Abstract
ADAMTS13, a metalloprotease, plays a pivotal role in preventing spontaneous microvascular thrombosis by cleaving hyperactive ultra large von Willebrand factor multimers into smaller, less active multimers. Reduced ADAMTS13 activity in plasma has been described in many diseases associated with systemic inflammation. It remains uncertain, however, whether ADAMTS13 contributes to disease pathogenesis or rather simply serves as an inflammation-associated marker. We hypothesized that, by decreasing vascular inflammation, ADAMTS13 reduces the development of early atherosclerotic plaques. Using intravital fluorescence microscopy, we observed excessive leukocyte adhesion and accelerated atherosclerotic plaque formation at the carotid sinus of Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice fed a high-fat Western diet. At 4 months of age, there was a significant increase in atherosclerosis in the aorta and aortic sinus of Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice. Interestingly, we detected a 2-fold increase in macrophage recruitment to the atherosclerotic plaque of the Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice, suggesting that the atherosclerotic lesions in these mice were not only larger but also more inflammatory. These findings reveal a new functional role for the antithrombotic enzyme ADAMTS13 in reducing excessive vascular inflammation and plaque formation during early atherosclerosis.
Introduction
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats-13) is a zinc-containing protease that is synthesized primarily by hepatic stellate cells1,2 and, to a lesser extent, by endothelial cells,3 both of which secrete ADAMTS13 into the bloodstream. The only known substrate for ADAMTS13 is von Willebrand factor (VWF), a hemostatic glycoprotein that is stored as ultra large VWF (ULVWF) multimers in platelet α-granules and endothelial storage granules called Weibel-Palade bodies.4 ULVWF multimers, which can be as large as 20 000 kDa and are considered to be the most hyperactive and thrombogenic form of VWF,4,5 are not present in blood plasma under normal conditions. However, on endothelial cell activation or injury, ULVWF multimers are released into the circulation from Weibel-Palade bodies. During the process of secretion, ULVWF remains transiently bound to the endothelial surface where they are cleaved by ADAMTS13 into smaller, less active VWF multimers, thereby reducing its thrombotic potential.6 The essential role of VWF in hemostasis is evidenced clinically in patients with von Willebrand disease, a bleeding disorder caused by a lack of functional VWF.7 In distinction, deficiency of ADAMTS13 increases plasma levels of ULVWF and causes thrombotic thrombocytopenic purpura, a disorder of thrombotic microangiopathy.8
Several epidemiologic studies have demonstrated reduced plasma ADAMTS13 activity in inflammatory conditions, including aging,9 systemic inflammation,10 pancreatitis,11 and sepsis.12,13 It remains uncertain, however, whether ADAMTS13 contributes to disease pathogenesis or rather simply serves as an inflammation-associated marker. Several recent findings led us to hypothesize that ADAMTS13 reduces inflammation during atherosclerosis through its proteolytic effect on ULVWF and/or VWF. First, platelets bound to ULVWF can support leukocyte tethering and rolling under high shear stress in vitro.14 Second, endothelium-derived VWF recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia,15 and platelet adhesion to activated endothelium is thought to play a crucial role in initiating lesion formation.16 Third, we have demonstrated that ADAMTS13 deficiency in mice promotes a VWF-dependent increase in leukocyte rolling, adhesion, and extravasation under acute inflammatory conditions,17 a process that also may promote inflammation in early atherosclerosis.
To determine the role of ADAMTS13 in atherosclerosis, we used intravital fluorescence microscopy to assess leukocyte adhesion and plaque formation at the carotid sinus, a lesion prone site, of Adamts13−/−/ApoE−/− mice and compared with ApoE−/− control mice. We also determined the role of ADAMTS13 in reducing atherosclerosis in the aorta and aortic sinus of ApoE−/− mice fed either a high-fat Western diet or a normal chow diet. Our findings reveal a critical role for ADAMTS13 in reducing inflammatory plaque formation during early atherosclerosis.
Methods
Animals
Adamts13−/− mice were backcrossed for more than 15 generations to the C57BL/6J background as described and characterized previously.18 To generate mice for this study, Adamts13−/− mice were intercrossed with ApoE−/− mice (C57BL/6J background). Adamts13−/−/ApoE−/− and littermate control ApoE−/− mice were generated and fed either a normal chow diet for 4 months or a high-fat Western diet (20% fat, 0.2% cholesterol) beginning at 6 weeks of age until they were killed at 4 months (ie, 10 weeks on high-fat Western diet) or 6.5 months (ie, 20 weeks on high-fat Western diet) of age. Both male and female mice were used. All experiments were approved by the University of Iowa Animal Care and Use Committee.
Intravital microscopy
Mice were anesthetized using 100 mg/kg ketamine/10 mg/kg xylazine. An incision was made, and the right common carotid artery and carotid bifurcation were carefully exposed at a distance of approximately 4 mm distal and approximately 6 to 7 mm proximal to carotid bifurcation. The exposed artery was kept moist by superfusion with warm (∼ 37°C) bicarbonate-buffered saline. Endogenous leukocytes were labeled by infusing 100 μL of 1 mg/mL rhodamine 6G (Sigma-Aldrich). We used the Nikon upright microscope with CF1 Fluor 10× and 20× water immersion objectives to visualize leukocyte-endothelial interactions. Spontaneous plaque formation was visualized with 10× and 20× water immersion objectives. All the movies were recorded through a high-speed EM camera and evaluated off-line using a Nikon computer-assisted image analysis program.
Extent and composition of atherosclerotic lesions
Before death, mice were anesthetized with 100 mg/kg ketamine/10 mg/kg xylazine and transcardially perfused with PBS followed by 4% paraformaldehyde under physiologic pressure, after which tissues were removed for histologic processing. To compare the extent of atherosclerosis among strains, whole aortae were isolated and stained with Oil Red O, and en face lesion area was measured morphometrically using National Institutes of Health (NIH) ImageJ Version 1.45 software. To quantify lesions in the aortic sinus, serial cross sections of 5 μm were cut through the aorta beginning at the origin of the aortic valve leaflets and stained with the VerHoeffs/Van Gieson method. Cross-sectional lesion area from each mouse was calculated using the mean value of 4 sections (each 80 μm apart, beginning at the aortic valve leaflets and spanning 320 μm) as described previously.19 NIH ImageJ Version 1.45 software was used for quantification.
Immunohistochemical staining
Tissue preparation and histochemical staining were performed as described.20 Antigen retrieval was performed before immunohistochemical staining. Briefly, 5-μm sections were incubated with blocking reagent followed by primary antibody (rat anti–mouse Mac-3, BD Biosciences PharMingen) or rat Ig (control) in the presence of 5% goat serum overnight at 4°C followed by biotin-conjugated goat anti–rat Ig, avidin-biotin complex, and 3,3′-diaminobenzidine as substrate (Vector Laboratories). Slides were counterstained with hematoxylin, dehydrated, and examined under a light microscope (Carl Zeiss). NIH ImageJ Version 1.45 software was used for lesion quantification and expressed as the area of positive immunostaining. A mean for each mouse was calculated using the mean value of 4 sections (each 80 μm apart, beginning at the aortic valve leaflets and spanning 320 μm).
Picrosirius red staining for type I and III collagen
To quantify interstitial collagen within the lesions of the aortic sinus, serial cross sections of 5 μm were cut through the aorta beginning at the origin of the aortic valve leaflets and stained with the Picrosirius red method. Briefly, serial formalin-fixed sections were incubated for 4 hours in a freshly prepared 0.1% solution of Sirius Red F3B (Sigma-Aldrich) in saturated aqueous picric acid. After rinsing twice in 0.01N HCl and distilled water, sections were dehydrated and mounted in Permount (Vector Laboratories). Picrosirius red staining was analyzed by polarization microscopy. NIH ImageJ Version 1.45 software with a defined threshold (minimum 100 and maximum 200) was used for quantification. A mean for each mouse was calculated from using the mean value of 4 sections (each 80 μm apart, beginning at the aortic valve leaflets and spanning 320 μm).
Determination of plasma lipid levels
Blood samples were collected in heparinized tubes by retro-orbital plexus puncture after overnight fasting. Plasma was separated by centrifugation and analyzed for total cholesterol (Thermo Electron) and triglyceride (Wako) levels using enzymatic colorimetric assays according to the manufacturer's instructions.
Statistical analysis
Values are expressed as mean ± SEM. Significance was analyzed using the Student 2-tailed unpaired t test. The proportion of mice that developed a spontaneous plaque at the carotid sinus was compared between different experimental groups using a contingency table and the χ2 test. A value of P < .05 was considered statistically significant.
Results
ADAMTS13 inhibits excessive leukocyte adhesion and atherosclerotic plaque formation at the carotid sinus
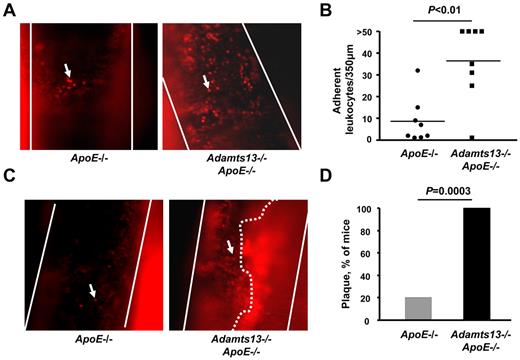
To test the hypothesis that ADAMTS13 reduces excessive leukocyte adhesion at arterial shear rates in the setting of atherosclerosis, we used intravital fluorescence microscopy to visualize basal leukocyte-endothelial interactions in the carotid sinus of Adamts13−/−/ApoE−/− and ApoE−/− mice fed a high-fat Western diet. At early time points (up to 10 weeks on Western diet and studied at 16 weeks of age), we did not observe spontaneous leukocyte adhesion, probably because of very small and/or absent atherosclerotic lesions at the carotid bifurcation (not shown). However, at a later time point (20 weeks on Western diet and studied at 26 weeks of age), we did observe spontaneous leukocyte adhesion to atherosclerotic lesions at the carotid sinus. The number of adherent leukocytes (adherent for > 60 seconds) was increased by approximately 4-fold at the carotid sinus of Adamts13−/−/ApoE−/− mice (mean ± SEM = 37 ± 6) compared with ApoE−/− mice (mean ± SEM = 9 ± 4, P < .01, Figure 1A-B; supplemental Videos 1-2). The mean number of adherent leukocytes in the Adamts13−/−/ApoE−/− mice is probably underestimated because 50% of the mice had more than 50 leukocytes adhering to the vessel wall (supplemental Video 2). Next, we visualized atherosclerotic plaque formed at the carotid sinus by intravital microscopy. Interestingly, we found that 100% (10 of 10) of the Adamts13−/−/ApoE−/− mice had detectable plaque at the carotid sinus that occluded the vessel by approximately 70% to 80% (Figure 1C-D; supplemental Video 3). In contrast, only 20% (2 of 10) of the ApoE−/− mice had plaque at the carotid sinus, and the plaques were small in size compared with Adamts13−/−/ApoE−/− mice (Figure 1C-D; supplemental Video 4). Together, these results suggest that ADAMTS13 reduces excessive leukocyte adhesion and plaque formation at the carotid sinus.
ADAMTS13 deficiency promotes excessive leukocyte adhesion and spontaneous plaque formation in the carotid sinus. (A) Representative photomicrographs of adhering leukocytes (> 60 seconds) at the carotid sinus, a lesion-prone site, as visualized by intravital upright microscope. Endogenous leukocytes were labeled with rhodamine 6G. White lines indicate the arteries; and arrows, adherent leukocyte. Original magnification ×200. (B) Dot plot shows the number of adherent leukocytes of each genotype (N = 8/group). Each dot represents a single mouse. Horizontal bars represent mean values. (C) Representative photomicrographs of spontaneous plaque formation as visualized by intravital upright microscope. Leukocytes were labeled with rhodamine 6G. White lines indicate the arteries; arrows, adherent leukocyte; and white dotted line, the spontaneous plaque, which occluded the vessel by approximately 70% to 80% (supplemental Video 4, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). (D) The percentage of mice with a spontaneous plaque at the carotid sinus (N = 10/group).
ADAMTS13 deficiency promotes excessive leukocyte adhesion and spontaneous plaque formation in the carotid sinus. (A) Representative photomicrographs of adhering leukocytes (> 60 seconds) at the carotid sinus, a lesion-prone site, as visualized by intravital upright microscope. Endogenous leukocytes were labeled with rhodamine 6G. White lines indicate the arteries; and arrows, adherent leukocyte. Original magnification ×200. (B) Dot plot shows the number of adherent leukocytes of each genotype (N = 8/group). Each dot represents a single mouse. Horizontal bars represent mean values. (C) Representative photomicrographs of spontaneous plaque formation as visualized by intravital upright microscope. Leukocytes were labeled with rhodamine 6G. White lines indicate the arteries; arrows, adherent leukocyte; and white dotted line, the spontaneous plaque, which occluded the vessel by approximately 70% to 80% (supplemental Video 4, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). (D) The percentage of mice with a spontaneous plaque at the carotid sinus (N = 10/group).
ADAMTS13 deficiency accelerates atherosclerosis in the aorta and aortic sinus
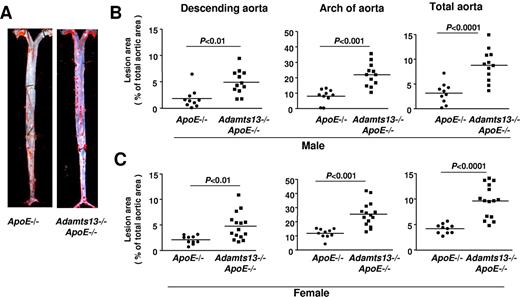
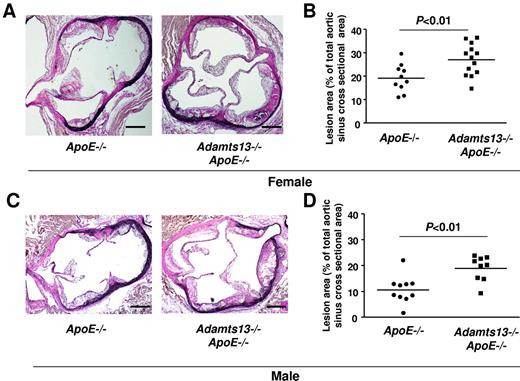
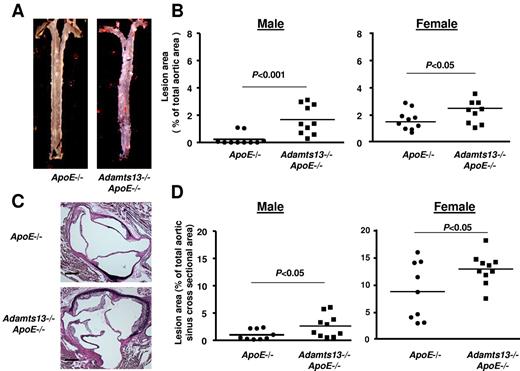
Next, we determined the effects of ADAMTS13 deficiency on atherosclerotic plaque formation in the aorta and aortic sinus of mice fed a Western diet for 10 weeks and studied at 16 weeks of age. We first compared the extent of atherosclerosis in whole aortae by staining with Oil Red O and calculating en face lesion area. Adamts13−/−/ApoE−/− male mice demonstrated significantly larger lesion areas in the descending aorta (P < .01), the arch of the aorta (P < .001), and the total aorta (P < .0001) compared with ApoE−/− mice (Figure 2A-B). Female Adamts13−/−/ApoE−/− mice also demonstrated accelerated larger lesions in the descending aorta (P < .01), arch of the aorta (P < .001), and total aorta (P < .0001) compared with female ApoE−/− mice (Figure 2C). Next, we quantified the cross-sectional area of lesions in the aortic sinus using the VerHoeffs/Van Gieson method. We observed a 2-fold increase in the mean lesion area in the aortic sinus of both male and female Adamts13−/−/ApoE−/− mice (P < .01) compared with ApoE−/− mice (Figure 3). As observed in many prior studies,21,22 the mean lesion area was larger in females than in males for both ApoE−/− and Adamts13−/−/ApoE−/− mice. Accelerated atherosclerosis was not associated with mortality in Adamts13−/−/ApoE−/− compared with ApoE−/− mice (not shown). No differences in total cholesterol or triglyceride levels were observed between ApoE−/− and Adamts13−/−/ApoE−/− mice fed the Western diet (Table 1). Together, these findings demonstrate that, irrespective of sex, ADAMTS13 deficiency accelerates atherosclerosis in the aorta and aortic sinus.
ADAMTS13 deficiency accelerates atherosclerotic lesion formation in the aorta. (A) Representative Oil red O-stained aortae from male mice on a high-fat Western diet beginning at 6 weeks until they were killed at 4 months of age (ie, 10 weeks on high-fat Western diet). (B) Quantification of en face lesion area from male mice (N = 10-12/group). (C) Quantification of en face lesion area from female mice (N = 10-15/group). Each dot represents a single mouse. Horizontal bars represent mean values.
ADAMTS13 deficiency accelerates atherosclerotic lesion formation in the aorta. (A) Representative Oil red O-stained aortae from male mice on a high-fat Western diet beginning at 6 weeks until they were killed at 4 months of age (ie, 10 weeks on high-fat Western diet). (B) Quantification of en face lesion area from male mice (N = 10-12/group). (C) Quantification of en face lesion area from female mice (N = 10-15/group). Each dot represents a single mouse. Horizontal bars represent mean values.
ADAMTS13 deficiency accelerates atherosclerosis in the aortic sinus. (A) Representative photomicrographs of VerHoeffs/Van Geison-stained aortic sinuses from female mice on a high-fat Western diet beginning at 6 weeks until they were killed at 4 months of age (ie, 10 weeks on high-fat Western diet). Bar represents 200μm. (B) Quantification of lesion in the cross-sectional area of aortic sinuses from female mice (N = 10-13/group). (C) Representative photomicrographs of VerHoeffs/Van Geison–stained aortic sinuses from male mice on a high-fat Western diet as described for female. Bar represents 200 μm. (D) Quantification of lesion in the cross-sectional area of aortic sinuses from male mice (N = 9 or 10/group). Each dot represents a single mouse. Horizontal bars represent mean values.
ADAMTS13 deficiency accelerates atherosclerosis in the aortic sinus. (A) Representative photomicrographs of VerHoeffs/Van Geison-stained aortic sinuses from female mice on a high-fat Western diet beginning at 6 weeks until they were killed at 4 months of age (ie, 10 weeks on high-fat Western diet). Bar represents 200μm. (B) Quantification of lesion in the cross-sectional area of aortic sinuses from female mice (N = 10-13/group). (C) Representative photomicrographs of VerHoeffs/Van Geison–stained aortic sinuses from male mice on a high-fat Western diet as described for female. Bar represents 200 μm. (D) Quantification of lesion in the cross-sectional area of aortic sinuses from male mice (N = 9 or 10/group). Each dot represents a single mouse. Horizontal bars represent mean values.
Plasma total cholesterol and triglyceride levels in mice fed on a high-fat Western diet and normal chow diet
| . | Male . | Female . | ||
|---|---|---|---|---|
| ApoE−/− . | Adamts13−/−/ApoE−/− . | ApoE−/− . | Adamts13−/−/ApoE−/− . | |
| High-fat Western diet | ||||
| Cholesterol levels, mg/dL | 790 ± 112 | 776 ± 52 | 570 ± 49 | 521 ± 26 |
| Triglyceride levels, mg/dL | 202 ± 18 | 221 ± 22 | 152 ± 11 | 167 ± 11 |
| Normal chow diet | ||||
| Cholesterol levels, mg/dL | 317 ± 57 | 357 ± 51 | 332 ± 33 | 402 ± 50 |
| Triglyceride levels, mg/dL | 132 ± 20 | 143 ± 17 | 118 ± 15 | 119 ± 13 |
| . | Male . | Female . | ||
|---|---|---|---|---|
| ApoE−/− . | Adamts13−/−/ApoE−/− . | ApoE−/− . | Adamts13−/−/ApoE−/− . | |
| High-fat Western diet | ||||
| Cholesterol levels, mg/dL | 790 ± 112 | 776 ± 52 | 570 ± 49 | 521 ± 26 |
| Triglyceride levels, mg/dL | 202 ± 18 | 221 ± 22 | 152 ± 11 | 167 ± 11 |
| Normal chow diet | ||||
| Cholesterol levels, mg/dL | 317 ± 57 | 357 ± 51 | 332 ± 33 | 402 ± 50 |
| Triglyceride levels, mg/dL | 132 ± 20 | 143 ± 17 | 118 ± 15 | 119 ± 13 |
Data are mean ± SEM; N = 9-11/group; P > .05 for all values using unpaired Student t test.
Increased macrophage and reduced interstitial collagen content in lesions of Adamts13−/−/ApoE−/− mice
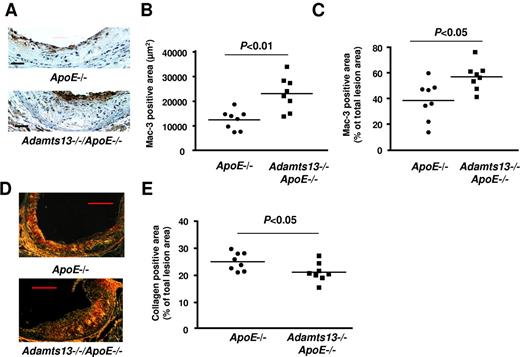
To test the hypothesis that ADAMTS13 reduces the inflammatory component of atherosclerotic lesions, we quantitated macrophage infiltration into lesions in the aortic sinus by immunohistochemistry. We observed increased numbers of Mac-3–positive macrophages in Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice fed a Western diet for 10 weeks (Figure 4A). Not only was the absolute Mac-3–positive area increased in Adamts13−/−/ApoE−/− mice than in ApoE−/− mice (Figure 4B), but the percentage of the total lesion area occupied by Mac-3–positive cells was also higher in Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice (Figure 4C). Next, we measured the interstitial collagen content. Picrosirius red staining of lesions in the aortic sinus revealed a significant reduction in collagen content in Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice at 10-week time point on high-fat Western diet (Figure 4D-E). This result suggests that ADAMTS13 deficiency may promote plaque vulnerability at later stages of atherogenesis. Together, these findings indicate that the atherosclerotic lesions in the aortic roots of Adamts13−/−/ApoE−/− mice were not only larger but also more inflammatory with reduced interstitial collagen.
ADAMTS13 deficiency enhances inflammation during early atherosclerosis. (A) Representative photomicrographs stained for macrophages (Mac-3–positive cells, brown) and counterstained with hematoxylin (blue). Bar represents 100 μm. (B-C) Quantification of Mac-3–positive area in the cross section of aortic sinuses from each genotype. (D) Representative photomicrographs stained for collagen as visualized by polarization microscope. Bar represents 20 μm. (E) Quantification of collagen-positive area in the cross sections of aortic sinus from each genotype. Each dot represents a single mouse (N = 8/group). Horizontal bars represent mean values.
ADAMTS13 deficiency enhances inflammation during early atherosclerosis. (A) Representative photomicrographs stained for macrophages (Mac-3–positive cells, brown) and counterstained with hematoxylin (blue). Bar represents 100 μm. (B-C) Quantification of Mac-3–positive area in the cross section of aortic sinuses from each genotype. (D) Representative photomicrographs stained for collagen as visualized by polarization microscope. Bar represents 20 μm. (E) Quantification of collagen-positive area in the cross sections of aortic sinus from each genotype. Each dot represents a single mouse (N = 8/group). Horizontal bars represent mean values.
ADAMTS13 abrogates early atherosclerosis in ApoE−/− mice fed a normal chow diet
To determine the effects of ADAMTS13 deficiency at an earlier stage of atherogenesis, we next measured the atherosclerotic lesion area in Adamts13−/−/ApoE−/− and ApoE−/− mice fed a normal chow diet for 10 weeks. Male Adamts13−/−/ApoE−/− mice demonstrated significantly greater en face lesion area in the total aorta (P < .001) than male ApoE−/− mice, in which lesions were largely absent (Figure 5A-B). Female Adamts13−/−/ApoE−/− mice also had a modest but statistically significant (P < .05) increase in lesion area in the aorta compared with female ApoE−/− mice (Figure 5B). Furthermore, both male and female Adamts13−/−/ApoE−/− mice showed significantly larger cross-sectional lesion areas in the aortic sinus compared with ApoE−/− mice (Figure 5C-D). Total cholesterol and triglyceride levels were similar in Adamts13−/−/ApoE−/− and ApoE−/− mice fed the normal chow diet (Table 1) Together, these findings suggest that, irrespective of diet, ADAMTS13 deficiency exacerbates atherosclerosis.
ADAMTS13 deficiency exacerbates atherosclerosis in ApoE−/− mice fed a normal chow diet. (A) Representative Oil red O-stained aortae from ApoE−/− and Adamts13−/−/ApoE−/− male mice fed a normal chow diet until 4 months of age. (B) Quantification of en face lesion area in the aorta (N = 10/group). (C) Representative photomicrographs of VerHoeffs/Van Geison-stained aortic sinuses from ApoE−/− and Adamts13−/−/ApoE−/− male mice fed a normal chow diet until 4 months of age. Bar represents 200 μm. (D) Quantification of lesion area in the cross sections of aortic sinus (N = 9 or 10/group). Each dot represents a single mouse. Horizontal bars represent mean values.
ADAMTS13 deficiency exacerbates atherosclerosis in ApoE−/− mice fed a normal chow diet. (A) Representative Oil red O-stained aortae from ApoE−/− and Adamts13−/−/ApoE−/− male mice fed a normal chow diet until 4 months of age. (B) Quantification of en face lesion area in the aorta (N = 10/group). (C) Representative photomicrographs of VerHoeffs/Van Geison-stained aortic sinuses from ApoE−/− and Adamts13−/−/ApoE−/− male mice fed a normal chow diet until 4 months of age. Bar represents 200 μm. (D) Quantification of lesion area in the cross sections of aortic sinus (N = 9 or 10/group). Each dot represents a single mouse. Horizontal bars represent mean values.
Discussion
Elevated VWF levels23-25 and reduced ADAMTS13 activity in plasma have been described in many conditions associated with systemic inflammation, such as acute respiratory distress, acute pancreatitis, sepsis and septic shock, sepsis-induced organ failure, and sepsis-induced disseminated intravascular coagulation.10,11,13,26,27 Elevated VWF levels in plasma in these syndromes may be the result of enhanced release of ULVWF and reduced processing of ULVWF and/or VWF by ADAMTS13. The importance of reduced ADAMTS13 activity in plasma in these diseases or other chronic inflammatory conditions is not known. This prompted us to investigate whether ADAMTS13 plays any functional role in the context of a chronic inflammatory disorder, such as atherosclerosis. In the present study, we document, for the first time, a key role for ADAMTS13 in reducing early atherosclerosis in ApoE−/− mice. Using intravital microscopy, we show that ADAMTS13 prevents excessive spontaneous leukocyte adhesion at the carotid sinus under chronic inflammatory conditions. This result extends a previous study in which we demonstrated that ADAMTS13 prevents increased leukocyte adhesion at venous shear rates under acute inflammatory conditions.17 Interestingly, we found that 100% of the Adamts13−/−/ApoE−/− mice had detectable plaque at the carotid sinus, a lesion-prone site, whereas only 20% of the ApoE−/− mice had plaques that were smaller in size. Next, we found that atherosclerotic lesions in the Adamts13−/−/ApoE−/− mice were not only larger but also more inflammatory with decreased interstitial collagen, suggesting that ADAMTS13 reduces vascular inflammation and subsequent atherosclerotic plaque formation.
VWF deficiency in low-density lipoprotein receptor–deficient mice has been shown to reduce atherosclerotic plaque formation.28 As VWF remains the only known substrate of ADAMTS13 in multiple experimental models,17,29,30 it is most likely that ADAMTS13 reduces excessive inflammation and atherogenesis through its proteolytic effect on hyperadhesive ULVWF and/or VWF. There are several possibilities by which ADAMTS13 deficiency could potentially contribute to excessive leukocyte adhesion and accelerated atherosclerosis. First, deficiency of ADAMTS13 may result in the recruitment of increased numbers of platelets and leukocytes to the activated endothelium, especially in regions of disturbed flow that are more prone to the atherosclerotic plaque formation. Endothelial VWF has been shown to recruit platelets to atherosclerotic lesion-prone sites.15 Recently, using intravital microscopy, we have demonstrated that ADAMTS13 regulates VWF-mediated platelet and leukocyte adhesion in activated vessels.17,18 Another study showed that quiescent platelets that are captured and firmly adhered to endothelial ULVWF multimers are activated and can support leukocyte rolling at high shear stress in vitro via leukocyte PSGL-1 and platelet P-selectin.14 Interestingly, activated platelets have been shown to promote acute inflammation by stimulating Weibel-Palade bodies and P-selectin-mediated leukocyte rolling31 and accelerate early atherosclerotic plaque development.16,32
Second, leukocytes have been shown to adhere and extravasate across inflamed endothelium through another VWF-bound/platelet interaction mediated via Mac-1 and platelet GPIbα.33 However, the role of GPIbα in atherosclerosis is still debatable because of 2 confounding studies. In the first report, an inhibitory anti–GPIbα antibody decreased development of atherosclerotic lesions in ApoE−/− mice fed a high-fat Western diet.16 In contrast, a second study in mice lacking the GPIb-V-IX complex found no obvious difference in atherosclerotic lesions in ApoE−/− mice fed on a normal chow diet.34 The discrepancy between these 2 studies remains unclear. Future studies using specific tools that inhibit VWF-GPIbα interactions without affecting platelet size and counts will be required to determine whether exacerbated atherosclerosis resulting from ADAMTS13 deficiency is platelet GPIbα-dependent.
Third, leukocytes can also interact with VWF directly, independently of platelets, via an interaction mediated by both neutrophil PSGL-1 and integrin αMβ2 in vitro.35 It is unlikely that exacerbated atherosclerosis in Adamts13−/−/ApoE−/− mice is platelet-independent, however, because elevated baseline leukocyte rolling observed in Adamts13−/− mice was clearly platelet-dependent.17 Inhibiting P-selectin completely abrogated leukocyte rolling in Adamts13−/− mice, suggesting that P-selectin and platelets are absolutely required to initiate leukocyte rolling, even in the presence of ULVWF multimers.17
Finally, although the aforementioned studies suggest that ADAMTS13 may reduce atherogenesis through its proteolytic effects on ULVWF and/or VWF, it remains possible that accelerated atherosclerosis in ADAMTS13 deficiency occurs via another unknown mechanism that is independent of VWF. Additional studies using Adamts13−/−/VWF−/−/ApoE−/− will be required to determine whether exacerbated atherosclerosis because of ADAMTS13 deficiency is VWF-dependent.
There are several limitations of this study. One limitation of this study is that patients with acute and chronic inflammatory conditions often have only partially reduced ADAMTS13 activity rather than complete ADAMTS13 deficiency.9,10,12,13,26,27 Future studies using ADAMTS13 inhibitor will be required to determine whether reduced ADAMTS13 activity accelerates early atherosclerotic plaque formation. Another limitation is that ApoE−/− mice, which are on C57BL6/J background express truncated and less active variant of ADAMTS13 that lacks the C-terminal CUB domains (ADAMTS13S/S).36 Evidence that ADAMTS13S/S variant may effect atherosclerotic lesion formation is, however, limited for 2 reasons. First, ADAMTS13S/S variant cleave ULVWF multimers with similar efficiency compared with full-length ADAMTS13 under steady state in vivo.37 Second, both Adamts13−/−/ApoE−/− and ApoE−/− mice are on C57BL/6J background. If ADAMTS13S/S variant was less active, then one should expect subtle or no difference in atherosclerotic plaque development in Adamts13−/−/ApoE−/− mice compared with ApoE−/− mice.
On the basis of our findings, we propose that, in conditions of ADAMTS13 deficiency and/or decreased ADAMTS13 activity, hyperactive ULVWF multimers accumulate on the endothelial surface mainly in the regions of disturbed flow, which are highly prone to atherosclerotic lesion development. Circulating quiescent platelets adhere and firmly bind to ULVWF multimers, presumably because of high-strength bond formation between ULVWF and GPIbα.5 Firmly adhered platelets become activated and release factors, such as proinflammatory cytokines and chemoattractants, which generate signals for recruitment and extravasation of monocytes, which accelerate atherosclerosis plaque formation.
In conclusion, we have demonstrated that the antithrombotic enzyme ADAMTS13 prevents excessive vascular inflammation and plaque formation during atherosclerosis, demonstrating an active functional role for ADAMTS13 in disease pathogenesis. These findings unravel a new function for ADAMTS13 in reducing early atherosclerosis in addition to its known function in preventing thrombotic thrombocytopenic purpura. Our studies suggest that measurement of ADAMTS13 activity might be a useful systemic biomarker of atherosclerosis in humans and may benefit from drugs aimed at reducing circulating ULVWF and/or increased VWF levels.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kristina W. Thiel for assistance in editing the manuscript.
This work was supported by the American Society of Hematology (ASH Scholar Award), the American Heart Association (national Scientist Development Grant; A.K.C.), and the NIH (grants HL063943, HL062984, and NS024621, S.R.L.).
National Institutes of Health
Authorship
Contribution: C.G. performed experiments and analyzed results; M.M.K. helped with the intravital microscopy experiments; S.R.L. contributed important intellectual insights and edited the manuscript; and A.K.C. directed the project, designed the study, did intravital microscopy, interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anil K. Chauhan, University of Iowa, Department of Internal Medicine, 25 S Grand Ave, 3160 Medical Labs, Iowa City, IA 52242; e-mail: anil-chauhan@uiowa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal