Abstract
Familial platelet disorder with a propensity to develop acute myeloid leukemia (FPD/AML) is a rare autosomal dominant disease characterized by thrombocytopenia, abnormal platelet function, and a propensity to develop myelodysplastic syndrome (MDS) and AML. So far, > 20 affected families have been reported. Recently, a second RUNX1 alteration has been reported; however, no additional molecular abnormalities have been found so far. We identified an acquired CBL mutation and 11q-acquired uniparental disomy (11q-aUPD) in a patient with chronic myelomonocytic leukemia (CMML) secondary to FPD with RUNX1 mutation but not in the same patient during refractory cytopenia. This finding suggests that alterations of the CBL gene and RUNX1 gene may cooperate in the pathogenesis of CMML in patients with FPD/AML. The presence of CBL mutations and 11q-aUPD was an important “second hit” that could be an indicator of leukemic transformation of MDS or AML in patients with FPD/AML.
Introduction
Familial platelet disorder with a propensity to develop acute myeloid leukemia (FPD/AML) is a rare autosomal dominant disease characterized by thrombocytopenia, abnormal platelet function, and a propensity to develop myelodysplastic syndrome (MDS) and AML.1,2 Since Song et al reported haploinsufficiency of the RUNX1/CBFA2 gene,3 more than 20 affected families have been reported.4-8 Notably, various types of mono-allelic mutations of the RUNX1 gene have been found in patients with AML secondary to FPD.3,7-9 RUNX1, which is a key regulator of definitive hematopoiesis and myeloid differentiation, is also commonly involved in sporadic cases of MDS and AML, by translocations in AML10 and by point mutations in AML11,12 and MDS.13 Recently, a second RUNX1 alteration has been reported8 ; however, no additional molecular abnormalities have been found so far.
In this regard, recent reports of somatic mutations of the CBL proto-oncogene in myeloid neoplasms are intriguing because these CBL mutations have been shown to result in aberrant tyrosine kinase signaling, which would also lead to the activation of RAS signaling pathways. So far, we and others have reported that CBL mutations occurred in a variety of myeloid neoplasms, including de novo AML,14,15 MDS,16,17 and myeloproliferative neoplasm,16,17 especially in chronic myelomonocytic leukemia (CMML)16,17 and juvenile myelomonocytic leukemia.18 The importance of CBL mutations for leukemogenesis has substantially increased, which prompted us to search for possible CBL mutations in this pedigree.
Here, we reported that CBL mutation developed at the time of diagnosis of CMML, but not during refractory cytopenia, in a Japanese patient with FPD/AML harboring a RUNX1 mutation.
Methods
RUNX1 mutation analysis
DNA and RNA were extracted from peripheral blood (PB) of the proband, her sister, and their mother after obtaining informed consent. We performed mutation analysis of the RUNX1 gene by PCR followed by direct sequencing with the use of an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). For further confirmation of deletion mutations, the PCR products were subcloned with the use of a TOPO TA Cloning Kit (Invitrogen) and then sequenced. Mutations were screened from exons 1-8 of the RUNX1 gene.
CBL mutation analysis
Because CBL mutations thus far reported almost exclusively involved exons 8-9 that encode Linker/RING finger domains, we confined our mutation analysis to these exons, which were subjected to direct sequencing. Because the frequency of 11q-acquired uniparental disomy (11q-aUPD) was reported as ∼ 85%-90% in CBL mutations, we also analyzed the sample with Affymetrix GeneChip 250K NspI.17-19 Genome-wide detection of copy number abnormalities or allelic imbalances was performed with CNAG/AsCNAR Version 3.0 software (http://www.genome.umin.jp), which enabled sensitive detection of copy number neutral loss of heterozygosity (or aUPD).19 In addition, we examined mutations of the following genes in the proband as previously reported: FLT3, KIT, RAS, JAK2, PTPN11, ASXL1, IDH1/2, and MPL.20-22 The study adhered to the principles of the Helsinki Declaration and was conducted under the regulations enacted by the Ethics Board of Gunma Children's Medical Center.
Results and discussion
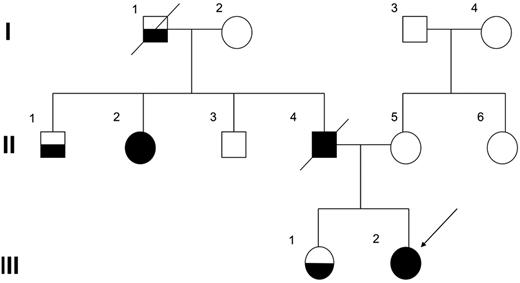
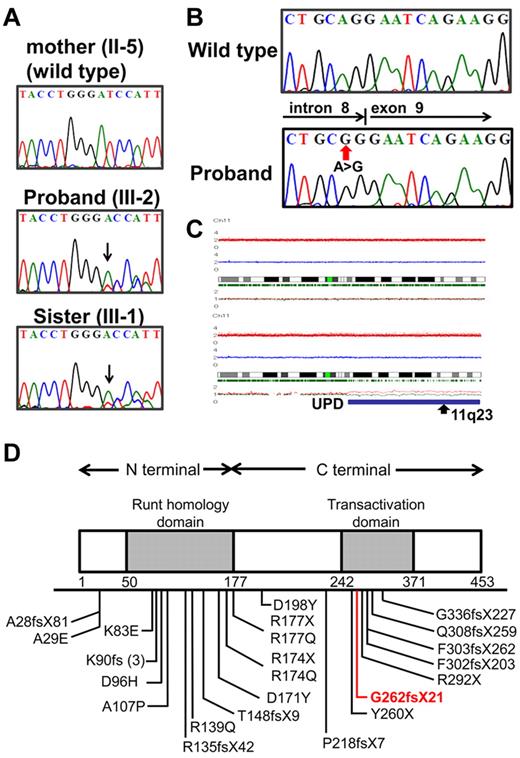
The proband (III-2), who was the second child of nonconsanguineous parents, underwent an 8-year follow-up of mild to moderate thrombocytopenia (50-80 × 103/μL), and at that age of 10 years, her condition was diagnosed as refractory cytopenia. Cytogenetic analysis found a normal karyotype, and FISH showed neither monosomy 7 nor trisomy 8. The proband had been closely observed without any therapy for 2 years and 9 months because she did not require transfusion and her disease remained stable; however, at the age of 12 years, leukocytosis and monocytosis developed and she became dependent on platelet transfusions. Finally, the disease evolved to CMML, and allogeneic bone marrow (BM) transplantation from an unrelated donor was performed. During the entire course, the number of blast cells in PB was constantly < 2%, and no additional symptoms were observed, such as hepatosplenomegaly. Her elder sister (III-1) was also followed for 10 years with mild thrombocytopenia; however, the morphologic findings of PB or BM were not compatible with myeloproliferative neoplasms.17 Because her platelet count has been gradually decreasing, allogeneic BM transplantation is being considered. Although her father (II-4) developed MDS at the age of 41 and died 2 years later, her paternal aunt (II-2) developed MDS at the age of 49 and has remained in complete remission for 11 years after successful allogeneic cord blood transplantation. Her paternal grandfather (I-1) and uncle (II-1) also had a history of thrombocytopenia (Figure 1). Direct sequencing analysis of RUNX1 found a one-base deletion of adenine at position 2364 within exon 7, resulting in a frameshift mutation that corresponded to AML1b transcript in the proband and her sister (Figure 2A). This resulted in a frameshift after amino acid change G262GfsX21. This mutation was not detected in their mother. All these data suggested that her paternal grandfather (I-1), uncle (II-1), aunt (II-2), and her father (II-4) were considered to have FPD/AML, carrying the same RUNX1 mutation.
The family pedigree. Squares indicate males and circles indicate females. Open symbols represent unaffected persons, half-filled symbols represent persons affected by thrombocytopenia, and closed symbols represent persons affected by FPD who developed MDS/AML. The proband (III-2) is indicated by an arrow.
The family pedigree. Squares indicate males and circles indicate females. Open symbols represent unaffected persons, half-filled symbols represent persons affected by thrombocytopenia, and closed symbols represent persons affected by FPD who developed MDS/AML. The proband (III-2) is indicated by an arrow.
Mutation analysis of RUNX1 and CBL genes in the pedigree. (A) Direct sequencing analysis of affected patients (III-1, III-2) and an unaffected family member (II-5) is shown. Arrow indicates a one-base deletion of adenine. (B) Mutated CBL is shown in the proband. (C) Identification of acquired uniparental disomy of 11q in the proband. Total copy number (tCN; red plot) is shown above the cytoband, and the results of allele-specific copy number analysis with anonymous references (AsCNAR) plots are shown below the cytoband. Larger allele is presented by a red line, and the smaller allele is presented by a blue line. Allele-specific analysis showed 11q-aUPD (blue line), which contained the CBL region (arrow). (D) Schematic representation of wild-type and mutated RUNX1. The affected RUNX1 is truncated at the C terminus of the transactivation domain (TAD). Part of TAD is lacking in this proband (red line).
Mutation analysis of RUNX1 and CBL genes in the pedigree. (A) Direct sequencing analysis of affected patients (III-1, III-2) and an unaffected family member (II-5) is shown. Arrow indicates a one-base deletion of adenine. (B) Mutated CBL is shown in the proband. (C) Identification of acquired uniparental disomy of 11q in the proband. Total copy number (tCN; red plot) is shown above the cytoband, and the results of allele-specific copy number analysis with anonymous references (AsCNAR) plots are shown below the cytoband. Larger allele is presented by a red line, and the smaller allele is presented by a blue line. Allele-specific analysis showed 11q-aUPD (blue line), which contained the CBL region (arrow). (D) Schematic representation of wild-type and mutated RUNX1. The affected RUNX1 is truncated at the C terminus of the transactivation domain (TAD). Part of TAD is lacking in this proband (red line).
Although no CBL mutations were found in the proband sample of refractory cytopenia before development of CMML, homozygous mutation of the CBL, which was located in the splice acceptor site of intron 8 (Figure 2B), was identified in the proband sample in the CMML. We also found 11q-aUPD (Figure 2C) in the proband sample, confirming a strong association of CBL mutations with 11q-aUPD, as previously described16-18 ; however, no mutations of any other genes, including FLT3, KIT, RAS, JAK2, PTPN11, ASXL1, IDH1/2, and MPL, were found and no additional somatic RUNX1 alterations. No CBL mutations were found in her sister's sample at this time.
Inherited RUNX1 mutations were clustered in the N-terminal region in exons 3-5, which affect the runt homology domain. Mutations in the C-terminal region, detected in the present pedigree, have been reported less frequently so far and are considered to affect the transactivation domain (Figure 2D).
It has been postulated that disruption of the RUNX1 gene is not sufficient to cause AML, as previously reported with monoallelic and biallelic inactivation of Runx1 in mice23,24 and in mice carrying the knocked-in Runx1-Eto chimeric gene. These data indicate that a second-hit mutation in addition to the dysfunction of RUNX1 is required for the development of AML. Minelli et al postulated that the mutations seen in FPD cases have a mutation effect that induces additional genetic abnormalities and promotes progression to hematologic malignancies.25
Marked associations between chromosome translocation and gene mutations have been reported: KIT mutation in core binding leukemia, t(8;21)/AML1-ETO and inv(16)(p13q22)/CBFβ-MYH11, FLT3-ITD in leukemia with t(15;17)/PML-RARα, or with t(6;9)/DEK-CAN. We consider that it is important to find an association to administer clinically relevant treatment. In addition to the germline RUNX1 mutation, we identified an acquired CBL mutation in the proband and assumed it to be a second hit mutation by which FPD evolved into CMML. To our knowledge, this is the first patient with FPD/AML in whom CBL mutation has developed. This finding suggests that alterations of the CBL gene and RUNX1 could cooperate in the pathogenesis of CMML or AML in patients with FPD/AML. The presence of 11q-aUPD provided evidence that loss of the wild-type copy of CBL with duplication of the mutant copy was an important second hit that could be an indicator of leukemic transformation in patients with FPD/AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Miss Sayaka Takeuchi for her excellent technical assistance.
This work was supported by a grant for cancer research and a grant for research on children and families from the Ministry of Health, Labor, and Welfare of Japan; a Grant-in-Aid for Scientific Research (B, C) and Exploratory Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by a research grant for Gunma Prefectural Hospitals.
Authorship
Contribution: Y.H. and C.O. designed the study; A.M., C.O., and D.H. provided critical reagents and samples; N.S., M.P., A.S.-O., and C.M. performed the experiments; H.A. and S.O. supervised the work; N.S. and M.P. analyzed the results; N.S. and D.H. constructed the figures; N.S. and Y.H. wrote the paper; and all the authors critically reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yasuhide Hayashi, Department of Hematology/Oncology, Gunma Children's Medical Center, 779, Shimohakoda, Hokkitsu, Shibukawa, Gunma, 377-8577, Japan; e-mail: hayashiy-tky@umin.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal