 (21) | 0.283 ± 0.083† |
| β-thalassemia intermedia‡ (transfused > 7/year) | 2 (4) | 0.203 ± 0.024* |
| HbE-β0 thalassemia | 6 (12) | 0.304 ± 0.098 |
| Sickle cell disease | 3 (6) | 0.293 ± 0.077 |
Plus or minus range for N = 2.
Significantly different from β-thalassemia major (P = .0036 by unpaired t test).
Receiving at least 7 transfusions annually before and during study period.
Patient demographic characteristics and key baseline values
| Characteristic . | FBS0701 14.5 mg/kg per d (N = 24) . | FBS0701 29 mg/kg per d (N = 27) . | P . | Total (N = 51) . |
|---|---|---|---|---|
| Age (y) | .8946 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 28.7 (8.60) | 28.4 (7.11) | 28.5 (7.77) | |
| Median | 26.5 | 27.0 | 27.0 | |
| Range | 19-48 | 18-47 | 18-48 | |
| Sex | 1.0000 | |||
| Male | 12 (50.0%) | 13 (48.1%) | 25 (49.0%) | |
| Female | 12 (50.0%) | 14 (51.9%) | 26 (51.0%) | |
| Race | .8256 | |||
| Asian | 6 (25.0%) | 9 (33.3%) | 15 (29.4%) | |
| Black or white | 2 (8.3%) | 2 (7.4%) | 4 (7.8%) | |
| White | 16 (66.7%) | 16 (59.3%) | 32 (62.7%) | |
| LIC, mg/g liver, dry weight | ||||
| Baseline (mean ± SD) | 13.2 (6.64) | 13.9 (7.82) | ||
| Serum ferritin, ng/mL* | ||||
| Baseline (median) | 2564 | 2624 | ||
| Baseline (mean ± SD) | 3335 (2644) | 3162 (1682) | ||
| Mean of previous (3) transfusions, pretransfusion hemoglobin, g/dL | .2813 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 9.6 (0.56) | 9.8 (0.79) | 9.7 (0.69) | |
| Median | 9.6 | 9.8 | 9.6 | |
| Range | 8.2-10.4 | 8.3-11.4 | 8.2-11.4 | |
| Daily transfusion iron intake, mg/kg, mean (range)* | 0.36 (0.22-0.84) | 0.38 (0.17-0.60) | ||
| Weight, kg | .1771 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 57.0 (11.22) | 52.9 (10.07) | 54.8 (10.71) |
| Characteristic . | FBS0701 14.5 mg/kg per d (N = 24) . | FBS0701 29 mg/kg per d (N = 27) . | P . | Total (N = 51) . |
|---|---|---|---|---|
| Age (y) | .8946 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 28.7 (8.60) | 28.4 (7.11) | 28.5 (7.77) | |
| Median | 26.5 | 27.0 | 27.0 | |
| Range | 19-48 | 18-47 | 18-48 | |
| Sex | 1.0000 | |||
| Male | 12 (50.0%) | 13 (48.1%) | 25 (49.0%) | |
| Female | 12 (50.0%) | 14 (51.9%) | 26 (51.0%) | |
| Race | .8256 | |||
| Asian | 6 (25.0%) | 9 (33.3%) | 15 (29.4%) | |
| Black or white | 2 (8.3%) | 2 (7.4%) | 4 (7.8%) | |
| White | 16 (66.7%) | 16 (59.3%) | 32 (62.7%) | |
| LIC, mg/g liver, dry weight | ||||
| Baseline (mean ± SD) | 13.2 (6.64) | 13.9 (7.82) | ||
| Serum ferritin, ng/mL* | ||||
| Baseline (median) | 2564 | 2624 | ||
| Baseline (mean ± SD) | 3335 (2644) | 3162 (1682) | ||
| Mean of previous (3) transfusions, pretransfusion hemoglobin, g/dL | .2813 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 9.6 (0.56) | 9.8 (0.79) | 9.7 (0.69) | |
| Median | 9.6 | 9.8 | 9.6 | |
| Range | 8.2-10.4 | 8.3-11.4 | 8.2-11.4 | |
| Daily transfusion iron intake, mg/kg, mean (range)* | 0.36 (0.22-0.84) | 0.38 (0.17-0.60) | ||
| Weight, kg | .1771 | |||
| N | 24 | 27 | 51 | |
| Mean (SD) | 57.0 (11.22) | 52.9 (10.07) | 54.8 (10.71) |
Supplemental Figure 3 shows the transfusion burdens, and supplemental Figure 4 shows the ferritin levels in detail.
Prior chelation therapy
Available chelation treatment at the time of screening is tabulated (Table 3). Only 1 patient, a 42-year-old with Hb S/S sickle cell disease, was completely naive to chelating therapy. Deferiprone was the most common agent (55%) as either monotherapy or in combination with deferasirox or deferoxamine. The chelating history for each patient is extensive; nevertheless, the majority of patients (78%) randomized to this study had moderate to severe iron overloading defined as LIC of 7 mg/g liver (dry weight) or greater. Only 11 patients who entered the study could be said to have clinically desirable LICs (ie, < 7 mg/g liver dry weight). These data are consistent with the general observation that clinical management for many patients remains suboptimal or that this study was biased in favor of enrollment of patients that were more difficult to manage for whatever reason.
Prior chelation therapy
| Chelation regimen at screening . | No. (%) . |
|---|---|
| Deferoxamine | 6 (12) |
| Deferasirox | 11 (21) |
| Deferiprone | 15 (29) |
| Deferoxamine + deferiprone | 12 (22) |
| Deferoxamine + deferasirox | 3 (6) |
| Deferiprone + deferasirox | 1 (2) |
| No chelation therapy at screening* | 2 (4) |
| Naive to chelation therapy | 1 (2) |
| Chelation regimen at screening . | No. (%) . |
|---|---|
| Deferoxamine | 6 (12) |
| Deferasirox | 11 (21) |
| Deferiprone | 15 (29) |
| Deferoxamine + deferiprone | 12 (22) |
| Deferoxamine + deferasirox | 3 (6) |
| Deferiprone + deferasirox | 1 (2) |
| No chelation therapy at screening* | 2 (4) |
| Naive to chelation therapy | 1 (2) |
Two patients with side effects to deferasirox were off chelator for at least 30 days before screening.
Safety evaluation
At least 1 AE was reported during the treatment period in 86% of the patients. A total of 51% of patients had AEs possibly or probably related to FBS0701 (treatment-related). All treatment-related AEs are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Five SAEs were reported in 2 patients: 4 in the first patient and 1 in the second patient. None of the SAEs (dyspnea, chest discomfort, sickle cell crisis, peripheral neuropathy, and hyperglycemia in the setting of diabetes mellitus) was considered by the investigators to be treatment related. No patients died during the study. A grade 2 elevation in ALT was reported in one patient who was subsequently shown to have acquired a new case of hepatitis C virus (HCV). In that patient, FBS0701 was discontinued and the patient withdrawn from the study. Two other subjects at the same site at the same time were also identified as having acquired a genotypically identical HCV infection, and the mechanism of infection is unknown; those 2 patients had moderate elevations in ALT and continued dosing for the duration of the study uneventfully.
All treatment-related AEs were mild or moderate in severity. The most common treatment-related AE was increased transaminases, which occurred in 8 patients (16%). Three of these patients (403, 404, and 405) became simultaneously infected with HCV while on study. Three other patients (304, 305, and 402) had increases of approximately 2- to 5-fold over their baseline value (∼ 7 times upper limit of normal), each of whom had abnormal transaminases at baseline, the highest elevations occurring in 2 patients (304 and 305; randomized to the lower dose); neither had a history of hepatitis. The third patient (402), dosed at 29 mg/kg, was HCV positive at the start of the study. FBS0701 treatment was discontinued in all 3 patients, with transaminases decreased during the 2- to 3-week drug interruption and restarted at the same dose whereupon ALT remained stable to the end of the study.
Treatment-related gastrointestinal AEs were reported in 29% of patients. The most common event was flatulence (12%, N = 6). Fewer than 5% of patients reported the following treatment-related gastrointestinal AEs: nausea, vomiting, abdominal pain, constipation, and colitis. All were mild and self-limited. One patient in each dose group reported mild transient arthralgias.
There were no trends or dose-dependent changes in serum creatinine (Figure 1). One patient (0506) with sickle cell disease randomized to the 29 mg/kg per day dose had a baseline serum creatinine of 0.93 mg/dL and a calculated creatinine clearance of 57 mL/min; serum creatinine gradually increased to 1.37 mg/dL. FBS0701 was discontinued, serum creatinine returned to normal, and the patient was restarted at the lower 14.5-mg/kg per day dose whereupon creatinine remained within 10% of baseline to the end of the study. A second patient (1106) had an increase in serum creatinine in the setting of diabetic nephropathy, glomerular nephritis with generalized granulomatous disease; the change in creatinine was not considered treatment-related by the investigator. There were no reductions in absolute neutrophil counts and no treatment-related rashes.
Creatinine measures by dose group. The mean creatinine values at each of the study visits shown are represented by mean ± SD. ○ represents the low-dose cohort; and ▵, the high-dose cohort. No significant difference between groups was noted at any time during the study, and the week 24 and study week 28 values did not differ from baseline. Most patients remained on drug for the week 28 measures, in the extension phase of the study. “Week 0” indicates baseline values before administration of FBS0701. Details of creatinine by patient and by treatment group are presented in supplemental Figure 1.
Creatinine measures by dose group. The mean creatinine values at each of the study visits shown are represented by mean ± SD. ○ represents the low-dose cohort; and ▵, the high-dose cohort. No significant difference between groups was noted at any time during the study, and the week 24 and study week 28 values did not differ from baseline. Most patients remained on drug for the week 28 measures, in the extension phase of the study. “Week 0” indicates baseline values before administration of FBS0701. Details of creatinine by patient and by treatment group are presented in supplemental Figure 1.
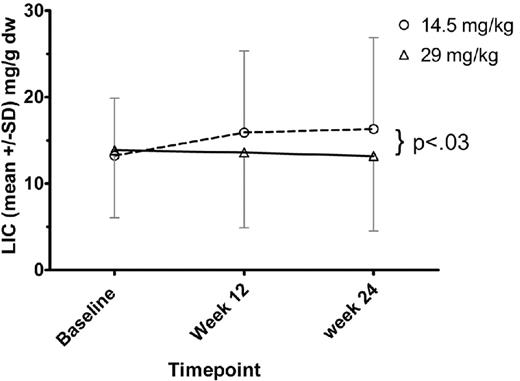
Dose-response evaluation
Twenty-four patients in the low-dose group and 25 of the high-dose group had baseline and 24 week R2 MRI (FerriScan) studies. (This analysis excludes patient 0713 with only baseline MRI data and patient 0403 with only baseline and week 12 MRI data.) The mean LIC for each treatment group at baseline, week 12, and week 24 is shown in Figure 2. In the low-dose group, 7 of 24 (29%) had a lower LIC at 24 weeks compared with baseline. The mean change in LIC in the low-dose group was a net gain of 3.1 mg/g liver (dry weight; SD, 5.55 mg/g liver [dry weight]). In the high-dose group, 11 of 25 (44%) had a lower LIC at 24 weeks compared with baseline. The mean change in LIC in the high-dose group was a net loss of 0.3 mg/g liver (dry weight; SD, 4.95 mg/g liver [dry weight]). The difference in the mean LIC change at 24 weeks between treatment groups was 3.4 mg/g liver (dry weight). This difference was statistically significant (P < .03).
LIC assessed by FerriScan (R2) MRI. For each dose cohort, mean ± SD of the LIC values are shown. ○ represents the low-dose cohort; and ▵, the high-dose cohort. At week 24 MRI assessment, the difference between dose cohorts was significant (P < .03).
LIC assessed by FerriScan (R2) MRI. For each dose cohort, mean ± SD of the LIC values are shown. ○ represents the low-dose cohort; and ▵, the high-dose cohort. At week 24 MRI assessment, the difference between dose cohorts was significant (P < .03).
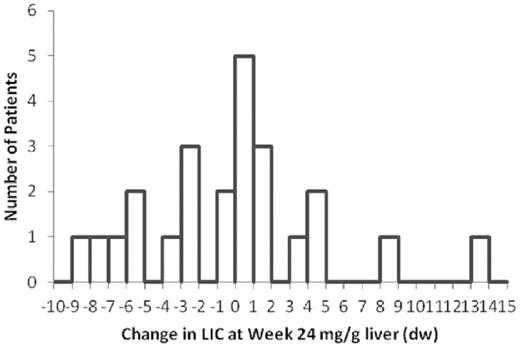
The distribution of the changes in LIC in the 29-mg/kg per day group is shown in Figure 3. Fourteen patients in this dose group showed an increase in LIC at 24 weeks. Nine of the 14 (64%) had an LIC increase of between 0.1 and 1.6 mg/g liver (dry weight).
Distribution of LIC changes compared with baseline in the 29 mg/kg per day dose cohort, assessed at week 24. Histogram depiction of the number of patients in the high-dose cohort who achieved specified change in LIC compared with baseline. A statistical analysis is presented in supplemental Figure 2.
Distribution of LIC changes compared with baseline in the 29 mg/kg per day dose cohort, assessed at week 24. Histogram depiction of the number of patients in the high-dose cohort who achieved specified change in LIC compared with baseline. A statistical analysis is presented in supplemental Figure 2.
A covariate analysis of factors that might influence response, including baseline LIC, primary diagnosis, compliance, study site, or patient demographics, did not identify any variables that might accurately predict the magnitude of response.
Discussion
The objectives in this phase 2 study were to assess the safety, tolerability, and hepatic iron-clearing response of 2 doses of FBS0701 in transfusionally iron-overloaded patients. The doses were selected based on rat and primate data in which iron clearing was measured. Those models predicted that 14.5 and 29 mg/kg of FBS0701 would induce the excretion of approximately 300 and 600 μg of iron/kg per day, representing the clinical goal of chelation therapy. This 2-fold dose difference was predicted to be sufficient to observe a difference in iron-clearing response over a treatment period of 24 weeks as assessed by R2 MRI (FerriScan).
FBS0701 was generally well tolerated. The most common treatment related AEs were elevated transaminases (16%, N = 8), flatulence (12%, N = 6), headache (6%, N = 3), abdominal pain (6%, N = 3), and chromaturia (6%, N = 3). The frequency or severity of AEs did not show dose dependency. Elevations in transaminases occurred in 8 patients, 3 of whom acquired HCV at one time, by unknown mechanism, at a single site (this non–drug-related, unexpected AE is under investigation). The other 5 patients (2 HCV+ at baseline) all had abnormal ALT values at baseline (day 1). Notably, there was a low incidence of each specific gastrointestinal treatment-related side effect; only flatulence was reported in more than 5% of patients. There were no dose-dependent changes in laboratory values, including creatinine or ALT and no rashes. Three patients had increases of serum creatinine beyond the ULN but none exceeding 40%; the change in creatinine in one patient was treatment related. All laboratory changes were reversible.
In the safety analysis, particular attention was paid to the effect of FBS0701 on renal function. Serum creatinine was determined weekly for the first 4 weeks of dosing to increase the sensitivity of the study to detect early changes in serum creatinine as it has been reported that deferasirox causes changes in serum creatinine shortly after the initiation of therapy. Over the 24-week course of the CTP-04 study, serum creatinine increased an average of 5%. The change from baseline to 24 weeks was not statistically different. Among the 49 patients who completed the 24-week dosing period, only 2 patients (4%) had an increase in serum creatinine of greater than 30% above baseline. Analysis of changes in serum creatinine between treatment arms (14.5 and 29 mg/kg per day) showed no dose dependency. The renal safety profile of FBS0701 is distinct from that published for deferasirox.7-11 These data demonstrate that the threshold for dose-dependent changes in renal function for FBS0701 exceeds 29 mg/kg per day.
With respect to hepatic iron clearing activity, there was a clear dose-dependent response that was statistically significant. Given the average transfusion burden in this study, without treatment, patients would have accumulated approximately 6 mg/g liver (dry weight); hence, both treatment arms resulted in clinically significant ongoing iron removal, even in patients without net iron reduction. Of the 14 patients in the 29-mg/kg per day treatment arm that had an LIC increase, 9 showed increase of 1.6 mg/g or less (Figure 3), suggesting that a small increase in dose may be sufficient to induce net hepatic iron loss in a significant proportion of patients. This hypothesis is currently being tested: patients who at 24 weeks had a suboptimal clinical response at their randomized dose were dose-adjusted to doses as high as 36 mg/kg per day (40 mg/kg salt form) in a 72-week extension of the current study. In addition, those patients randomized to the 14.5 mg/kg per day treatment arm who showed an insufficient change in LIC at week 24 were dose-adjusted to 32 or 40 mg/kg per day. Future analyses of the extension study will reveal to what extent these increases succeed in net iron removal from all of the patients with elevated LIC.
Patients were stratified by their historical average daily transfusion iron intake to minimize the influence this variable is known to have on response.6 However, we did not observe a significant correlation between dose-response and transfusional iron intake, perhaps because the distribution of TII was relatively narrow (data not shown). Indeed, there were no covariants that might accurately predict the magnitude of response. The cardiac measures of iron concentrations were not statistically different from baseline in both dose groups; 87% of the patients had normal baseline values (ie, > 20 ms). This parameter will be reassessed during the 72-week extension phase of this study.
In conclusion, FBS0701 was generally well tolerated for 24 weeks in iron-overloaded patients. A clear dose-response was observed. Given the absence of dose-dependent AEs, including changes in serum creatinine and transaminases, higher doses of FBS0701 might be expected to safely decrease LICs in a greater proportion of patients.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maggie Neptune and Laura Eichorn of FerroKin BioSciences; Jennifer Eile and Pamela Boardman of Children's Hospital (Boston, MA); Jacqueline Madden and Elliott Vichinsky of Children's Hospital & Research Center (Oakland, CA); Dr Maciej Garbowski of University Hospital London (United Kingdom); Dr Bunchoo Pongtanakul, Siriraj Hospital (Bangkok, Thailand); Sue Robson and Emma Prescott of Whittington Hospital (London, United Kingdom); Dr Patrizia Porqueddu, Università degli Studi di Cagliari (Italy); Dr Silvia Caviglia, Centro della Microcitemia e delle Anemia Congenite-Ematologia, Ospedale Galliera (Genoa, Italy); Dr Silvia Fracchia, Scienze Cliniche Biologiche (Torino, Italy); and Raziye Işın, Ege University Hospital, Izmir, Turkey.
This study was sponsored by FerroKin BioSciences Inc. The project was also supported in part by NIH/NCRR CTSA grants UL1RR024131 (P.H.), and UL1RR025758 (E.J.N. and R.F.G.).
“Medical Dictionary for Regulatory Activities” terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturer and Associations (IFPMA).
Authorship
Contribution: H.Y.R., E.J.N., R.G., J.C.W., G.L.F, A.P., F.T.S., Y.A., A.J., and J.P. designed the study; J.P., A.J., and H.Y.R. managed study conduct; H.Y.R. and E.J.N. wrote the manuscript; H.Y.R., J.C.W., and E.J.N analyzed the data; E.J.N., H.Y.R., J.C.W., A.J., and J.P. edited the paper; and E.J.N., F.T.S., R.G., V.V., Y.A., R.F.G., A.P., G.L.F., P.H., F.T.S., and J.B.P. served as investigators on this trial, enrolled patients, and reviewed and provided comments on the manuscript.
Conflict-of-interest disclosure: J.B.P. received consulting fees, research grant funding, and lecture fees from Novartis Pharmaceuticals and consulting fees from Vifor International and Mundipharma. Y.A. received research grant support, consulting fees, and lecture fees from Novartis Pharmaceuticals. R.G. received consulting fees from Apopharma. V.V. received research grant support and lecture fees from Novartis Pharmaceuticals and research grant support from GPO-L-ONE and the National Research University and BIOTEC, Thailand. J.C.W., G.L.F., and P.H. received research support from Novartis. H.Y.R., A.J., and J.P. are employees of the sponsor, FerroKin BioSciences Inc, whose product was studied in the present work, and hold equity in the company. The remaining authors declare no competing financial interests.
Correspondence: Hugh Young Rienhoff, FerroKin Biosciences Inc, 2729 Debbie Ct, San Carlos, CA 94070; e-mail: hugh@ferrokin.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal