Abstract
Two novel oral anticoagulants, dabigatran and rivaroxaban, have recently been approved. They differ in many ways from warfarin, including rapid onset of action, shorter half-life, fewer drug-drug interactions, lack of need for monitoring, and no need for titration or dose adjustments. These novel agents represent a landmark shift in anticoagulant care; however, many aspects of their use will be unfamiliar to practicing clinicians, despite the imminent widespread use of these agents in the community. The management of these anticoagulants when transitioning from or back to warfarin, around surgery or in case of major hemorrhage, requires knowledge of their pharmacokinetics and mechanism of action. Unfortunately, there is a limited evidence base to inform decisions around management of these agents. We present our practice in these settings supported, where available, with literature evidence.
Introduction
Dabigatran has become available for long-term use in the United States and Canada at the end of 2010 and is the first novel antithrombotic agent approved for stroke prophylaxis in atrial fibrillation since the introduction of warfarin more than 50 years ago.1 The Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) study provided high-quality evidence that, when administered at a dose of 150 mg twice daily, dabigatran is associated with a reduced risk of stroke and a similar risk of bleeding as warfarin when targeted to an international normalized ratio (INR) of 2.0 to 3.0.2 When administered at a dose of 110 mg twice daily, the risk of stroke with dabigatran is similar to that of warfarin while producing less bleeding. This dose is approved in Canada but not in the United States because it did not reduce the risk of stroke compared with warfarin and stroke prevention was considered more important than the benefit of decreased risk for extracranial major bleeding. The dose of 75 mg twice daily is, however, recommended in the United States for patients with severe renal failure (calculated creatinine clearance 15-30 mL/min). Dabigatran has also been approved for the prevention of deep vein thrombosis after orthopedic surgery in Canada, Europe,3 and several other countries, and has been studied in the setting of long-term secondary prevention for venous thromboembolism.4 Peer organization guidelines now recommend the use of dabigatran for many patients with atrial fibrillation.5 The oral factor Xa inhibitor, rivaroxaban, has also been approved for prevention of stroke in atrial fibrillation and of venous thrombosis after orthopedic surgery.6
Clinicians are intimately familiar with warfarin. It has 100% name recognition internationally, monitoring for its anticoagulant effect is routinely available, and physicians are aware of the need to interrupt warfarin for many interventional procedures.7 There are established and highly effective treatment strategies for bleeding in patients receiving warfarin and for the management of unanticipated excess anticoagulant effect.8,9 Dabigatran and rivaroxaban, as novel agents, are relatively unknown to the practicing community. Further, there is little or no evidence to guide practical management when patients present with a need for any interventional procedure or who present with bleeding complications.
Despite the perceived inconvenience of warfarin, studies of anticoagulated patients have generally failed to demonstrate any significant negative impact of this treatment on quality of life.10,11 Furthermore, substantial variability in health state valuations and treatment preferences between patients indicates the need for individual tailoring of anticoagulant therapy.12
This review is designed to provide clinicians with a practical approach to the management of oral anticoagulation in stroke prophylaxis, including suggestions for the choice of agent, and guidance regarding switching between agents, perioperative discontinuation and resumption of the new agents, and treatment of bleeding. We acknowledge that there is little evidence in this domain and that our recommendations are based largely on expert opinion.
Selection of patient groups for warfarin or the new anticoagulants
For warfarin
Good level of control.
The level of control of warfarin therapy and its interaction with the treatments in the RE-LY study was recently analyzed.13 The authors measured the level of control of the INR as the percent time in therapeutic range at the study center level (cTTR) and divided the cTTR results into quartiles, corresponding to less than 57.1%, 57.1% to 65.5%, 65.5% to 72.6%, and more than 72.6%. The last 2 groups would be considered having good or excellent control, respectively. Table 1 shows the hazard ratios for different outcomes for these 2 groups of high cTTR for 150 mg of dabigatran twice daily versus warfarin. In these well-controlled patients, dabigatran (150 mg twice daily) was not superior to warfarin for prevention of stroke. Dabigatran was associated with a doubling of the risk of major gastrointestinal bleeding and a lower risk of intracranial bleeding. The American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (among other groups) has recently published a supplementary report on the use of dabigatran for atrial fibrillation.5 This guideline uses rigorous methodology and has a robust conflict of interest policy. The guideline provides a class I, level of evidence B recommendation for dabigatran as an agent to prevent stroke in patients with atrial fibrillation. However, the guideline notes, “Because of the twice daily dosing and greater risk of nonhemorrhagic side effects … patients already taking warfarin with excellent INR control may have little to gain by switching to dabigatran.” This recommendation suggests that patient values and preferences should influence the decision to initiate dabigatran.
Risk of important clinical outcomes with dabigatran 150 mg twice daily versus adjusted warfarin therapy that has a good or excellent level of control
| Outcome, dabigatran vs warfarin . | cTTR of 65.5%-72.6%, HR (95% CI) . | cTTR of > 72.6%, HR (95% CI) . |
|---|---|---|
| Stroke and systemic embolism (primary outcome) | 0.69 (0.44-1.09) | 0.95 (0.65-1.48) |
| Stroke, systemic embolism, pulmonary embolism, myocardial infarction, or cardiovascular death | 0.94 (0.72-1.22) | 1.19 (0.90-1.57) |
| Total death | 0.98 (0.75-1.28) | 1.08 (0.81-1.44) |
| Major bleeding | 1.13 (0.87-1.48) | 1.16 (0.88-1.54) |
| Intracranial bleeding | 0.35 (0.15-0.82) | 0.39 (0.18-0.84) |
| Major gastrointestinal bleeding | 2.26 (1.50-3.40) | 2.00 (1.25-3.21) |
| Outcome, dabigatran vs warfarin . | cTTR of 65.5%-72.6%, HR (95% CI) . | cTTR of > 72.6%, HR (95% CI) . |
|---|---|---|
| Stroke and systemic embolism (primary outcome) | 0.69 (0.44-1.09) | 0.95 (0.65-1.48) |
| Stroke, systemic embolism, pulmonary embolism, myocardial infarction, or cardiovascular death | 0.94 (0.72-1.22) | 1.19 (0.90-1.57) |
| Total death | 0.98 (0.75-1.28) | 1.08 (0.81-1.44) |
| Major bleeding | 1.13 (0.87-1.48) | 1.16 (0.88-1.54) |
| Intracranial bleeding | 0.35 (0.15-0.82) | 0.39 (0.18-0.84) |
| Major gastrointestinal bleeding | 2.26 (1.50-3.40) | 2.00 (1.25-3.21) |
Modified from Wallentin et al13 with permission.
cTTR indicates percentage of time in the therapeutic range at center level; and HR, hazard ratio.
A recent trial, in which patients with a stable warfarin dose were randomized to 4-weekly or 12-weekly INR testing demonstrated that the longer interval was noninferior for the primary outcome of TTR,14 confirming prior observational data.15 This reduced INR monitoring frequency for selected patients further reduces the perceived inconvenience of warfarin treatment.
Renal failure.
Patients with severe renal failure (creatinine clearance < 30 mL/min) were excluded from the RE-LY2 and Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF)16 trials of dabigatran and rivaroxaban, respectively. Dabigatran is mainly (80%) eliminated via the kidneys, and in severe renal failure bioaccumulation occurs.17 Rivaroxaban is less dependent on renal elimination.18 In the ROCKET AF trial, patients with a creatinine clearance of 30 to 49 mL/min received a reduced dose of 15 mg daily.16 These observations suggest that warfarin remains the treatment of choice for patients with a calculated creatinine clearance close to or less than 30 mL/min.
Mechanical heart valve replacement.
These new drugs have not been evaluated in patients with mechanical heart valve prosthesis. Their safety and efficacy profiles in such patients cannot be determined, and at present all such patients should remain on warfarin.
Gastrointestinal disease and elderly patients.
In the analysis of bleeding outcomes in various subgroups, patients 75 years of age or older in RE-LY had similar or a higher risk of extracranial bleeding on dabigatran as on warfarin.19 When this complication is of particular concern, for example in patients with a history of recurrent extracranial bleeding or with preexisting coagulopathy, anticoagulation with warfarin may be preferable because this drug is rapidly reversible. This concern may not be true for the factor Xa inhibitors, for which subgroup analyses have not yet been published.
Lower gastrointestinal bleeding is significantly increased with dabigatran compared with warfarin. This is probably because of low bioavailability, which results in high concentrations of active drug in the feces.20 Treatment with rivaroxaban was also associated with a significant increase of the risk for gastrointestinal bleeding16 ; hence, patients with intestinal angiodysplasia, inflammatory bowel disease, or diverticulosis, or those with a history of other forms of gastrointestinal bleeding may experience a deterioration on treatment with dabigatran or rivaroxaban.
In the RE-LY trial, patients receiving warfarin or dabigatran had a similar rate of discontinuation of study medication (17% vs 21%).2 Discontinuation of dabigatran was more frequent as a result of gastrointestinal distress, and 11.3% to 11.8% of patients on this drug complained of dyspepsia. This has been attributed to both tartaric acid contained in the capsule (required for absorption) and to a high concentration of active drug in the colon. This again suggests that dabigatran may not be an ideal agent for patients with a history of gastrointestinal diseases.
Poor compliance.
Patients with documented poor adherence to the treatment with vitamin K antagonists are particularly problematic. Dabigatran has to be administered twice daily for patients with atrial fibrillation,2 which suggests that it should not be seen as an alternate to warfarin in patients who have been poorly compliant with warfarin. The inability to monitor dabigatran, coupled with its short half-life (14-17 hours), would imply that patients who are poorly compliant are at high risk of stroke because failure to take the medication will quickly experience a complete loss of antithrombotic efficacy. Rivaroxaban has an even shorter half-life (11-13 hours in the elderly),18 and a missed dose might be equally hazardous in such patients. For patients treated with warfarin and who undergo INR monitoring, the clinician is at least aware of inadequate levels, suggesting that aggressive measures to increase compliance can be put in place. With novel agents, the first marker of noncompliance is probably stroke or other thrombotic complications.
Drug cost.
Acquisition costs of novel agents will be higher than for warfarin; thus, many patients who do not have drug coverage will probably remain on warfarin, despite evidence that from a broader perspective novel agents are cost-neutral or cost-effective in many settings.21
For the new anticoagulants
Unexplained poor warfarin control.
Warfarin-experienced patients who continue to have variable INR results, corresponding to a TTR of less than 65%, have lower rates of stroke and other complications when treated with dabigatran 150 mg twice daily.13 If drug costs are not a deterrent, dabigatran or another novel agent approved for atrial fibrillation should be considered. However, it is crucial to determine the reasons for instability. As previously discussed, if instability is the result of noncompliance, warfarin remains the anticoagulant of choice.
Poor level of control because of unavoidable drug-drug interactions.
The new anticoagulants have, as opposed to vitamin K antagonists, few interactions with other drugs (see “Drug interactions”). Patients with frequent need for antibiotic treatment, chemotherapy, amiodarone, frequent use of acetaminophen, azathioprine, or a large number of concomitant medications, particularly if the exposure to these medications varies, will probably do better with the new anticoagulants.
New patients on anticoagulant therapy for atrial fibrillation.
For warfarin-naive patients who can afford newer agents, it is very tempting to go straight for the new anticoagulants to avoid the initial several weeks of frequent dose adjustments of warfarin. Patients should be informed of the advantages and disadvantages of the alternatives, including monitoring, dose adjustments, interactions, bleeding risks, availability of antidote, and costs, allowing them to make an informed decision on their preferred therapy.
Drug interactions
The only classes of drugs that dabigatran is known to interact with are inhibitors or inducers of the transporter P-glycoprotein (P-gp). Rivaroxaban is subject to interaction via P-gp as well as to inducers and inhibitors of the microsomal enzyme CYP3A4, which is responsible for the metabolism of this drug. Dabigatran is dependent on P-gp for its transport across the intestinal wall. There are at least 64 drugs that have been described to influence the P-gp, but only a few drugs cause clinically important changes in the drug exposure (Table 2).22 The strong P-gp inhibitor quinidine increases the exposure to dabigatran by 53% in healthy volunteers. During the phase 3 trials with dabigatran, an amendment of the protocols was therefore introduced, prohibiting concomitant use of quinidine.23 Quinidine should be administered at least 2 hours after dabigatran. Verapamil and amiodarone are moderate inhibitors of P-gp, and multiple doses of verapamil resulted in a 50% to 60% increase in dabigatran concentration.24 The increase varied depending on the dosing schedule of verapamil and its formulation, and the effect can be minimized by dosing dabigatran at least 2 hours before verapamil.25 The strongest interaction is with ketoconazole, which increases the exposure to dabigatran as well as to rivaroxaban by 150% to 160% and therefore is contraindicated with either anticoagulant. Rifampicin, a strong inducer of P-gp and of CYP3A4, causes at least a 50% reduction of the exposure to the new anticoagulants, and it is recommended to avoid concomitant use. St John's wort is another strong inducer of P-gp and of CYP3A4 and must be avoided with novel agents. Many of these drugs also interact with warfarin, but the availability of the INR levels allows dose adjustment, which mitigates the risk of concomitant treatment.
Drug interactions with at least 50% change in the exposure to dabigatran or rivaroxaban
| Mechanism . | Dabigatran . | Rivaroxaban . | ||
|---|---|---|---|---|
| Interacting drug . | Δ exposure, % . | Interacting drug . | Δ exposure, % . | |
| P-gp inhibition | Ketoconazole* | 150 | Ketoconazole* | 160 |
| Quinidine | 53 | |||
| Amiodarone | 60 | |||
| Verapamil | ≃ 50† | |||
| P-gp induction | Rifampicin | −67 | Rifampicin | −50 |
| St John's wort | ND | St John's wort | ND | |
| CYP3A4 inhibition | Ketoconazole* | 160 | ||
| Clarithromycin | 50 | |||
| Ritonavir | 50 | |||
| CYP3A4 induction | Rifampicin | −50 | ||
| St John's wort | ND | |||
| Mechanism . | Dabigatran . | Rivaroxaban . | ||
|---|---|---|---|---|
| Interacting drug . | Δ exposure, % . | Interacting drug . | Δ exposure, % . | |
| P-gp inhibition | Ketoconazole* | 150 | Ketoconazole* | 160 |
| Quinidine | 53 | |||
| Amiodarone | 60 | |||
| Verapamil | ≃ 50† | |||
| P-gp induction | Rifampicin | −67 | Rifampicin | −50 |
| St John's wort | ND | St John's wort | ND | |
| CYP3A4 inhibition | Ketoconazole* | 160 | ||
| Clarithromycin | 50 | |||
| Ritonavir | 50 | |||
| CYP3A4 induction | Rifampicin | −50 | ||
| St John's wort | ND | |||
ND indicates not determined.
Contraindicated.
Variable depending on the formulation of verapamil.
Concomitant use of antiplatelet agents
Studies with dabigatran, rivaroxaban, and apixaban in patients with acute coronary syndromes, receiving combined antiplatelet therapy with aspirin and clopidogrel, have generally shown a dose-dependent increase in the risk of major bleeding and any bleeding.26-30 Nevertheless, there is a possibility for a net clinical benefit of concomitant therapy as recently shown for rivaroxaban.27
Conversion from warfarin to dabigatran or rivaroxaban
As noted, patient stabilized on warfarin may prefer to remain on this medication. However, given the added convenience and the potential for enhanced efficacy and reduced risk for intracranial bleeding of dabigatran or rivaroxaban, many patients will choose to transition from warfarin to one of the new anticoagulants. Although not evidence-based, we recommend starting medication with dabigatran or rivaroxaban when warfarin has been discontinued and the INR has decreased to less than 2.3. In the trials where patients could have warfarin before starting on dabigatran (RE-LY, RE-SONATE, and RE-MEDY),2,31,32 the highest INR permitted at the time of transition was 2.3, whereas in ROCKET AF it was 3.0 for starting rivaroxaban.16 There was no signal in these trials that the overlap was associated with an increased risk for bleeding. Point-of-care INR monitors should not be used to assess the INR during transitions between dabigatran and warfarin, given the potential for dabigatran to increase the “baseline INR.”33
Conversion from dabigatran or rivaroxaban to warfarin
For patients who are unable to continue on dabigatran or rivaroxaban and need to transition to warfarin, it is necessary to take into account the expected onset of warfarin (∼ 5 days) as well as the half-life of dabigatran, which varies from 14 to 17 hours with normal renal function34 to 18 to 27 hours in moderate to severe renal impairment.17 Based on these data, we suggest transition strategies as shown in Table 3. The first INR should be obtained on day 3 of warfarin medication but only for the purpose of identifying an excessive level, resulting in more caution with the warfarin dosing. For patients with a creatinine clearance of less than 30 mL/min, there are no data available to support a recommendation regarding transition to the new anticoagulants. Point-of-care INR monitors should not be used to assess the INR during transitions between rivaroxaban and warfarin, given the observation that rivaroxaban will increase the prothrombin time to differing extents, based on difference in the sensitivity of the coagulometer and the prothrombin reagent.35,36
Suggested strategy for conversion from dabigatran or rivaroxaban to warfarin
| Calculated creatinine clearance, mL/min . | Dabigatran: start day with warfarin* . | Rivaroxaban: start day with warfarin* . |
|---|---|---|
| > 50 | Day −3 | Day −4 |
| 31-50 | Day −2 | Day −3 |
| 15-30 | Day −1 | Day −2 |
| Calculated creatinine clearance, mL/min . | Dabigatran: start day with warfarin* . | Rivaroxaban: start day with warfarin* . |
|---|---|---|
| > 50 | Day −3 | Day −4 |
| 31-50 | Day −2 | Day −3 |
| 15-30 | Day −1 | Day −2 |
Dabigatran/rivaroxaban is stopped on day 0. The longer overlap with rivaroxaban is justified by its half-life being shorter than that of dabigatran and by the concern about thromboembolic events shortly after transitioning from rivaroxaban to warfarin.6
Periprocedural management of dabigatran or rivaroxaban
The principles that are important for the perioperative management with the new anticoagulants are as follows: (1) the half-life is shorter than with warfarin; and (2) the onset of effect is within 2 hours, provided that intestinal absorption is normal.
Preoperative management
The manufacturer of dabigatran issued guidance for the investigators regarding preoperative discontinuation of the drug halfway through the phase 3 trials on stroke prophylaxis in atrial fibrillation and on treatment of venous thromboembolism. This guide was based on pharmacokinetic data and on considerations regarding the differential risk of bleeding. In patients with normal renal function the half-life of 14 to 17 hours34 suggests that interruption of dabigatran for 48 hours should be sufficient to ensure adequate hemostasis. This short period of interruption does not require bridging therapy with heparin or low-molecular-weight heparin. For procedures with a low risk of bleeding, where an INR of 1.5 for patients on warfarin would be acceptable, it is reasonable to stop dabigatran 24 hours in advance. Such procedures include cardiac catheterization, diagnostic endoscopy, and minor orthopedic surgery. Thus, for elective surgery in patients with normal renal function, it is probably safe to recommend the patient simply to interrupt therapy for 1 to 2 days (depending on the type of procedure) before their planned procedure (Table 4). With decreasing renal function, the period of interruption should be longer. Our routine has not been to use any bridging therapy for these patients (Table 4). Similar reasoning should apply for rivaroxaban. The study protocol for ROCKET AF suggested interruption of the drug approximately 2 days before a procedure. If desired, a normal thrombin clotting (TCT) time rules out the presence of important levels of dabigatran (see “Emergency testing of hemostatic function: dabigatran”); this test could be used preoperatively in patients in whom there is a potential for persistent anticoagulant effect or for those undergoing surgery with a high risk of complications from bleeding, such as spinal surgery or neurosurgery. There is no equivalent, widely available, test for persistence of rivaroxaban.
Timing of interruption of dabigatran or rivaroxaban before surgery or invasive procedures
| Calculated creatinine clearance, mL/min . | Half-life, hours . | Timing of last dose before surgery . | |
|---|---|---|---|
| Standard risk of bleeding* . | High risk of bleeding† . | ||
| Dabigatran | |||
| > 80 | 13 (11-22) | 24 h | 2 d |
| > 50- ≤ 80 | 15 (12-34) | 24 h | 2 d |
| > 30- ≤ 50 | 18 (13-23) | 2 d | 4 d |
| ≤ 30 | 27 (22-35) | 4 d | 6 d |
| Rivaroxaban | |||
| > 30 | 12 (11-13) | 24 h | 2 d |
| < 30 | Unknown | 2 d | 4 d |
| Calculated creatinine clearance, mL/min . | Half-life, hours . | Timing of last dose before surgery . | |
|---|---|---|---|
| Standard risk of bleeding* . | High risk of bleeding† . | ||
| Dabigatran | |||
| > 80 | 13 (11-22) | 24 h | 2 d |
| > 50- ≤ 80 | 15 (12-34) | 24 h | 2 d |
| > 30- ≤ 50 | 18 (13-23) | 2 d | 4 d |
| ≤ 30 | 27 (22-35) | 4 d | 6 d |
| Rivaroxaban | |||
| > 30 | 12 (11-13) | 24 h | 2 d |
| < 30 | Unknown | 2 d | 4 d |
Examples are cardiac catheterization, ablation therapy, colonoscopy without removal of large polyps, and uncomplicated laparoscopic procedures, such as cholecystectomy.
Examples are major cardiac surgery, insertion of pacemakers or defibrillators (resulting from the risk for pocket hematoma), neurosurgery, large hernia surgery, and major cancer/urologic/vascular surgery.
Patients receiving dabigatran and who require emergency surgery will either have to wait until the anticoagulant effect has spontaneously diminished or undergo their procedure with the knowledge that they have an increased risk of bleeding. Rivaroxaban may be reversible with a 4-factor prothrombin complex concentrate (PCC). See also “Managing overdose and bleeding complications.”
Postoperative management
The time point for resumption of dabigatran or rivaroxaban depends almost exclusively on the postoperative risk of bleeding. The risk for major bleeding complications after surgery clearly exceeds the risk for thromboembolic complications.37 For major abdominal surgery or urologic surgery with incomplete hemostasis, resumption should be delayed until there is no drainage or other evidence of active bleeding. For procedures with good hemostasis shortly after the end of the procedure, we suggest resumption the same evening a minimum of 4 to 6 hours after surgery. For dabigatran, we suggest starting with a half dose (75 mg) for the first dose, and thereafter the usual maintenance dose, extrapolating from the trials in orthopedic surgery. A similar strategy could be followed for rivaroxaban where a 10-mg dose could be used as the first dose. Patients with bowel paralysis may require bridging with a parenteral anticoagulants given their inability to take their oral anticoagulant.38
Emergency testing of hemostatic function
Dabigatran
In the setting of emergency surgery, a test that predicts whether the patient has acceptable hemostasis is desirable. The TCT has a linear correlation with the dabigatran concentration (r2 = 0.86)39 but prolongs rapidly at low levels of drug and is not routinely available in every hospital. If the TCT is normal, it is safe to assume that the level of dabigatran is very low and that the patient's risk of bleeding is similar to that of other patients undergoing the procedure. The prothrombin time (PT, usually expressed as the INR) is insensitive to dabigatran and not useful in this context. Point-of-care testing INR values may be inaccurate in patients receiving dabigatran and should not be used to judge drug effect.33 The activated partial thromboplastin time (aPTT) shows a curvilinear response to the concentration of dabigatran. At high dabigatran concentrations, the aPTT becomes incoagulable. The aPTT is prolonged 2-fold compared with the control value at peak drug levels during chronic treatment and 1.5-fold at 12 hours (r2 = 0.85).39 The sensitivity of the aPTT reagent may result in more or less pronounced differences between trough and peak. The hematologist needs to take into account the time elapsed since the last dose of dabigatran as well as the responsiveness of the local reagent to make a correct interpretation of the aPTT.
Rivaroxaban
The PT shows a linear dose-response to rivaroxaban and is prolonged to a similar extent as the degree of inhibition of factor Xa.35 However, the PT response to rivaroxaban is assay dependent. Assay specific calibration curves can be developed and, when used with standard calibrators, produce a rivaroxaban level.36
In summary, although aPTT or PT cannot provide precise information on the level and thus anticoagulant effect of dabigatran or rivaroxaban, respectively, they are probably useful as a qualitative indicator of drug presence. Thus, a normal aPTT or PT in the setting of dabigatran or rivaroxaban, respectively, would suggest that hemostatic function is not impaired because of the drug. Given the risk of epidural hemorrhage, epidural or spinal anesthesia should only be performed in patients in whom there is a high degree of certainty that their novel anticoagulant is completely cleared. The predictive ability of the PT or the aPTT has not been clinically proven; additional research is required to confirm their utility in this setting.
Monitoring compliance
In the RE-LY and ROCKET AF studies, the anticoagulant effect of dabigatran and rivaroxaban, respectively, was not monitored.2,16 Monitoring is not required because of the wide therapeutic window and predictable pharmacokinetic profiles of these products. Therefore, when used for atrial fibrillation, orthopedic prophylaxis and long-term secondary prevention of recurrent venous thromboembolism dabigatran and rivaroxaban do not require routine anticoagulant monitoring. If monitoring is thought to be required, a number of options are available. As noted previously, the TCT is very sensitive to the presence of dabigatran and the aPTT is usually prolonged in its presence.39 The PT should be prolonged in patients receiving rivaroxaban, and an antifactor Xa assay, either that normally used to measure the activity of heparin or low-molecular-weight heparin, or an assay specifically calibrated for rivaroxaban, should also be increased because both heparin and rivaroxaban produce their anticoagulant effect through inhibition of factor Xa.36 Only the anti-Xa assay specifically calibrated for rivaroxaban reliably predicts drug levels. Routine testing with the aPTT, TCT, or anti–factor Xa levels is not recommended for patients receiving the new anticoagulants.
Specialized laboratories may wish to set up functional dabigatran or rivaroxaban levels. Commercial calibrators are available that allow accurate reporting of the amount of drug present.40,41 At present, “target drug levels” have not been promulgated, and levels are highly dependent on time since the drug was taken. Thus, the development of a “therapeutic drug level” is going to require both correlation of observed drug levels with clinical events and development of an accepted adjustment for the level based on the time since blood draw.
Monitoring of renal function
Dabigatran is eliminated to 80% via the kidneys; therefore, renal function has to be assessed before starting the treatment to determine the appropriate dose. Reassessment of renal function should be performed annually and whenever a decline in renal function is suspected.
Managing overdose and bleeding complications
When bleeding occurs, the event should be risk stratified: Minor bleeding (such as epistaxis, ecchymosis, or menorrhagia) can be managed with simple withdrawal of the anticoagulant for 1 or more days, allowing definitive interventions (where available) to be applied. The drug could then be restarted at a lower dose (dabigatran at 75 mg per day) for a short period of time. Moderate bleeding (such as upper or lower gastrointestinal bleeding) should be managed with withdrawal of the anticoagulant, careful clinical monitoring, interventions to identify and definitively treat the bleeding source, and consideration of an extended period of withdrawal of the oral anticoagulant (perhaps with the addition of a low dose of a parenteral anticoagulant for patients at particularly high risk of thrombosis) to allow healing. Transfusion therapy with red blood cells might be required to treat symptomatic anemia. Major and life-threatening bleeding should be treated with immediate anticoagulant withdrawal, aggressive clinical monitoring, transfusion of packed red blood cells in response to proven or anticipated severe anemia, aggressive interventions to identify and treat the bleeding source (requiring endoscopy, interventional radiology, or surgery), and consideration of lifesaving therapies, such as inotropes, ventilation, and intensive care unit admission to stabilize the patient. Other blood products, such as plasma or cryoprecipitate, do not reverse the anticoagulant effect of novel agents. Interventional therapy may be lifesaving in the case of major bleeding. Such therapy cannot await reversal of the anticoagulant effect of the novel agents; thus, interventionalists will be required to provide therapeutic interventions despite the presence of significant anticoagulant effect and the associated risk of bleeding.
Dabigatran
If detected soon after ingestion, the absorption of dabigatran may be reduced by gastric lavage and/or administration of charcoal.39 In vitro experiments have verified that the lipophilic drug in solution is completely absorbed by activated charcoal.39
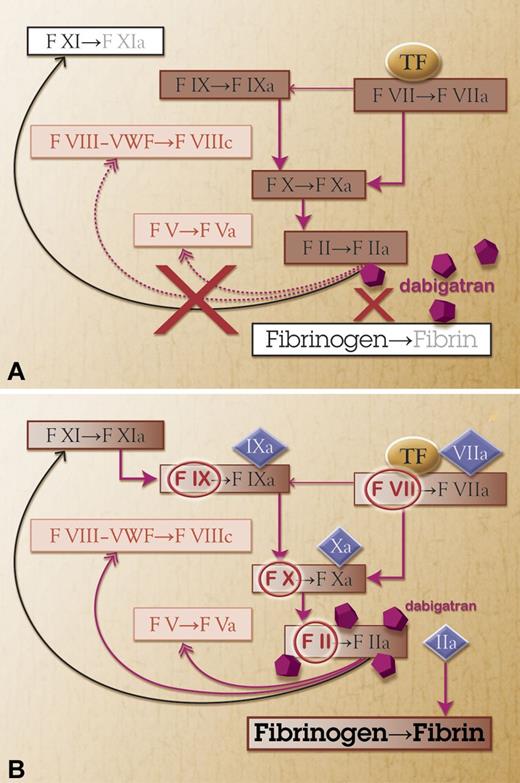
There is currently no antidote for the anticoagulant effect of dabigatran. Fresh frozen plasma or PCC is not known to mitigate its anticoagulant effect, as measured by different coagulation tests.42 However, in a mouse model with induced intracerebral hemorrhage and a high dose of dabigatran (9 mg/kg), a high dose of PCC (100 IU/kg) prevented further expansion of the hematoma.43 On the other hand, in another study with induced intracerebral hemorrhage in mice, doses of dabigatran of 37.5 to 112.5 mg/kg did not cause any expansion of the hematoma, as opposed to pretreatment with warfarin, heparin, fondaparinux, or lepirudin.44 Thus, the implications of these results for the clinical management remain unclear until we obtain information on the reversal of bleeding in humans. Alternate agents, such as recombinant activated factor VII (rFVIIa), have not been demonstrated to reverse bleeding complications. Although suggested in the package insert for treatment of dabigatran-associated bleeding, rFVIIa has a very short half-life, is expensive, and its use is associated with otherwise avoidable thromboembolism.45 Furthermore, in a study in healthy volunteers receiving another oral thrombin inhibitor (melagatran), rFVIIa had no effect on most coagulation parameters.46 In the aforementioned mouse model, rFVIIa did not produce the stabilizing effect on hematoma expansion seen with PCC.43 The lack of effect of this agent is explained by the fact that dabigatran inhibits the last enzymatic step of the coagulation cascade. Any agent that stimulates this cascade or replaces coagulation factors proximal to thrombin will not compensate for the profound terminal defect in hemostasis. Activated prothrombin complex concentrate may improve hemostasis by providing small amounts of thrombin (Figure 1). In an animal model of bleeding, activated prothrombin complex concentrate reduced bleeding after treatment with another thrombin inhibitor (melagatran).47
Possible reversal of dabigatran. (A) Treatment with a direct thrombin inhibitor (dabigatran) blocks factor IIa (thrombin) and thereby the fibrin formation. (B) Activated prothrombin complex concentrate provides additional factor II, VII, IX, and X (circled red) and also these factors in activated form (diamonds). Factor IIa may then overcome the thrombin inhibitor.
Possible reversal of dabigatran. (A) Treatment with a direct thrombin inhibitor (dabigatran) blocks factor IIa (thrombin) and thereby the fibrin formation. (B) Activated prothrombin complex concentrate provides additional factor II, VII, IX, and X (circled red) and also these factors in activated form (diamonds). Factor IIa may then overcome the thrombin inhibitor.
In extreme cases, and where it is available, acute hemodialysis should be considered because only 35% of dabigatran is bound to plasma proteins. This strategy has been evaluated in patients with end-stage renal disease, who received 1 dose of 50 mg dabigatran and then had hemodialysis. The mean drug concentration in the inlet and outlet to the dialyzer was 12.6 and 4.4 ng/mL, respectively. In our experience from reversal of postoperative bleeding in one patient, who had no effect from a variety of blood products, dialysis for 6 hours reduced the TCT from approximately 150 seconds to 68 seconds.
Rivaroxaban
Because of a high degree of albumin binding in plasma (92%-95%), rivaroxaban is not dialyzable. All its measurable anticoagulant effects are, however, reversed by a 4-factor PCC, as studied in healthy volunteers.42 Clinical data are still lacking, but it seems reasonable to give a dose of 50 IU/kg in case of acute, major bleeding (Figure 2). Whether this needs to be repeated is unknown. In the United States, there are no available 4-factor PCCs; whether one of the available 3-factor PCCs is effective for reversal of rivaroxaban is unknown.
Possible reversal of factor Xa inhibitors. (A) Treatment with a direct factor Xa inhibitor (rivaroxaban) blocks factor Xa and thereby the next step in the fibrin formation. (B) Prothrombin complex concentrate provides additional factor II, VII, IX, and X (circled red) and the Xa inhibitor is overcome.
Possible reversal of factor Xa inhibitors. (A) Treatment with a direct factor Xa inhibitor (rivaroxaban) blocks factor Xa and thereby the next step in the fibrin formation. (B) Prothrombin complex concentrate provides additional factor II, VII, IX, and X (circled red) and the Xa inhibitor is overcome.
Other considerations
Dabigatran tablets are hygroscopic and should thus not be removed from their original packaging. Bottles, once opened, are only approved for 60 days of use, after which residual tablets should be thrown away (http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm249005.htm). Individual tablets should not be placed into dosettes because they will lose potency. Blister-packaged dabigatran should be used in all settings where individual tablets are dispensed for other than immediate use.
Although subject to a rigorous phase 3 evaluation program, rare complications of dabigatran or rivaroxaban may yet occur. When suspected, these events should be reported to national regulatory authorities, as such reports are a seminal component of safety monitoring for uncommon complications.
Introduction of the new agents at your hospital or clinic
With the different characteristics of the new anticoagulants compared with warfarin, it is probable that mistakes will occur. In Canada, where dabigatran is marketed under the name Pradax, there have already been warnings that it has been inadvertently interchanged with Plavix. It is the responsibility of the hematologists to inform colleagues from other specialties on proper management of patients receiving novel anticoagulants. Ideally, the hematologist should offer a service to assist with treatment of major bleeding complications and with the perioperative management of anticoagulation. Cardiologists, internists, emergency physicians, and general practitioners should be the primary targets for grand rounds or similar educational events. A local newsletter or protocol should be prepared without further delay and posted on the internet, for example in collaboration with the Pharmacy and Therapeutics Committee (http://healthcare.utah.edu/Thrombosis/newagents/TS.Dabi_lab.monitor.pdf; accessed October 16, 2011, for an example). Booklets with suitable information for the patients on each of the new drugs should also be made available.
In conclusion, dabigatran and rivaroxaban offer promise as the first new oral anticoagulants in more than 50 years. Large studies suggest that they are relatively safe and highly effective. Primary care clinicians as well as specialists will need to develop a familiarity with these drugs given their potential for hemorrhage, potential to interfere with perioperative management, and the requirement for specific management in the case of overdose or bleeding. Warfarin is, however, still a good choice for several subsets of patients, and new patients should be able to participate actively in an informed decision on the type of anticoagulant treatment.
Authorship
Contribution: S.S. and M.A.C. wrote and reviewed the manuscript and reviewed and approved each other's sections.
Conflict-of-interest disclosure: S.S. has received honoraria from Boehringer Ingelheim, Bayer Healthcare, Sanofi-Aventis, CSL Behring, Merck, and Glaxo-Smith-Kline. M.A.C. has received honoraria from Leo Laboratories, Pfizer, Bayer, Boehringer Ingelheim, CSL Behring, Octapharma, and Artisan Pharma and has provided expert testimony for Bayer.
Correspondence: Sam Schulman, Thrombosis Service, HHS-General Hospital, 237 Barton Street East, Hamilton, ON, L8L 2X2, Canada; e-mail: schulms@mcmaster.ca.