Abstract
Morbidity and mortality in thalassemia are associated with iron burden. Recent advances in organ-specific iron imaging and the availability of oral deferasirox are expected to improve clinical care, but the extent of use of these resources and current chelation practices have not been well described. In the present study, we studied chelation use and the change in iron measurements in 327 subjects with transfusion-dependent thalassemia (mean entry age, 22.1 ± 2.5 years) from 2002-2011, with a mean follow-up of 8.0 years (range, 4.4-9.0 years). The predominant chelator currently used is deferasirox, followed by deferoxamine and then combination therapies. The use of both hepatic and cardiac magnetic resonance imaging increased more than 5-fold (P < .001) during the study period, leading to an 80% increase in the number of subjects undergoing liver iron concentration (LIC) measurements. Overall, LIC significantly improved (median, 10.7 to 5.1 mg/g dry weight, P < .001) with a nonsignificant improvement in cardiac T2* (median, 23.55 to 34.50 ms, P = .23). The percentage of patients with markers of inadequate chelation (ferritin > 2500 ng/mL, LIC > 15 mg/g dry weight, and/or cardiac T2* < 10 ms) also declined from 33% to 26%. In summary, increasing use of magnetic resonance imaging and oral chelation in thalassemia management has likely contributed to improved iron burden.
Introduction
Survival in thalassemia is strongly influenced by iron burden and adherence to chelation therapy.1,2 Deferoxamine (DFO), the first available chelator, has been in widespread clinical use since the 1970s. Improved survival of patients with thalassemia major born after 1970 compared with those born before provided strong indirect evidence of the impact of DFO on overall mortality.3,4 Although DFO is an effective chelator, parenteral administration of DFO often hinders adherence, with resultant premature deaths related to iron overload. Markers of inadequate chelation, including serum ferritin levels persistently above 2500 ng/mL and liver iron concentration (LIC) above 15 mg/g dry weight (dw), are associated with reduced survival in thalassemia.3,5,6
Recent advances in the assessment of iron burden in thalassemia patients enable closer monitoring of organ-specific iron stores with more timely adjustments in chelation regimens. Magnetic resonance imaging (MRI) of hepatic iron content using R2 and R2* techniques has been shown to provide accurate and reproducible quantitation of LIC in thalassemia.7-9 In contrast to liver biopsy, which is invasive and associated with risks including bleeding and procedure-related pain, hepatic MRI is generally more acceptable to patients, which permits more frequent monitoring. Similarly, T2* MRI now enables indirect assessment of cardiac iron loading.8,10 T2* values below 20 ms, and especially those less than 6 ms, predict an increased risk of arrhythmia and congestive heart failure.11 Given that hepatic iron does not always predict cardiac iron accurately—especially for patients receiving chelation, because the kinetics of removal of heart and liver iron differ greatly12 —serial measurements of cardiac iron may also aid in the personalization of chelation. In the United Kingdom, a significant reduction in mortality due to iron overload was seen after 1999, when cardiac T2* MRI became available, providing indirect evidence that the use of MRI, and likely the resulting adjustments in chelation therapy, has improved survival.13 Whether such advances have affected care in North America has not been studied.
Expanded options for chelation therapy are now available for patients with thalassemia. Deferiprone (DFP), an oral chelator first introduced into clinical studies in the 1980s, is approved for use in Europe and other countries, but was available in North America only through research or compassionate use protocols during the study period. Deferasirox (DFX), taken orally once daily, was approved by the Food and Drug Administration (FDA) in the United States in November 2005. Although it was anticipated that adherence to oral chelation would be superior to that with parenteral DFO, this may not be true in clinical practice. In addition, monotherapy with DFP or DFX may not be ideal for all patients, either because of inadequate iron removal or intolerable toxicities.
The impact of expanded monitoring and chelation options on iron burden in thalassemia patients has not been studied extensively. The overall goals of the present study were to assess the current chelator use among patients with thalassemia in North America and in London, United Kingdom, to assess patterns of MRI use, to determine whether control of iron burden has improved in recent years, and to compare the effectiveness of the chelators and the degree of adherence to treatment. We hypothesized that iron burden would be better controlled now than in the past, that most patients would currently be treated with DFX, and that iron burden would be better controlled and adherence with therapy would be superior with DFX compared with DFO treatment.
Methods
The Thalassemia Clinical Research Network (TCRN) is a National Institutes of Health/National Heart, Lung, and Blood Institute–sponsored clinical trial network of 16 thalassemia centers in North America and London. The Thalassemia Longitudinal Cohort (TLC) study of the TCRN, launched in 2007, with continued new patient enrollment through 2009, is a longitudinal registry designed to measure the prevalence and incidence of complications of thalassemia. Institutional review board approval was obtained from each site and written informed consent and assent, where applicable, were obtained in accordance with the Declaration of Helsinki. Inclusion criteria for the TLC were a diagnosis of thalassemia, including β-thalassemia major and intermedia, hemoglobin E–β-thalassemia, and clinically significant α-thalassemia syndromes (ie, 4 α gene deletion or hemoglobin H disorders with steady-state hemoglobin level < 9 g/dL). In general, this led to the exclusion of patients with relatively few clinical sequelae that did not require as frequent monitoring for complications of thalassemia and its treatment.
Baseline data collection included a 5-year retrospective component, with clinical, radiographic, and laboratory data from 2002-2004 on, followed by annual prospective data collection. Detailed information regarding chelation therapy was collected, including chelator agent(s) used, dose adjustments, indications for chelator/dose changes, and adverse effects. A small subset of individuals receiving combination therapy with DFO and DFX were participating in a pilot clinical trial of this therapy.14 Adherence with therapy was estimated by the nurse coordinator based on patient interview and chart review. Assessments of degree of iron overload included ferritin levels (up to 4 values per year, measured when the patients were not acutely ill), cardiac iron assessment by T2* MRI,15 and liver iron measurements obtained by R2 (FerriScan; Resonance Health) or R2* MRI, superconducting quantum interference device (SQUID), or liver biopsy. R2 and R2* MRI LIC measurements correlate well with liver biopsy measurements.7,9 In vivo LIC values assessed by SQUID biosusceptometry were converted to dw iron concentration by a factor of 6.16
The current analyses are limited to subjects who were receiving regular transfusions and had a history of chelation therapy. Data from the first year of the retrospective portion (historical values, 2002-2004), the study entry assessment (baseline values, 2007-2009), and prospective follow-up data (2008-2011) were used to determine the changing patterns of iron assessment (by biopsy and MRI) and overall change in iron burden. These dates were chosen to include time points spanning the initiation of DFX, initially in clinical trials (2003) and then in routine clinical use (approved by the FDA in November 2005, by the European Union in August 2006, and by Health Canada in October 2006). DFP was not commercially available in North America during the study period but had limited availability for compassionate use, whereas DFP was available as a second-line agent in the United Kingdom. T2* MRI has been used in the United Kingdom for hepatic and cardiac iron estimation since 1999, whereas R2 MRI (FerriScan) has been used in routine clinical practice since 2008. Most TCRN North American sites began using T2* techniques between 2003 and 2005, R2 and/or R2* MRI methods for liver iron have been in use routinely since 2003 at selected sites, and FerriScan R2 MRI methodology for liver iron assessment was placed into routine clinical practice at variable times ranging from 2006-2010. Registry enrollment occurred earlier in North American sites than in United Kingdom sites, resulting in a relatively larger contribution of data from North America.
Statistical analyses
Differences in categorical variables were tested by χ2 and Fisher exact tests and differences in continuous variables were tested by t test, ANOVA, and the Wilcoxon 2-sample test. Generalized estimating equations with the logit link function and exchangeable correlation structure were used to compare the changes in proportions for the use of liver iron measurements and cardiac T2* over time. Changes in LIC, ferritin, and cardiac T2* measurements between the time closest to the current chelation start date and the last available assessment were computed. Linear mixed models with participant-specific intercepts and slopes controlled for time effects were used to test for changes in LIC, ferritin, and cardiac T2* measurements. Log-transformation was used for LIC, ferritin, and cardiac T2* that were not normally distributed. All analyses were performed at the Data Coordinating Center of New England Research Institutes (Watertown, MA) with SAS Version 9.2 statistical software (SAS Institute) and R Version 2.11.1 software (R Foundation for Statistical Computing, http://www.r-project.org/). P < .05 was considered statistically significant.
Results
Subjects
A total of 428 subjects were enrolled in the TLC study; 101 subjects were excluded from the current analyses because of history of prior stem cell transplantation (n = 12) or not having been chronically transfused or received chelation (n = 89). The contribution of patients by country is as follows: United States, n = 233; Canada, n = 60; and United Kingdom, n = 34. Patient characteristics of the 327 subjects included in the analyses are shown in Table 1. The mean follow-up time for the cohort was 8.0 years (range, 4.4-9.0), with a mean prospective follow-up of 2.2 years (range, 0.7-3.4).
Patient characteristics (N = 327)
| Characteristic . | Baseline n (%) . | Follow-up n (%) . |
|---|---|---|
| Age at enrollment, y, mean (SD) | 22.2 (12.5) | |
| Sex, n (%) | ||
| Male | 156 (47.7) | |
| Female | 171 (52.3) | |
| Race, n (%)* | ||
| White | 160 (48.9) | |
| Black | 8 (2.4) | |
| Asian | 146 (44.6) | |
| Other | 9 (2.8) | |
| Missing | 4 (1.2) | |
| Diagnosis, n (%) | ||
| β-thalassemia, ≥ 8 transfusions/previous y | 291 (89.0) | |
| E-β-thalassemia, ≥ 8 transfusions/previous y | 31 (9.5) | |
| Homozygous α-thalassemia | 5 (1.5) | |
| Chelation, n (%) | ||
| DFO | 81 (24.8) | 43 (13.1) |
| DFX | 192 (58.7) | 172 (52.6) |
| DFP | 9 (2.8) | 9 (2.8) |
| DFO/DFP | 22 (6.7) | 34 (10.4) |
| DFO/DFX | 10 (3.1) | 18 (5.5) |
| No ongoing chelation | 13 (4.0) | 4 (1.2) |
| Missing chelation data | 47 (14.4) | |
| Iron assessment | ||
| Median ferritin, ng/mL (range) | 1271 (67.7-23 712) | 1103 (46.0-17 900) |
| Mean LIC, mg/g dw (SD) | 10.6 (8.9) | 7.70 (8.2) |
| Mean cardiac T2*, ms (SD) | 25.0 (13.7) | 26.7 (12.4) |
| Cardiac complications, n (%) | ||
| Congestive heart failure | 30 (9.2) | 31 (9.5)* |
| Supraventricular arrhythmia | 13 (4.0) | 15 (4.6)* |
| Ventricular arrhythmia | 14 (4.3) | 17 (5.2)* |
| Characteristic . | Baseline n (%) . | Follow-up n (%) . |
|---|---|---|
| Age at enrollment, y, mean (SD) | 22.2 (12.5) | |
| Sex, n (%) | ||
| Male | 156 (47.7) | |
| Female | 171 (52.3) | |
| Race, n (%)* | ||
| White | 160 (48.9) | |
| Black | 8 (2.4) | |
| Asian | 146 (44.6) | |
| Other | 9 (2.8) | |
| Missing | 4 (1.2) | |
| Diagnosis, n (%) | ||
| β-thalassemia, ≥ 8 transfusions/previous y | 291 (89.0) | |
| E-β-thalassemia, ≥ 8 transfusions/previous y | 31 (9.5) | |
| Homozygous α-thalassemia | 5 (1.5) | |
| Chelation, n (%) | ||
| DFO | 81 (24.8) | 43 (13.1) |
| DFX | 192 (58.7) | 172 (52.6) |
| DFP | 9 (2.8) | 9 (2.8) |
| DFO/DFP | 22 (6.7) | 34 (10.4) |
| DFO/DFX | 10 (3.1) | 18 (5.5) |
| No ongoing chelation | 13 (4.0) | 4 (1.2) |
| Missing chelation data | 47 (14.4) | |
| Iron assessment | ||
| Median ferritin, ng/mL (range) | 1271 (67.7-23 712) | 1103 (46.0-17 900) |
| Mean LIC, mg/g dw (SD) | 10.6 (8.9) | 7.70 (8.2) |
| Mean cardiac T2*, ms (SD) | 25.0 (13.7) | 26.7 (12.4) |
| Cardiac complications, n (%) | ||
| Congestive heart failure | 30 (9.2) | 31 (9.5)* |
| Supraventricular arrhythmia | 13 (4.0) | 15 (4.6)* |
| Ventricular arrhythmia | 14 (4.3) | 17 (5.2)* |
The number of cardiac complications at follow-up includes the number of events at baseline.
Use of MRI
Overall, the number of subjects with available liver iron measurements increased significantly from 160 at historical measurement (2002-2004) to 290 at baseline (study entry), and 270 at follow-up (P < .001). Furthermore, a significant shift toward more frequent use of MRI to estimate LIC in recent years has occurred (11.9% of LIC historically to 77.9% at baseline to 85.2% at follow-up, P < .001). In contrast, the use of liver biopsy significantly declined (65.6% historically to 9.3% at baseline to 1.8% at follow-up, P < .001), and the use of SQUID, mostly (67%) from subjects at a single United States center where the technology is available, also decreased (22.5% historically to 12.8% at baseline and 13.0% at follow-up, P = .04). The routine use of cardiac T2* MRI has also significantly increased (only 33 subjects had historical measurements between 2002 and 2004, compared with 189 at baseline and 213 at follow-up, P < .001).
Chelation use
The distribution of chelator use at both enrollment (baseline) and latest follow-up is shown in Table 1. The most commonly used chelator currently was DFX. Most subjects who switched to (or initiated chelation with) DFX chose to use the oral drug once it became commercially available, whereas 51 subjects initially used this chelator in the context of a clinical trial.17,18 Subjects also switched to DFX because of inadequate control of iron burden or adverse effects with DFO (Table 2). Switches from DFX back to DFO also occurred because of rising iron burden or adverse effects (Table 2).
Reasons for changing chelator
| Reason for switch, n (%) . | Chelator switch DFO to DFX (N = 55) . | Chelator switch from DFX to DFO (N = 34)* . |
|---|---|---|
| Ferritin/LIC too high | 34 (61.8) | 10 (29.4) |
| Ferritin/LIC too low | 8 (14.5) | 1 (2.9) |
| Audiologic toxicity | 2 (3.6) | 0 (0.0) |
| Ophthalmologic toxicity | 0 (0.0) | 0 (0.0) |
| Nephrotoxicity | 0 (0.0) | 3 (8.8) |
| Cardiac disease or cardiac iron loading requiring intensification | 0 (0.0) | 4 (11.8) |
| Elevated alanine aminotransferase | 3 (5.5) | 5 (14.7) |
| Gastrointestinal side effects (nausea, diarrhea, abdominal pain) | 1 (1.8) | 5 (14.7) |
| Dislike taste/texture | 0 (0) | 1 (2.9) |
| Gastrointestinal bleeding | 0 (0.0) | 1 (2.9) |
| Local skin reactions/central access difficulty | 3 (5.5) | 0 (0.0) |
| Rash | 1 (1.8) | 2 (5.9) |
| Anxiety/palpitations | 0 (0) | 1 (2.9) |
| Missing | 3 (5.5) | 1 (2.9) |
| Reason for switch, n (%) . | Chelator switch DFO to DFX (N = 55) . | Chelator switch from DFX to DFO (N = 34)* . |
|---|---|---|
| Ferritin/LIC too high | 34 (61.8) | 10 (29.4) |
| Ferritin/LIC too low | 8 (14.5) | 1 (2.9) |
| Audiologic toxicity | 2 (3.6) | 0 (0.0) |
| Ophthalmologic toxicity | 0 (0.0) | 0 (0.0) |
| Nephrotoxicity | 0 (0.0) | 3 (8.8) |
| Cardiac disease or cardiac iron loading requiring intensification | 0 (0.0) | 4 (11.8) |
| Elevated alanine aminotransferase | 3 (5.5) | 5 (14.7) |
| Gastrointestinal side effects (nausea, diarrhea, abdominal pain) | 1 (1.8) | 5 (14.7) |
| Dislike taste/texture | 0 (0) | 1 (2.9) |
| Gastrointestinal bleeding | 0 (0.0) | 1 (2.9) |
| Local skin reactions/central access difficulty | 3 (5.5) | 0 (0.0) |
| Rash | 1 (1.8) | 2 (5.9) |
| Anxiety/palpitations | 0 (0) | 1 (2.9) |
| Missing | 3 (5.5) | 1 (2.9) |
All but 1 subject switched from DFO to DFX to DFO.
A center effect was evident regarding the prevalence of use of certain chelators (Table 3). DFP, either as a single agent or in combination therapy, was used significantly more frequently in the United Kingdom sites, whereas DFX was used more commonly in the US sites at baseline and at both United States and Canadian sites at follow-up, compared with the United Kingdom sites. Similarly, age was associated with the type of chelator regimen used (Table 3): children younger than 6 years of age were more likely to receive DFO monotherapy than older subjects, whereas older subjects were more likely to receive both DFX monotherapy and combination therapies compared with younger patients (P = .01).
Site and age effect on chelator utilization at baseline
| Factor site . | Chelator regimen, n (%) . | P* . | |||||
|---|---|---|---|---|---|---|---|
| DFO . | DFX . | DFP . | DFO/ DFP . | DFO/ DFX . | None . | ||
| United States | 50 (21.5) | 147 (63.1) | 4 (1.7) | 13 (5.6) | 10 (4.3) | 9 (3.9) | < .001 |
| Canada | 28 (46.7) | 29 (48.3) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 2 (3.3) | |
| United Kingdom | 3 (8.8) | 16 (47.1) | 5 (14.7) | 8 (23.5) | 0 (0.0) | 2 (5.9) | |
| Age group, y | |||||||
| ≤ 6 | 12 (46.1) | 14 (53.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .01 |
| 7-16 | 25 (23.8) | 71 (67.6) | 0 (0.0) | 2 (1.9) | 3 (2.9) | 4 (3.8) | |
| > 16 | 44 (22.5) | 107 (54.6) | 9 (4.6) | 20 (10.2) | 7 (3.6) | 9 (4.6) | |
| Factor site . | Chelator regimen, n (%) . | P* . | |||||
|---|---|---|---|---|---|---|---|
| DFO . | DFX . | DFP . | DFO/ DFP . | DFO/ DFX . | None . | ||
| United States | 50 (21.5) | 147 (63.1) | 4 (1.7) | 13 (5.6) | 10 (4.3) | 9 (3.9) | < .001 |
| Canada | 28 (46.7) | 29 (48.3) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 2 (3.3) | |
| United Kingdom | 3 (8.8) | 16 (47.1) | 5 (14.7) | 8 (23.5) | 0 (0.0) | 2 (5.9) | |
| Age group, y | |||||||
| ≤ 6 | 12 (46.1) | 14 (53.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .01 |
| 7-16 | 25 (23.8) | 71 (67.6) | 0 (0.0) | 2 (1.9) | 3 (2.9) | 4 (3.8) | |
| > 16 | 44 (22.5) | 107 (54.6) | 9 (4.6) | 20 (10.2) | 7 (3.6) | 9 (4.6) | |
For overall comparisons.
The mean dose of DFO at enrollment was 39.3 mg/kg/d and did not change significantly by the time of last follow-up (39.7 mg/kg/d), whereas the mean DFX dose at baseline was 25.3 mg/kg/d and increased to 26.9 mg/kg/d at last follow-up (P = .04). Individual dosing was variable but, in general, higher chelator doses were prescribed to those with higher LIC and/or low cardiac T2* values with DFX but not DFO. In addition, the mean dose of DFX generally increased from baseline to follow-up for patients with higher LIC, as expected given evidence that doses below 20 mg/kg do not reduce liver iron in the majority of thalassemia patients17 ; DFO dosing did not increase in a similar manner (Table 4).
Chelator dose at baseline and follow-up by liver and iron burden
| Chelator . | Chelator dose, mg/kg/d, mean (SD) . | ||||||
|---|---|---|---|---|---|---|---|
| Baseline LIC, mg/g dw . | Baseline cardiac T2*, ms . | ||||||
| < 4 . | 4 to < 7 . | 7 to < 15 . | ≥ 15 . | < 10 . | 10 to < 20 . | ≥ 20 . | |
| DFX | |||||||
| Baseline | 21.8 (10.6) | 23.3 (6.0) | 27.1 (6.3) | 28.5 (8.9) | 29.8 (10.9) | 30.7 (8.9) | 24.9 (8.0) |
| n | 28 | 37 | 69 | 35 | 12 | 22 | 69 |
| Follow-up | 25.1 (14.5) | 28.4 (7.1) | 29.0 (10.7) | 36.5 (18.8) | 39.0 (28.5) | 34.5 (15.0) | 26.9 (7.9) |
| n | 20 | 26 | 58 | 28 | 10 | 18 | 53 |
| P* | .47 | .004 | .28 | .06 | .42 | .31 | .11 |
| DFO | |||||||
| Baseline | 38.0 (13.2) | 40.7 (11.0) | 38.6 (12.1) | 36.7 (12.1) | 44.7 (5.4) | 43.0 (15.5) | 41.8 (10.5) |
| n | 16 | 17 | 23 | 14 | 6 | 8 | 24 |
| Follow-up | 39.7 (15.9) | 40.8 (8.5) | 35.9 (6.8) | 42.0 (9.7) | 41.5 (2.1) | 37.2 (17.2) | 43.9 (9.1) |
| n | 7 | 6 | 11 | 7 | 2 | 5 | 17 |
| P* | .44 | .47 | .36 | .25 | .50 | .68 | .66 |
| Chelator . | Chelator dose, mg/kg/d, mean (SD) . | ||||||
|---|---|---|---|---|---|---|---|
| Baseline LIC, mg/g dw . | Baseline cardiac T2*, ms . | ||||||
| < 4 . | 4 to < 7 . | 7 to < 15 . | ≥ 15 . | < 10 . | 10 to < 20 . | ≥ 20 . | |
| DFX | |||||||
| Baseline | 21.8 (10.6) | 23.3 (6.0) | 27.1 (6.3) | 28.5 (8.9) | 29.8 (10.9) | 30.7 (8.9) | 24.9 (8.0) |
| n | 28 | 37 | 69 | 35 | 12 | 22 | 69 |
| Follow-up | 25.1 (14.5) | 28.4 (7.1) | 29.0 (10.7) | 36.5 (18.8) | 39.0 (28.5) | 34.5 (15.0) | 26.9 (7.9) |
| n | 20 | 26 | 58 | 28 | 10 | 18 | 53 |
| P* | .47 | .004 | .28 | .06 | .42 | .31 | .11 |
| DFO | |||||||
| Baseline | 38.0 (13.2) | 40.7 (11.0) | 38.6 (12.1) | 36.7 (12.1) | 44.7 (5.4) | 43.0 (15.5) | 41.8 (10.5) |
| n | 16 | 17 | 23 | 14 | 6 | 8 | 24 |
| Follow-up | 39.7 (15.9) | 40.8 (8.5) | 35.9 (6.8) | 42.0 (9.7) | 41.5 (2.1) | 37.2 (17.2) | 43.9 (9.1) |
| n | 7 | 6 | 11 | 7 | 2 | 5 | 17 |
| P* | .44 | .47 | .36 | .25 | .50 | .68 | .66 |
For change in dose from baseline to follow-up for each chelator.
Iron burden
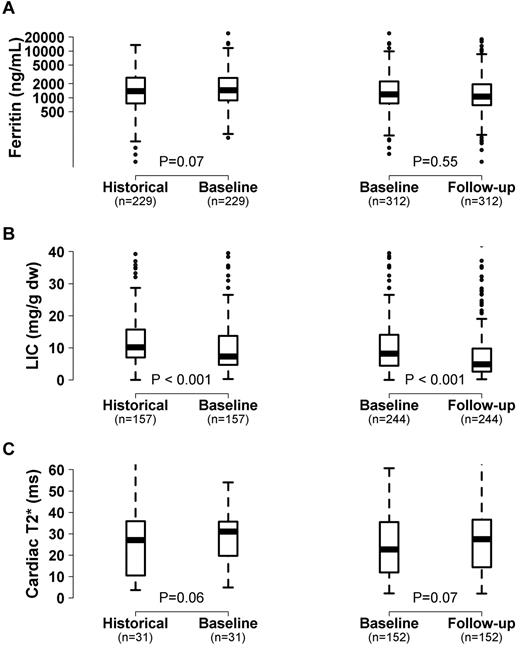
Overall, LIC significantly improved and there was a trend toward an increase in cardiac T2* and improvement in ferritin levels in this patient cohort from the historical to baseline measurement obtained at a mean of 5.7 years later (Figure 1). From baseline to the last follow-up (mean time, 2.2 years; range, 0.7-3.4 years), LIC continued to improve and the trend toward improvement in cardiac T2* also continued (Figure 1). Of 246 subjects with at least 2 LIC measurements, 190 (77%) improved by the last measurement, and of 153 subjects with 2 or more cardiac T2* values, 95 (62%) improved.
Change in iron measurements in chelated subjects over time. Box and whisker plot showing the change in ferritin level (A), LIC (B), and cardiac T2* (C) among patients receiving chelation from the historical measurement (2002-2004) to baseline (2007-2009) to follow-up visit (2008-2011). The line within the box represents the median value.
Change in iron measurements in chelated subjects over time. Box and whisker plot showing the change in ferritin level (A), LIC (B), and cardiac T2* (C) among patients receiving chelation from the historical measurement (2002-2004) to baseline (2007-2009) to follow-up visit (2008-2011). The line within the box represents the median value.
At baseline (2007-2009), both LIC and cardiac T2* values were better in the United Kingdom cohort compared with the North-American subjects (Table 5). Iron burden improved over time in both the United Kingdom and North America and, by follow-up assessment, the difference in iron burden was no longer significant (Table 5), although small numbers of subjects in the United Kingdom cohort at follow-up may have limited these analyses.
Liver and cardiac iron at baseline and follow-up in UK and North-American sites
| . | Baseline (2007-2009) . | Follow-up (2008-2011) . | ||||
|---|---|---|---|---|---|---|
| United Kingdom . | North America . | P* . | United Kingdom . | North America . | P* . | |
| LIC (mg/g dw) | n = 29 | n = 260 | n = 19 | n = 251 | ||
| Median (range) | 4.1 (0.5-40.6) | 9.0 (0.3-68) | < .001 | 3.4 (0.5-20.1) | 5.0 (0.2-45.8) | .15 |
| Cardiac T2* (ms) | n = 32 | n = 157 | n = 14 | n = 199 | ||
| Mean (SD) | 30.9 (12.6) | 23.8 (13.6) | < .001 | 30.5 (17.1) | 26.5 (12.0) | .53 |
| . | Baseline (2007-2009) . | Follow-up (2008-2011) . | ||||
|---|---|---|---|---|---|---|
| United Kingdom . | North America . | P* . | United Kingdom . | North America . | P* . | |
| LIC (mg/g dw) | n = 29 | n = 260 | n = 19 | n = 251 | ||
| Median (range) | 4.1 (0.5-40.6) | 9.0 (0.3-68) | < .001 | 3.4 (0.5-20.1) | 5.0 (0.2-45.8) | .15 |
| Cardiac T2* (ms) | n = 32 | n = 157 | n = 14 | n = 199 | ||
| Mean (SD) | 30.9 (12.6) | 23.8 (13.6) | < .001 | 30.5 (17.1) | 26.5 (12.0) | .53 |
For comparison of United Kingdom values with North-American values.
Changes in ferritin level and LIC with monotherapy with DFO or DFX are shown in Table 6. Individual results were highly variable. There was no significant difference in the change in LIC or cardiac T2* between subjects taking DFO compared with DFX after adjusting for time. Thirty-three percent of subjects receiving DFO or DFX monotherapy at study entry (baseline) had ferritin > 2500 ng/mL, LIC > 15 mg/g dw, and/or cardiac T2* < 10 ms, all indices of severe iron overload; this significantly improved to 26% (P = .01) at follow-up (33% to 27% for DFO and 32% to 24% for DFX). Adherence to treatment was higher in the DFX group than in the DFO group (Table 6).
Change in iron burden on DFO or DFX monotherapy
| Measurement . | DFO . | Mean time, y (SD)† . | P‡ . | DFX . | Mean time, y (SD)† . | P‡ . | P DFO vs DFX§ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Historical* . | Baseline* . | Follow-up* . | Historical* . | Baseline* . | Follow-up* . | ||||||
| Ferritin (ng/mL) | n = 64 | n = 78 | n = 67 | n = 168 | n = 188 | n = 180 | |||||
| Mean (SD) | 1964 (2275) | 1664 (2162) | 1376 (1282) | 4.1 (2.0) | .37 | 2202 (2082) | 1906 (2197) | 1859 (2353) | 3.7 (1.4) | .001 | .004 |
| LIC (mg/g dw) | n = 35 | n = 56 | n = 52 | n = 63 | n = 147 | n = 136 | |||||
| Mean (SD) | 12.3 (8.4) | 10.2 (9.0) | 9.5 (8.8) | 4.0 (1.5) | .06 | 13.7 (13.4) | 10.4 (8.7) | 6.6 (7.0) | 3.8 (1.4) | < .001 | .42 |
| Cardiac T2*, ms | n = 17 | n = 39 | n = 37 | n = 43 | n = 92 | n = 85 | |||||
| Mean (SD) | 30.9 (14.7) | 25.9 (13.8) | 24.6 (12.7) | 3.3 (1.2) | .09 | 25.0 (14.1) | 26.3 (13.3) | 27.4 (13.6) | 3.8 (1.4) | .76 | .46 |
| Adherence, n (%) | |||||||||||
| < 25% | 1 (1.7) | 1 (1.4) | 1 (2.1) | .12 | 1 (1.1) | 1 (0.6%) | 2 (1.5) | .72 | .004¶ | ||
| 25%-49% | 6 (0.0) | 2 (2.9) | 1 (2.1) | 3 (3.3) | 2 (1.1%) | 1 (0.8) | |||||
| 50%-74% | 11 (18.3) | 11 (15.7) | 7 (14.6) | 6 (6.6) | 15 (8.3%) | 13 (9.8) | |||||
| ≥ 75% | 42 (70.0) | 56 (80.0) | 39 (81.3) | 81 (89.0) | 162 (90.0%) | 117 (88.0) | |||||
| Measurement . | DFO . | Mean time, y (SD)† . | P‡ . | DFX . | Mean time, y (SD)† . | P‡ . | P DFO vs DFX§ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Historical* . | Baseline* . | Follow-up* . | Historical* . | Baseline* . | Follow-up* . | ||||||
| Ferritin (ng/mL) | n = 64 | n = 78 | n = 67 | n = 168 | n = 188 | n = 180 | |||||
| Mean (SD) | 1964 (2275) | 1664 (2162) | 1376 (1282) | 4.1 (2.0) | .37 | 2202 (2082) | 1906 (2197) | 1859 (2353) | 3.7 (1.4) | .001 | .004 |
| LIC (mg/g dw) | n = 35 | n = 56 | n = 52 | n = 63 | n = 147 | n = 136 | |||||
| Mean (SD) | 12.3 (8.4) | 10.2 (9.0) | 9.5 (8.8) | 4.0 (1.5) | .06 | 13.7 (13.4) | 10.4 (8.7) | 6.6 (7.0) | 3.8 (1.4) | < .001 | .42 |
| Cardiac T2*, ms | n = 17 | n = 39 | n = 37 | n = 43 | n = 92 | n = 85 | |||||
| Mean (SD) | 30.9 (14.7) | 25.9 (13.8) | 24.6 (12.7) | 3.3 (1.2) | .09 | 25.0 (14.1) | 26.3 (13.3) | 27.4 (13.6) | 3.8 (1.4) | .76 | .46 |
| Adherence, n (%) | |||||||||||
| < 25% | 1 (1.7) | 1 (1.4) | 1 (2.1) | .12 | 1 (1.1) | 1 (0.6%) | 2 (1.5) | .72 | .004¶ | ||
| 25%-49% | 6 (0.0) | 2 (2.9) | 1 (2.1) | 3 (3.3) | 2 (1.1%) | 1 (0.8) | |||||
| 50%-74% | 11 (18.3) | 11 (15.7) | 7 (14.6) | 6 (6.6) | 15 (8.3%) | 13 (9.8) | |||||
| ≥ 75% | 42 (70.0) | 56 (80.0) | 39 (81.3) | 81 (89.0) | 162 (90.0%) | 117 (88.0) | |||||
Historical values are retrospective values obtained at the time point closest to starting (or continuing) the chelator at the time of enrollment, baseline values are values at the time point closest to the baseline visit, and follow-up values are the last available follow-up values while still receiving that chelator.
Time refers to total time on chelator (historical to follow-up).
For comparison of historical and follow-up values within each chelator type.
For comparison of change in iron burden between DFO and DFX after adjusting for duration of time on the chelator.
For overall comparison of adherence rates after adjusting for time effect.
Thirty-two patients were receiving combination chelation therapy with either DFO/DFP (n =22) or DFO/DFX (n = 10) at study enrollment. The mean age of subjects receiving DFO/DFP was 29.5 years (range, 13-51 years) and they had been receiving this combination for a mean of 1.29 ± 1.05 years at baseline (range, 0-4.42 years). Subjects receiving DFO/DFP had significantly lower cardiac T2* compared with subjects not receiving this combination (median cardiac T2* = 10.5 vs 26 ms, P = .0004), but similar ferritin levels (median, 1727 vs 1257 ng/mL, P = nonsignificant) and LIC (median, 5.55 vs 8.3 mg/g dw, P = nonsignificant). Among 8 patients with follow-up after a mean of 2.5 years of DFO/DFP combination treatment, cardiac T2* significantly improved from mean 9.1 ± 5.3 to 12.7 ± 8.4 ms, P = .04, whereas LIC did not significantly change (10.0 ± 11.2 to 11.2 ± 6.6 mg/g dw, P = .28, n = 7). Subjects receiving DFO/DFX had a mean age of 21.4 years (range, 10-30 years) and had received this combination for a mean duration of 1.04 ± 1.16 years (range, 0-3.3 years) at baseline. Their cardiac T2* was similar to subjects not receiving DFO/DFX (median 23.5 vs 24.7 ms, P = nonsignificant), but ferritin levels (median, 3380 vs 1271 ng/mL, P = .01) and LIC (median 17.2 vs 8 mg/g dw, P = .003) were significantly higher. Among the 4 subjects treated with DFO/DFX with follow-up of a mean of 2.1 years, a trend toward improvement in cardiac T2* (21.1 ± 9.2 to 26.2 ± 4.8 ms, P = .06) was evident, whereas LIC was not significantly changed (14.1 ± 4.2 to 11.4 ± 7.3 mg/g dw, P = .47).
Cardiac complications and mortality
From baseline to the last follow-up, 6 new cardiac events occurred in 4 subjects (congestive heart failure, n = 1; ventricular arrhythmia, n = 3; and supraventricular arrhythmia, n = 2; Table 1). Among subjects more than 15 years of age, the rate of any new cardiac event was 5.59 cases per 1000 patient-years. Seven deaths occurred from study entry (baseline) to the last follow-up, including 5 from cardiac causes and 1 sudden death (Table 7). The overall mortality rate was 6.30 cases/1000 person-years, whereas the mortality rate among subjects more than 15 years of age was 9.88 cases per 1000 person-years. There was no significant difference in the most recent cardiac T2* or LIC between the groups with new cardiac events and/or cardiac deaths and the remainder of the subjects.
Deaths in the longitudinal cohort study
| Patient no. . | Sex . | Age at death, y . | Cause of death . | Time of radiology studies prior to death, mo . | Cardiac T2*, ms . | LIC, mg/g dw . |
|---|---|---|---|---|---|---|
| 1 | F | 32.2 | Cardiomyopathy | 16 | 7 | 5.8 |
| 2 | F | 32.3 | Right-sided heart failure | 2 | NA | 14.4 |
| 3 | F | 33.1 | Congestive heart failure | 24 | NA | 6.2 |
| 4 | M | 40.4 | Congestive heart failure; hepatocellular carcinoma | 26 | 24.2 | 10 |
| 5 | F | 53.9 | Malignancy | 9 | 29.9 | 17.9 |
| 6 | M | 35.9 | Congestive heart failure | NA | NA | NA |
| 7 | M | 41.5 | Sudden death | 19 | 27 | 1.4 |
| Patient no. . | Sex . | Age at death, y . | Cause of death . | Time of radiology studies prior to death, mo . | Cardiac T2*, ms . | LIC, mg/g dw . |
|---|---|---|---|---|---|---|
| 1 | F | 32.2 | Cardiomyopathy | 16 | 7 | 5.8 |
| 2 | F | 32.3 | Right-sided heart failure | 2 | NA | 14.4 |
| 3 | F | 33.1 | Congestive heart failure | 24 | NA | 6.2 |
| 4 | M | 40.4 | Congestive heart failure; hepatocellular carcinoma | 26 | 24.2 | 10 |
| 5 | F | 53.9 | Malignancy | 9 | 29.9 | 17.9 |
| 6 | M | 35.9 | Congestive heart failure | NA | NA | NA |
| 7 | M | 41.5 | Sudden death | 19 | 27 | 1.4 |
NA indicates not available.
Discussion
In this large, diverse cohort, we describe current chelation use, monitoring practices, and iron burden among thalassemia patients in North America and London. In recent years, MRI techniques have nearly replaced invasive biopsy for the measurement of liver iron in this patient population. In parallel, a significantly greater number of patients have undergone liver and cardiac iron measurements, likely reflecting the greater availability of MRI techniques and greater patient acceptance of noninvasive iron measurements. Overall, there was an improvement in LIC and a trend toward improvement in cardiac iron loading at last follow-up compared with measurements from a mean of 8 years earlier. Among patients with follow-up data, more than three-quarters had a reduction in LIC and more than 60% showed improvement in cardiac T2*. The lack of significant change in serum ferritin level in the same time frame underscores the need for liver and cardiac iron assessment in the management of these patients. Therefore, it appears that improved organ-specific iron imaging and expanded chelator options have had a positive impact on patient care.
The overall mortality rate in our patient population was 6.3 per 1000 patient-years, similar to the rate reported from 2000-2004 in the Cyprus thalassemia cohort (approximately 5 per 1000 patient-years). However, in our cohort, cardiac disease contributed to at least 70% of deaths, compared with only approximately 50% of deaths in the Cyprus cohort.19 Three of the 7 patients who died in our cohort had not had prior cardiac T2* measurements. Increasing use of MRI with appropriate treatment adjustments may decrease morbidity and mortality over time. Combination therapy with DFP/DFO also was not available for routine clinical use in most TCRN sites during the study period, whereas it was available after 2000 in the Cyprus cohort,19 which could have contributed to the difference in cardiac deaths. The recent FDA approval of DFP as a second-line agent in the United States will likely result in a significant change in chelator use patterns, particularly in patients with low cardiac T2* values.
Similarly, in a preliminary report of a cohort of UK patients monitored for a decade with cardiac T2* MRI, the mortality rate was only 1.65 per 1000 patient-years.20 Cardiac mortality was low and the percentage of patients with T2* below 20 ms decreased from 60% to 17% over the decade.20 Interestingly, within our TCRN cohort, both LIC and cardiac T2* values were significantly better in the UK patients at baseline compared with North-American patients. T2* MRI techniques have been used clinically in the United Kingdom for at least 5 years longer than in the North American sites. Furthermore, DFP was used significantly more frequently in the United Kingdom sites during the study period. These data support a positive effect of MRI use and of expanded chelation options on patient outcome. The difference in iron burden between the United Kingdom and North America was not evident at follow-up, perhaps because of smaller patient numbers available for analysis or increased use of MRI and/or oral chelation in the North American cohort over time.
DFX is currently the predominant chelator used in patients with thalassemia in the TCRN. In a recent report from an Italian cohort,21 DFX also was used most commonly, but was used in approximately 29% compared with more than 50% of patients in our cohort. The clinical availability of DFP in Italy likely contributed to this difference, because DFP monotherapy was used in approximately 15% and combination DFO/DFP in 25% of the Italian cohort.21 In our patient population, the majority of subjects chose to switch to DFX either through participation in clinical trials or once the drug became clinically available, likely because of the easier mode of administration. Switches from DFX back to DFO related to rising iron burden and to known drug toxicities, including gastrointestinal upset, elevation in hepatic transaminases, renal dysfunction, and gastrointestinal bleeding, also occurred.
Adherence to chelation therapy was significantly better among subjects receiving DFX compared with DFO. This result is perhaps not surprising, given that oral administration should be more acceptable to patients than the parenteral route required for DFO. A previous report showed that in a large phase 3 clinical trial, thalassemia patients randomized to treatment with DFX reported significantly higher treatment satisfaction levels and found the regimen to be more convenient than patients randomized to DFO.22 The results of our present study suggest that these benefits of DFX translate into better adherence to chelation therapy in clinical practice.
A significant reduction in LIC occurred between the 2002-2004 starting point for retrospective review and the last follow-up from 2008-2011 among patients who were receiving DFX. In the same time period, LIC also improved among subjects taking DFO, but the change was not statistically significant. Smaller numbers of subjects receiving DFO may have limited the analyses. Better adherence to DFX therapy could also have resulted in improved iron levels with this chelator. In addition, the mean DFX dose increased both with increasing hepatic and cardiac iron burden and over time, whereas DFO dosing was more constant in relation to cardiac and hepatic iron burden (Table 4). No significant change in cardiac T2* values was seen with either DFO or DFX over the study period, but small numbers of paired cardiac T2* studies may have limited these analyses. Future analysis of the longitudinal data should allow such comparisons, given that the rate of use of cardiac T2* MRI in our cohort has significantly improved over time.
Treatment with more than one chelator used in combination is currently being used in only approximately 15% of our patient cohort. DFP combined with DFO is most commonly used in subjects with low cardiac T2* values. Combination therapy with DFX and DFO appears to be prescribed more commonly in response to high ferritin levels and/or LIC, rather than for evidence of cardiac iron deposition.
There are several limitations to the present study. First, several different techniques were used to assess LIC, including biopsy, R2 and R2* MRI, and SQUID measurements, and the correlation of LIC results between measures is not precise. Nonetheless, LIC obtained by R2 and R2* MRI have been shown to correlate well with biopsy results for a wide range of LIC measurements7,9 and, although SQUID measurements have underestimated LIC obtained by biopsy in some clinical trials,17 the error is minimized when an appropriate conversion factor is used.16 Furthermore, because the MRI iron measurements were obtained clinically, local readings were used and central validation was not performed in the current study. Secondly, chelation regimens were not uniform and dosing of DFX in particular was variable, with probable underdosing of some patients in the early years before appropriate dosing strategies were recognized.17,23 Finally, adherence was estimated through provider/patient interaction and not by the measurement of drug levels or formal pill/vial counts, which could bias results.24
In this large cohort of North American and United Kingdom patients with thalassemia, clinical use of MRI estimates of liver and cardiac iron loading and of oral chelation have increased substantially over the past decade and an overall improvement in iron burden has occurred in parallel. These results strongly support the routine use of MRI measurements of iron and oral chelation in the management of iron overload. Consensus recommendations among the thalassemia treatment centers participating in this study were to obtain at least annual cardiac iron T2* MRI beginning at age 10 years (with more frequent measurements considered for patients with T2* < 10-20 ms) and annual liver iron measurements, without a specified starting age, for transfusion-dependent patients and to adjust chelation in response to these measurements. Nonetheless, in approximately 25% of subjects, LIC of 15 mg/g dw or greater, serum ferritin levels of 2500 ng/mL or higher, and/or cardiac T2* less than 10 ms still were observed at last follow-up. The risk of cardiac disease and death is higher among patients with such elevated iron stores,3,5,6,11,25 and cardiac causes contributed to more than 70% of deaths in our series. Therefore, close monitoring of iron burden with appropriate chelator and dose adjustments, routine assessment of potential side effects and other obstacles to optimal adherence, as well as ongoing encouragement and support of adherence are essential.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following National Heart, Lung, and Blood Institute/National Institutes of Health (NIH) cooperative agreements: U01-HL65232 and NIH/NCRR UL1-RR024134 to the Children's Hospital of Philadelphia; U01-HL72291 and Harvard Catalyst CTSC UL1-RR-025758 to Children's Hospital, Boston; U01-HL65233 to University Health Network Toronto General Hospital; U01-HL65239 and CTS1 ULI-RR024131 to Children's Hospital & Research Center Oakland; U01-HL65244 and CTSC UL1-RR024996 to Weill Medical College of Cornell University; and U01-HL65238 to the New England Research Institutes.
National Institutes of Health
Authorship
Contribution: J.L.K. assisted in study design, provided study and patient data, contributed to data analysis and interpretation, and wrote the manuscript; H.-Y.K. contributed to data analysis and interpretation and edited the manuscript; A.A.T., C.T.Q., B.U.M., I.O., P.J.G., E.P.V., J.M.B., A.R.C., J.B.P., T.C., and N.F.O. provided study and patient data and edited the manuscript; and E.J.N. designed the study, provided study and patient data, contributed to data analysis and interpretation, and edited the manuscript.
Conflict-of-interest disclosure: C.T.Q. is on the advisory board for ApoPharma. B.U.M. receives research funding from Novartis. E.P.V. receives funding and has other relationships with Novartis and with ApoPharma. A.R.C. received reimbursement for travel expenses for an annual meeting of the safety committee from ApoPharma. J.B.P. receives research funding from and is on the advisory board and speakers' bureau for Novartis. T.C. receives research funding from and is a consultant and speaker for Novartis and is a consultant for ApoPharma. E.J.N. receives research funding from Novartis and from FerroKin BioSciences. The remaining authors declare no competing financial interests.
Correspondence: Janet L. Kwiatkowski, MD, MSCE, The Children's Hospital of Philadelphia, Division of Hematology, 34th St and Civic Center Blvd, Colket Translational Research Bldg, Rm 11024, Philadelphia, PA 19104; e-mail:kwiatkowski@e-mail.chop.edu.