Abstract
Human immunodeficiency virus (HIV) infection is characterized by a progressive loss of memory CD4+ T cells in multiple tissues, especially at mucosal surfaces where most of these cells reside. Although antiretroviral therapy (ART) suppresses viral replication and promotes the recovery of peripheral CD4+ T cells, HIV-infected patients fail to fully reconstitute the CD4+ T-cell pool at mucosal sites. IL-15 has been shown to preferentially expand memory-phenotype T cells and promote their migration to nonlymphoid tissues. Here we examined IL-15 treatment in combination with highly active ART in chronically SIV-infected rhesus macaques and found that IL-15 delayed viral suppression and failed to enhance ART-induced total and antigen-specific CD4+ T-cell reconstitution at mucosal and lymphoid sites. IL-15 was able to induce the transient proliferation of SIV-specific, CMV-specific, and total memory CD8+ T cells, but not of SIV-specific or total CD4+ T cells. Moreover, upon treatment interruption, macaques receiving combined IL-15+ART lost CD4+ T cells faster than those receiving ART alone. These results suggest that the combination of IL-15 with highly active ART is not more efficient than ART alone in promoting CD4+ T-cell recovery in HIV-infected individuals and may accelerate CD4+ T-cell loss after treatment interruption.
Introduction
Infection with HIV or its simian equivalent SIV induces a progressive loss of CD4+ T cells and eventual immunodeficiency. In HIV-infected subjects, highly active antiretroviral therapy (HAART) considerably decreases morbidity and mortality as well as opportunistic infections, likely by halting CD4+ T-cell loss and dysfunction.1 Earlier studies focused on the loss of CD4+ T cells in the peripheral blood and lymphoid tissues; more recently, reports demonstrated that the gastrointestinal tract is a major site of CD4+ T-cell depletion during the course of HIV and SIV infections in all phases of the disease.2,3 In particular, during the second week of infection with SIV, at peak viral load, 30%-60% of memory CD4+ T cells throughout the body are infected, and the majorityof those are lost within a few days.4,5 Importantly, the extent of CD4+ T-cell loss during the acute phase of infection predicts the progression of the disease and death.6 Similarly, the rate of CD4+ T-cell loss during the chronic phase is highly correlated with progression to AIDS or death.7,8 HAART promotes immune reconstitution in the peripheral blood and in the lymph nodes through the indirect induction of homeostatic memory T-cell proliferation and the production of new lymphocytes.9 However, despite efficient suppression of viral replication and CD4+ T-cell reconstitution by prolonged HAART treatment in the blood and in lymphoid tissues, CD4+ T-cell restoration at mucosal sites generally does not occur10 or is partial,11 even though optimal restoration of gut CD4+ T cells has been described in a single report.12 Factors such as ongoing T-cell activation or a low nadir of the CD4+ T-cell count before therapy initiation are predictive of this restoration.13
IL-15 belongs to the γ-chain family of cytokines and can mediate the expansion of CD4+ and CD8+ T cells through the induction of cell cycling.14 It preferentially targets effector memory (TEM)-phenotype T cells, but, to a lesser extent, also expands T cells at earlier stages of differentiation such as central memory (TCM) and transitional memory (TTM) cells.15,16 We recently demonstrated that upon administration of IL-15 to rhesus macaques, T-cell proliferation occurs in multiple lymphoid and nonlymphoid tissues of the body, including the gut.16 In particular, TCM cells from lymphoid tissues further differentiate16 and migrate to nonlymphoid sites upon IL-15 administration.17 IL-15 can exert a dual role in the context of SIV infection depending on the phase of infection. Increased IL-15 production is observed in acute SIV infection and renders CD4+ T cells more susceptible to SIV infection,18 and, at pharmacologic doses, can alter viral set point.19,20 Conversely, chronic SIV viral loads are not affected by IL-15 administration, however the response threshold of T cells to IL-15 at this stage markedly differs: optimal CD8+ T-cell proliferation requires 5× higher doses and CD4+ T cells no longer respond in viremic monkeys. When viral replication is suppressed, a suboptimal proliferation of TEM-phenotype CD4+ T cells which are preferentially depleted by SIV15 is induced. As such, therapeutic administration of IL-15 has been proposed as a strategy to expand memory CD4+ T cells in the tissues, promote their differentiation and migration to mucosal sites, and thereby favor the replenishment of the CD4+ T-cell pool. In addition, ongoing investigation using transpresentation of IL-15 in the presence of soluble IL-15Rα appears to enhance CD4+ T-cell targeting over CD8+ T cells (E.L. and M.R., unpublished observations, June 2011).
Here we report on the administration of IL-15 in combination with antiretroviral therapy (ART) in an effort to promote CD4+ T-cell immune reconstitution in rhesus macaques chronically infected with SIV. We show that, although IL-15 was able to transiently promote the proliferation of total memory and antigen-specific CD8+ T cells in the peripheral blood, it failed to do so in CD4+ T cells. Furthermore, IL-15 failed to boost ART-induced CD4+ T-cell recovery both in the blood and in peripheral tissues. IL-15 with ART did not promote the expansion of antigen-specific CD4+ T cells nor did it affect the quality of their cytokine production beyond that of ART alone. Conversely, after ART interruption, previous IL-15 treatment resulted in a faster drop in peripheral CD4+ T cells despite comparable viral load rebounds. These findings suggest that IL-15 treatment alone may be insufficient to promote CD4+ T-cell recovery in HAART-treated HIV-infected patients.
Methods
Animals and experimental design
The study protocol and the procedures described in this article were approved by the Vaccine Research Center (NIAID, National Institutes of Health) Institutional Animal Care and Use Committees. A total of 16 SIV mac251 chronically infected Indian rhesus macaques (housed at Bioqual Inc) were used for this study. These animals were recycled from an experimental vaccine protocol that did not alter chronic viremia nor SIV-specific immunity (and no vaccine group–related differences were observed in our experiments); they had been challenged with SIV 9 months before initiation of this study. The animals were randomized into 3 different treatment groups on the basis of sex, baseline viral load, and pre-infection vaccination protocol: ART alone (n = 6), IL-15 alone (n = 4), and ART+IL-15 (n = 6). ART consisted of 20 mg/kg/d Tenofovir (PMPA, given subcutaneously; from Gilead Sciences), 40 mg/kg/d emtricitabine (FTC, given subcutaneously; from Gilead), and 100 mg/kg/BID raltegravir (Isentress, mixed with food; from Merck). Simian IL-15 was produced as described previously21 and was given subcutaneously at 80 μg/kg twice a week. ART was administered from day (d) 0 through d46. The first IL-15 injection occurred on day 0 (d0) and the last on d42. Biopsies for the isolation of jejunal (Jej) and inguinal lymph node (ILN) specimens were performed 13 days before day 0 (d−13) and d28. Bronchoalveolar lavage (BAL) was performed on the same time points and on d59. Animals were euthanized on d70 through d74 (24-28 days after therapy interruption; indicated as d70 in the text and figures) for detailed analysis of cells from tissues throughout the body.
Isolation of MNC from blood and tissues
Mononuclear cells from the peripheral blood (PBMC) and from tissues were isolated as previously described.16 Either fresh (BAL, LN, and Jej samples) or frozen (PBMC) cells were used for phenotyping and stimulation. PBMCs were frozen in FBS + 10% DMSO and stored in liquid phase nitrogen until analysis.
Antibodies and flow cytometry
Purified monoclonal antibodies (BD Biosciences) were conjugated in our laboratory (http://www.drmr.com/abcon). For phenotypic analysis, frozen PBMCs were thawed and stained immediately with the Violet Viability Dye (ViViD; Invitrogen) in PBS for 15 minutes at room temperature, followed by staining with the combination of antibodies indicated in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) for 20 minutes at room temperature. For the analysis of intracellular Ki-67 and BrdU, cells were incubated with FacsLyse (BD Biosciences) for 7 minutes at room temperature, then washed and fixed and permeabilized with the Cytofix/Cytoperm kit (BD Sciences), followed by incubation with 2 mg/mL DNAse, Ki-67 and BrdU antibodies for 30 minutes at 37°C.
For the analysis of spontaneous apoptosis, fresh PBMC were cultured in complete medium for 16 hours at 37°C. Phosphatidylserine expression was revealed by annexin V-PE (BD Biosciences) staining in annexin buffer (10mM HEPES, 140mM NaCl, 2.5mM CaCl2; pH 7.4).
Labeled cells were analyzed using a modified LSR II, equipped to detect up to 18 colors. Flow cytometric data were compensated and analyzed with FlowJo 8.3.6 software (Treestar Inc). Data were formatted with Pestle software (Version 1.6.2) and presented with SPICE 5.1 software.22
T-cell intracellular cytokine staining
Frozen cells were thawed and rested for 4 hours at 37°C before stimulation. Approximately 1 × 106 frozen PBMC were stimulated with peptide pools derived from rhesus CMV pp65 (rhCMV; 1.3 μg/mL/peptide; New England Peptide), SIV Gag, or SIV Env (2 μg/mL/peptide; National Institutes of Health AIDS Research & Reference Reagent Program). Fresh cells were used to assess response to C albicans extract (2.5 μg/mL; Greer labs) or heat inactivated C albicans (5 × 106/well). Stimulations occurred in the presence of GolgiPlug, anti–human CD49 days (2.5 μg/mL) and Cy5PE anti–human CD28 (all from BD Biosciences) for 15 hours. In the case of C albicans, GolgiPlug was added 2 hours after the stimulation. Cells were then surface stained with monoclonal antibodies to lineage antigens, fixed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) and stained with antibodies directed to intracellular cytokines.
Delayed type hypersensitivity (DTH) test
C albicans skin test antigen for cellular hypersensitivity (Candin; Allermed Laboratories Inc) was performed by intradermal injection of 0.1 mL of the antigen solution into the forearm or the upper arm. At 48 hours, the skin test was read by visual inspection of the test site and the diameter of the response was measured in millimeters. Measurements were averaged for 2 diameters of the reaction site as recommended by the manufacturer.
Measurement of plasma IL-15
Measurement of plasma IL-15 levels were performed by the Quantiglo human IL-15 chemiluminescent kit (R&D systems), according to the manufacturer's instructions.
In vivo BrdU labeling
BrdU (5-bromo-2′-deoxyuridine; Sigma-Aldrich) in HBSS (Invitrogen), pH 7.2, was administered intravenously at 30 mg/kg/d for 4 days. Two cycles of BrdU injections occurred: from d−6 to d−3 (before initiation of therapy) and from d43 to d46 (before cessation of therapy). Detection of BrdU incorporation by flow cytometry was performed as described in “Antibodies and flow cytometry.”
Measurement of viral load
Viral RNA in plasma was measured as previously described.23 To quantify viral replication, a real-time RT-PCR (TaqMan; Applied Biosystems) assay was used as described.23,24 All RT-PCR reactions were run on an ABI Prism 7700 Sequence Detection System, and ABI sequence detection software was used to determine viral RNA copy numbers in test samples (Applied Biosystems).
Statistical analysis
Statistical analyses were performed using Prism Version 5 (GraphPad Software) and SPICE 5.1 software. For most comparisons a nonparametric Wilcoxon rank test was used to compare distributions, unless specified. When possible, a non parametric paired Student t test was used. In some cases, nonparametric 1-way ANOVA (Kruskal-Wallis test) was used to compare 3 or more groups. Comparisons of distributions (shown with pie charts) were performed in SPICE using 10 000 permutations.22 P values were considered significant when < .05.
Results
IL-15 delays but does not abrogate ART mediated suppression of viral replication and immune activation
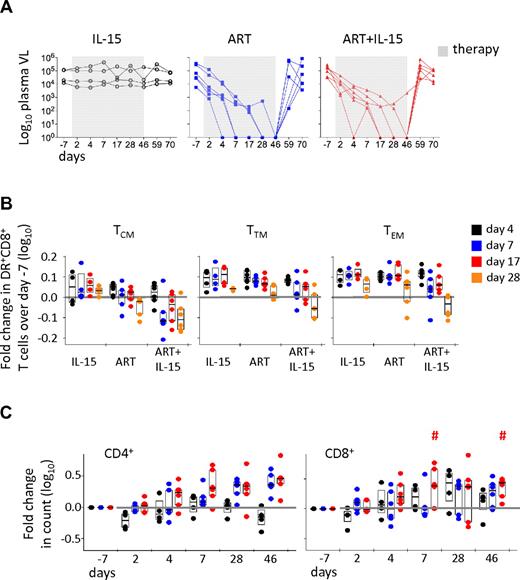
Recent works demonstrated that IL-15 administration during acute SIV infection had no effect on peak viral load but altered the establishment of the set point, suggesting that the cytokine influences the dynamics of SIV replication.19,25 However, IL-15 treatment of chronically SIV-infected rhesus macaques did not affect CD4+ T cells in non-ART–treated animals.25 We thus combined ART and IL-15 therapies to suppress viral replication and, simultaneously, induce expansion of T cells; the combined therapy was compared with treatment with either regimen alone. As shown in Figure 1A, IL-15 alone did not alter viral replication in a twice-a-week dose regimen. In contrast, the highly active ART used here efficiently suppressed viremia to below detection levels in 11 of 12 animals treated. There was some variability in the kinetics of suppression achieved by ART alone (Figure 1A). The same was observed for the combined IL-15+ART therapy, but the addition of IL-15 appeared to delay the time to suppression. Similar to a previous report,26 the combination of PMPA, FTC and Raltegravir suppressed viral loads below detection in 3 of 6 ART alone treated animals within 1 week of treatment. In contrast, in ART+IL-15 treated animals complete suppression was first achieved only after d17 (P = .046; ART vs ART+IL15; Student t test). On average it took 3 weeks for complete suppression in the ART alone group and 4 weeks for ART+IL-15 group. Notably, the presence of IL-15 did not affect viral rebound after ART was discontinued. Similarly, combined therapy did not affect the suppression of CD8+ T-cell activation (ie, HLA-DR expression) in multiple memory CD8+ subsets observed in the ART group. Moreover, HLA-DR expression decay tended to occur faster in the ART+IL15 group, that is at d7 (Figure 1B). In contrast, IL-15 alone did not have any effect on the level of CD8+ T-cell activation (Figure 1B). The expression of CD38 was also investigated and was found to be up-regulated by IL-15 therapy in multiple CD8+ T-cell memory subsets,16 and could thus not be considered a reliable marker of the effect of ART on T-cell activation (data not shown).
Suppression of viral replication and CD8+ T-cell activation do not favor IL-15–induced CD4+ T-cell expansion. (A) Plasma SIV RNA copies at different days after introduction (gray box) or cessation of therapies. Each line represents a different animal. (B) Fold change in HLA-DR+CD8+ T cells after treatment initiation versus baseline (d−7) in TCM, TTM, and TEM cells. Bars show the interquartile range, and the wide bar shows the median, of the distribution. Each point represents an animal. (C) Fold change in CD4+ and CD8+ absolute T-cell counts in the peripheral blood in the different treatment groups. Data are expressed as in panel B. The horizontal gray line indicates no change versus baseline. #P < .05 vs ART; Wilcoxon rank-sum test.
Suppression of viral replication and CD8+ T-cell activation do not favor IL-15–induced CD4+ T-cell expansion. (A) Plasma SIV RNA copies at different days after introduction (gray box) or cessation of therapies. Each line represents a different animal. (B) Fold change in HLA-DR+CD8+ T cells after treatment initiation versus baseline (d−7) in TCM, TTM, and TEM cells. Bars show the interquartile range, and the wide bar shows the median, of the distribution. Each point represents an animal. (C) Fold change in CD4+ and CD8+ absolute T-cell counts in the peripheral blood in the different treatment groups. Data are expressed as in panel B. The horizontal gray line indicates no change versus baseline. #P < .05 vs ART; Wilcoxon rank-sum test.
IL-15 does not improve CD4+ T-cell reconstitution upon ART treatment
We next analyzed the fold change in CD4+ and CD8+ T-cell counts in the peripheral blood of animals receiving the different therapies. As expected, ART promoted CD4+ T-cell recovery in the peripheral blood by inducing a 2-fold increase in the CD4+ T-cell count by d28, which then remained stable through d46 (Figure 1C). Combined ART+IL-15 therapy appeared to accelerate CD4+ T-cell recovery at d7, but this increase was not statistically significant. Importantly, ART+IL-15 was able to expand CD8+ T cells (P < .05 vs ART at d7 and d46; Wilcoxon rank-sum test), suggesting that viral suppression does not impede the ability of IL-15 to expand CD8+ T cells.
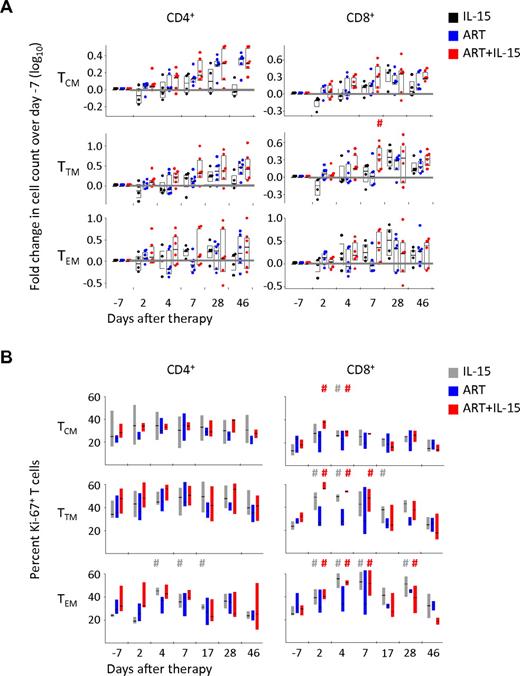
Absence of expansion of selective memory T-cell subsets by IL-15+ART despite the induction of cell cycle
We and others have previously shown that IL-15 is able to preferentially expand memory cells in vivo with the following hierarchy: TEM > TTM > TCM. By combining selective markers of memory T-cell differentiation, ie CD45RA, CD95, CCR7 and CD28 we were able to track changes in the counts of these subsets.16 In this study, frequencies of CD4+ T-cell subpopulations were not significantly different between the different treatment groups at the beginning of the treatment period (naive CD4+ T cells: IL-15: 38 ± 12%, ART: 46 ± 9.8%, IL-15+ART: 54 ± 7.8%; TCM: IL-15: 26 ± 5.0%, ART: 26 ± 3.3%, IL-15+ART: 28 ± 5.4%; TTM: IL-15: 9.7% ± 1.1, ART: 13 ± 5.2%, IL-15+ART: 7.0 ± 1.5%; TEM: IL-15: 10 ± 7.1%, ART: 3.9 ± 1.2%, IL-15+ART: 2.7 ± 1.1%). As shown in Figure 2A, by d28 IL-15 alone was able to induce a 3-fold and a 2-fold expansion of CD4+ TTM and TEM, respectively, but had a weak effect on other memory subsets. Conversely, ART only expanded CD4+ TCM (2-fold at d28 and 46 vs d−7). However, ART+IL-15 did not improve the expansion of CD4+ TCM and had no effect on the other memory subsets. IL-15 alone induced increases in TTM and TEM CD8+ T cells (Figure 2A) but the addition of ART to IL-15 decreased TEM expansion by d28. ART+IL-15 showed a transient increase in all 3 memory CD8+ T-cell populations on d7 compared with d−7. Similarly, both CD4+ and CD8+ TN cells, which are known to express low levels of the IL-2/15Rβ chain and thus to poorly respond to IL-15, were expanded at d7 by ART+IL-15 (∼ 2- and ∼ 3-fold in CD4+ and CD8+ T cells, respectively) and remained higher even at d46 (supplemental Figure 1).
Absence of proliferation and expansion of selective CD4+ T-cell memory subsets by IL-15. (A) Fold change in CD4+ and CD8+ TCM, TTM, and TEM absolute counts and (B) percent of Ki-67+ cells in the same T-cell subsets in the peripheral blood during treatment with IL-15, ART, or ART+IL-15. The horizontal gray line indicates no change versus baseline. #P < .05 vs d−7; Wilcoxon rank-sum test. The color of each “#” symbol refers to the group whose distribution is significantly different from the reference group. Data are expressed as in Figure 1B. In Figure 1C, individual animals are not shown.
Absence of proliferation and expansion of selective CD4+ T-cell memory subsets by IL-15. (A) Fold change in CD4+ and CD8+ TCM, TTM, and TEM absolute counts and (B) percent of Ki-67+ cells in the same T-cell subsets in the peripheral blood during treatment with IL-15, ART, or ART+IL-15. The horizontal gray line indicates no change versus baseline. #P < .05 vs d−7; Wilcoxon rank-sum test. The color of each “#” symbol refers to the group whose distribution is significantly different from the reference group. Data are expressed as in Figure 1B. In Figure 1C, individual animals are not shown.
To test whether IL-15–induced expansion was because of promotion of cell cycle, we measured intracellular Ki-67 expression. Because both TN CD4+ and CD8+ T cells increased in blood by ART+IL-15 in the absence of measureable proliferation (data not shown), these increases in peripheral blood numbers likely reflect redistribution of these cells from LN.
In contrast, IL-15 alone (Figure 2B), but not ART or ART+IL-15, increased Ki-67 expression in CD4+ TEM by d4 after therapy initiation, but the percentage of Ki-67+ cells declined over time. IL-15 in the presence or absence of ART induced dramatic increases of CD8+ TTM and TEM cells expressing Ki-67; for TEM CD8+ T cells this was sustained through d28. Analysis of the half-life of BrdU dilution in memory subsets of CD4+ and CD8+ T cells revealed that the different treatments had negligible effects on their turnover, although ART tended to decrease the turnover of TCM and TEM CD8+ T cells (supplemental Figure 2). These data thus confirm that IL-15 had biologic activity on CD8+ but not CD4+ T cells and that the increase of CD4+ TCM cells can be ascribed solely to ART and likely reflects ART-induced redistribution of these cells in the body.
Chronically SIV-infected animals had slight increases in IL-15 levels in plasma compared with uninfected controls (supplemental Figure 3); it is possible that this could influence the effect of administered IL-15 in vivo because of chronic stimulation of the T-cell targets. We exclude this possibility by finding no correlation between baseline IL-15 levels and the fold expansion of total CD4+ or CD8+ T cells at d46 after treatment with ART+IL-15 (supplemental Figure 3).
IL-15–induced local proliferation is not sufficient to promote CD4+ T-cell recovery in peripheral tissues
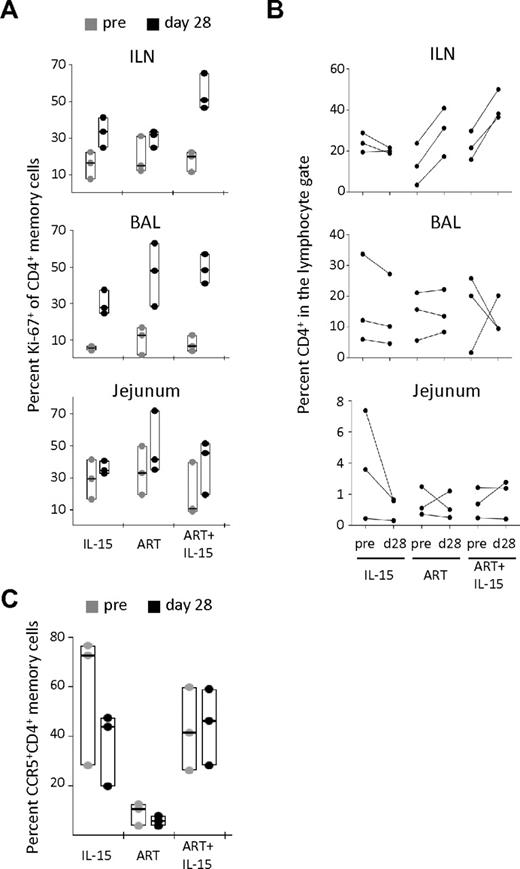
To test whether IL-15 therapy could be inducing expansion of CD4+ T cells in peripheral tissues, we analyzed Ki-67 expression on CD95+ memory cells. At d28 of therapy, both IL-15 and ART alone showed increased percentages of Ki-67+CD4+ memory T cells in the ILN and BAL, but not in the jejunum (Figure 3A). ART+IL-15 combined therapy potently boosted memory CD4+ T-cell proliferation in the ILN, and to a lesser degree in BAL and jejunal samples (Figure 3A). Indeed, ART+IL-15 induced a nearly 5-fold increase in memory CD4+ T-cell proliferation in ILN, which was higher than the effect of ART or IL-15 alone.
IL-15 fails to promote CD4+ T-cell recovery in peripheral tissues despite the induction of local proliferation. (A) Percent Ki-67+ among memory (CD95+) CD4+ T cells and (B) percent of total CD4+ T cells in the lymphocyte gate in the ILN, BAL, and jejunum at pretherapy (d−13) and at d28 after therapy initiation. (C) Percent CCR5+ CD4+ memory T cells in the BAL at the same time points after therapy initiation. In panels A and C, data are expressed as in Figure 1B. In panel B, each line represents an animal.
IL-15 fails to promote CD4+ T-cell recovery in peripheral tissues despite the induction of local proliferation. (A) Percent Ki-67+ among memory (CD95+) CD4+ T cells and (B) percent of total CD4+ T cells in the lymphocyte gate in the ILN, BAL, and jejunum at pretherapy (d−13) and at d28 after therapy initiation. (C) Percent CCR5+ CD4+ memory T cells in the BAL at the same time points after therapy initiation. In panels A and C, data are expressed as in Figure 1B. In panel B, each line represents an animal.
To estimate relative expansions of cells, we quantified the proportion of CD4+ T cells among total lymphocytes in ILN and found that ART, but not IL-15 alone, effected an increase. However, addition of IL-15 to ART did not improve this effect (Figure 3B top panel). We observed no effect on CD4+ T-cell frequencies with any treatment group at mucosal surfaces, ie in the BAL and in the jejunum (Figure 3B middle and bottom panels). On the other hand, IL-15 alone tended to decrease the percentage of CD4+ T cells (Figure 3B) and, simultaneously, to increase CD8+ T cells (supplemental Figure 4). We also quantified the proportion of CD4+CCR5+ memory T cells, which are preferentially depleted in SIV infection and therefore may be a specific indicator of immune reconstitution. Treatment with IL-15 alone decreased the percentage of these cells in the BAL (Figure 3C) but not in other sites (data not shown). The presence of ART inhibited this effect, suggesting that the loss of CD4+CCR5+ memory T cells in IL-15 treated animals is because of an enhanced virus-mediated depletion (Figure 3C). Together, these data show that IL-15 was not able to reconstitute the CD4+ T-cell pool in peripheral tissues despite the induction of CD4+ T-cell proliferation at these sites.
Dynamics of antigen-specific T cells upon IL-15 treatment
To determine whether IL-15 was able to improve ART-induced CD4+ T-cell reconstitution, we quantified the magnitude and quality of the CD4+ T-cell response, that is the pattern of cytokine secretion, against different antigens. Analysis of PBMC cytokine response to C albicans revealed that only 2 animals in the whole cohort had a detectable response and no changes were observed with treatment. Similarly, a skin reaction in response to the subcutaneous injection of C albicans could be detected only in 3 animals and did not show any correlation with the type of treatment.
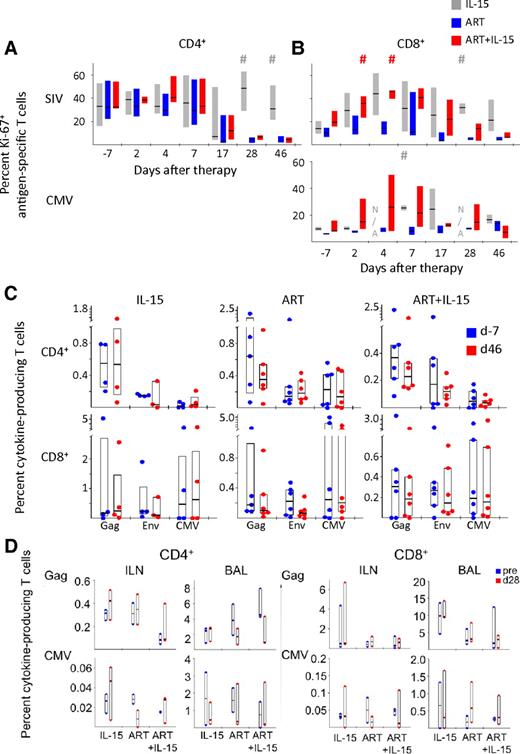
We measured the proliferation state of SIV- and CMV-specific cells in response to treatment by quantifying Ki-67 expression in antigen-specific T cells (Figure 4A-B). Because Gag- and Env-specific T cells responded similarly, for simplicity we aggregate their responses and refer to them as SIV-specific. Introduction of ART decreased the proportion of proliferating SIV-specific CD4+ T cells and addition of IL-15 to ART did not have any effect on their proliferation (Figure 4A). Few animals had a detectable CMV-specific CD4+ T-cell response and, thus, analysis of Ki-67 expression in these cells is not shown. Conversely, SIV-specific CD8+ T cells tended to up-regulate Ki-67 in response to IL-15 treatment, either in the presence or absence of ART (Figure 4B). As for total CD8+ T cells, this effect was only transient in the ART+IL-15 group and followed the same kinetic—although the proportion of Ki-67+ cells was still higher than in the ART alone group. A similar trend could be observed for CMV-specific CD8+ T cells (Figure 4B). Despite induction of antigen-specific CD8+ T-cell proliferation, IL-15, either alone or associated with ART, was not able to increase the magnitude of cells responding to Gag, Env, or CMV at d46 after therapy initiation (Figure 4C) or at earlier time points (data not shown). No changes were found in magnitude of CD4+ T-cell responses.
IL-15 mediates the expansion of antigen-specific CD8+ but not CD4+ T cells. (A) Percent Ki-67+ SIV-specific (Gag and Nef averaged) and CMV-specific CD4+ T-cells and (B) CD8+ T cells before and after therapy introduction. N/A indicates data not available because of low number of responding cells. Data are expressed as in Figure 2B. #P < .05 vs ART; Wilcoxon rank-sum test. (C) Percent of Gag-, Env-, or CMV-specific CD4+ (top) and CD8+ (bottom) T cells producing cytokines (IL-2 or TNF-α or IFN-γ) in the different treatment groups in the peripheral blood at d−7 or at d46 after therapy initiation. Data are expressed as in Figure 1B. (D) As in panel C but in the ILN and the BAL at baseline (“pre”) and at d28. Data from the jejunum are not depicted because very low responses were detected.
IL-15 mediates the expansion of antigen-specific CD8+ but not CD4+ T cells. (A) Percent Ki-67+ SIV-specific (Gag and Nef averaged) and CMV-specific CD4+ T-cells and (B) CD8+ T cells before and after therapy introduction. N/A indicates data not available because of low number of responding cells. Data are expressed as in Figure 2B. #P < .05 vs ART; Wilcoxon rank-sum test. (C) Percent of Gag-, Env-, or CMV-specific CD4+ (top) and CD8+ (bottom) T cells producing cytokines (IL-2 or TNF-α or IFN-γ) in the different treatment groups in the peripheral blood at d−7 or at d46 after therapy initiation. Data are expressed as in Figure 1B. (D) As in panel C but in the ILN and the BAL at baseline (“pre”) and at d28. Data from the jejunum are not depicted because very low responses were detected.
The magnitude of antigen-specific CD4+ and CD8+ T-cell responses were also measured in different sites of the body, including the ILN or the BAL and the effect of the different treatments was assessed at d28 of treatment. Similar to what we observed in the peripheral blood, IL-15, either alone or in combination with ART, did not affect the magnitude of the T cells responding to peptide pools (Figure 4D). These data thus demonstrate the ineffectiveness of IL-15 in preferentially expanding antigen-specific cells in chronically SIV-infected macaques, even in the context of viral suppression.
Quality of the SIV-specific CD4+ T-cell response
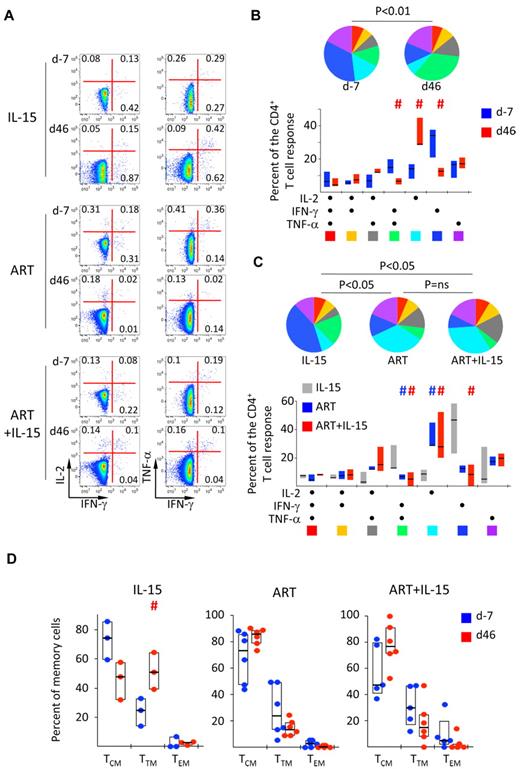
Analysis of the quality of cytokine production, as shown in a representative example in Figure 5A, revealed that Gag-specific CD4+ T cells in blood were mainly producing IFN-γ alone or in combination with TNF-α across all treatment groups at baseline. A similar pattern could be observed for Env-specific CD4+ T cells, as previously noted. Treatment with ART, but not IL-15, changed the quality of SIV-specific CD4+ T-cell response by selectively increasing cells producing only IL-2 (P < .01 in d46 vs d−7; Wilcoxon rank-sum test; Figure 5B). IL-15+ART had a similar IL-2 favoring effect (Figure 5C), however overall CD4+ T-cell responses were decreased. This was not the case for CMV-specific CD4+ T cells (supplemental Figure 5), thus suggesting that the presence of antigen is a major determinant of the quality of SIV-specific CD4+ T-cells. Conversely, this effect was not seen in animals treated with IL-15 alone (P < .05 in ART vs IL-15 at d46; χ2 permutation test22 ; Figure 5C top).
IL-15 allows the induction of IL-2-producing SIV-specific CD4+ T cells under ART but does not improve antigen-specific CD8+ T-cell function. (A) Representative example of the expression of IFN-γ, IL-2, and TNF-a in response to Gag peptide pool at d−7 and at d46 after therapy initiation. Numbers indicate the percentage of cells in the quadrants. (B) Pie charts and bars representing the quality of the SIV-specific (Gag and Env averaged) CD4+ T-cell response at baseline and at d46 after ART treatment. Each section of the pie chart represents a specific combination of cytokines, as indicated by the color at the bottom of the bar graph. Data were expressed as in Figure 2B. #P < .05 vs d−7; Wilcoxon rank-sum test. (C) As in panel B but in the different treatment groups at d46 after treatment initiation. #P < .05 vs IL-15; Wilcoxon rank-sum test. (D) Percentage of SIV-specific (Gag and Env averaged) CD4+ T-cells with TCM, TTM, or TEM cell phenotype of total memory cells at d−7 and at d46 after treatment initiation. Data are expressed as in Figure 1B.
IL-15 allows the induction of IL-2-producing SIV-specific CD4+ T cells under ART but does not improve antigen-specific CD8+ T-cell function. (A) Representative example of the expression of IFN-γ, IL-2, and TNF-a in response to Gag peptide pool at d−7 and at d46 after therapy initiation. Numbers indicate the percentage of cells in the quadrants. (B) Pie charts and bars representing the quality of the SIV-specific (Gag and Env averaged) CD4+ T-cell response at baseline and at d46 after ART treatment. Each section of the pie chart represents a specific combination of cytokines, as indicated by the color at the bottom of the bar graph. Data were expressed as in Figure 2B. #P < .05 vs d−7; Wilcoxon rank-sum test. (C) As in panel B but in the different treatment groups at d46 after treatment initiation. #P < .05 vs IL-15; Wilcoxon rank-sum test. (D) Percentage of SIV-specific (Gag and Env averaged) CD4+ T-cells with TCM, TTM, or TEM cell phenotype of total memory cells at d−7 and at d46 after treatment initiation. Data are expressed as in Figure 1B.
Because IL-2 is mainly produced by TCM cells while more differentiated cells preferentially produce IFN-γ, we investigated the T-cell differentiation state of SIV-specific CD4+ T cells as described in Figure 2A. IL-15 therapy alone induced the differentiation of these cells from the TCM to the TTM cell phenotype at d46 after therapy introduction (Figure 5D). ART, either alone or in addition to IL-15, had the opposite trend, with an increase of TCM cells, in concordance with the increased IL-2 production by SIV-specific CD4+ T cells (Figure 5D).
Conversely, no major changes occurred in the quality of antigen-specific CD8+ T cells at d7, when the peak of CD8+ T-cell proliferation occurred, or at d46 after treatment for any of the stimuli in any of the groups (supplemental Figure 6). Moreover, no major changes were detected in the differentiation state of these cells (data not shown).
Effect of ART interruption in animals treated with ART+IL-15
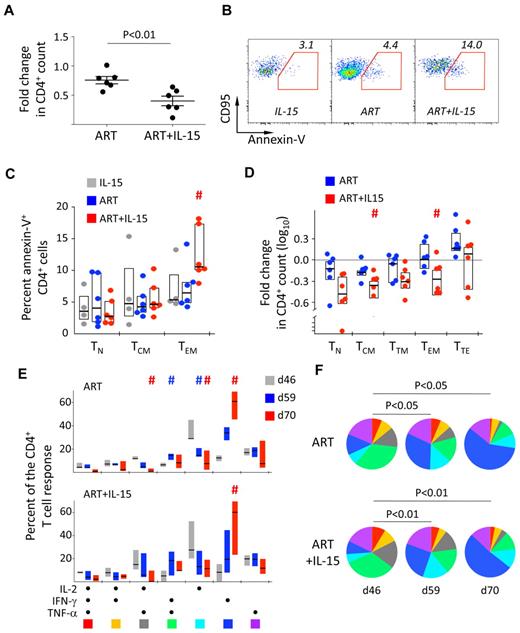
We next sought to determine whether previous IL-15 treatment affected virologic and immunologic parameters upon ART interruption. As shown in Figure 1A, IL-15 treatment did not have any effect on viral rebound and dynamics after ART interruption. On the other hand, we observed a much faster drop in the CD4+ T-cell count by d59 (d13 post ART interruption) in the ART+IL-15 compared with the ART alone group (P < .01; Mann Whitney; Figure 6A). This was accompanied by increased spontaneous apoptosis (ie, annexin-V+) of the CD4+ TEM subset from the ART+IL-15 compared with the ART alone group (Figure 6B-C). However, analysis of the changes in the absolute counts revealed that CD4+ T cells were lost in the same way irrespectively of differentiation status at d59 compared with d46 in the ART+IL-15 (Figure 6D). Of note, no differences where found in the activation status, (ie, expression of Ki-67, HLA-DR, and CD25) of CD4+ and CD8+ T-cell subsets before and after treatment interruption in the 3 groups (not shown). Similarly, no changes were found in the magnitude of antigen-specific CD8+ T-cell responses described in “Dynamics of antigen-specific T cells upon IL-15 treatment.” Conversely, changes in the composition of SIV-specific CD4+ T-cell response were observed. On ART removal, independently of previous IL-15 therapy, the quality of SIV-specific CD4+ T-cell response returned to baseline, as we observed a contraction of early-differentiated cells producing IL-2 only or in combination with TNF-α and a concomitant increased proportion of cells making IFN-γ alone (Figure 6E-F). This effect was also accompanied by the differentiation of SIV-specific CD4+ TCM to TTM cells (supplemental Figure 7). Notably, upon removal of IL-15 in the IL-15 alone group, SIV-specific CD4+ T cells tended to revert to their initial TCM cell phenotype at the expense of TTM cells (supplemental Figure 7).
Post treatment effects of IL-15 therapy. (A) Fold change in the absolute count of total CD4+ T cells in the peripheral blood at d59 (d13 posttherapy interruption) in the ART and ART+IL-15 groups. Each point represents an animal. The median ± SEM is depicted. (B) Representative example of the expression of phosphatidylserine on the cell surface by d59 TEM CD4+ T cells after overnight culture. (C) Summary of the data as in panel B but also from TN and TCM CD4+ T cells. #P < .05 vs ART; Wilcoxon rank-sum test. (D) Fold change in the absolute count of CD4+ T cells with different differentiation phenotypes in the peripheral blood at d59 (d13 posttherapy interruption) in the ART and ART+IL-15 groups. Data are depicted as in Figure 2A. #P < .05 vs ART; Wilcoxon rank-sum test. (E) Bar graph and (F) pie charts representing the quality of the SIV-specific (Gag and Env averaged) CD4+ T-cell response at days 46, 59, and 70 in the ART and ART+IL-15 groups. Colors in the pie charts corresponding to different cytokine combinations are indicated at the bottom of panel C. #P < .05 vs d46. (E) Percentage of SIV-specific (Gag and Env averaged) CD4+ T cells with TCM, TTM, or TEM phenotype of total memory cells before treatment interruption (d46) and at days 59 and 70. Data were expressed as in Figure 1B. # = P < .05 vs d46.
Post treatment effects of IL-15 therapy. (A) Fold change in the absolute count of total CD4+ T cells in the peripheral blood at d59 (d13 posttherapy interruption) in the ART and ART+IL-15 groups. Each point represents an animal. The median ± SEM is depicted. (B) Representative example of the expression of phosphatidylserine on the cell surface by d59 TEM CD4+ T cells after overnight culture. (C) Summary of the data as in panel B but also from TN and TCM CD4+ T cells. #P < .05 vs ART; Wilcoxon rank-sum test. (D) Fold change in the absolute count of CD4+ T cells with different differentiation phenotypes in the peripheral blood at d59 (d13 posttherapy interruption) in the ART and ART+IL-15 groups. Data are depicted as in Figure 2A. #P < .05 vs ART; Wilcoxon rank-sum test. (E) Bar graph and (F) pie charts representing the quality of the SIV-specific (Gag and Env averaged) CD4+ T-cell response at days 46, 59, and 70 in the ART and ART+IL-15 groups. Colors in the pie charts corresponding to different cytokine combinations are indicated at the bottom of panel C. #P < .05 vs d46. (E) Percentage of SIV-specific (Gag and Env averaged) CD4+ T cells with TCM, TTM, or TEM phenotype of total memory cells before treatment interruption (d46) and at days 59 and 70. Data were expressed as in Figure 1B. # = P < .05 vs d46.
Discussion
Based on several reports, IL-15 is promising as an immunotherapeutic strategy to mediate T-cell expansion via induction of cell cycle14 and differentiation of TCM into effector-phenotype T-cell subsets.16,17,27,28 For this reason, it has been proposed as an adjuvant to replenish the CD4+ TEM pool at mucosal surfaces in patients with HIV infection.29 However, here we demonstrate that IL-15 treatment combined with highly active ART is ineffective in mediating this effect in chronically SIV-infected macaques. As expected, we observed CD8+ T-cell proliferation and concomitant expansion of the CD8+ population in the periphery (∼ 3-fold compared with baseline) as well as SIV-specific CD8+ T-cell proliferation. This was observed as soon as d7 after ART+IL-15, but was transient and lost after the first week. In contrast, IL-15+ART treatment was not superior to ART alone in mediating CD4+ T-cell expansion in the periphery, despite the tendency of CD4+ T cells to increase faster (ie, at d7) in the former group. We suggest that this is the consequence of a mild effect of IL-15 on CD4+ T-cell redistribution during immune reconstitution, as no proliferation was induced in CD4+ T cells. Conversely, long-term effect of the cytokine on CD4+ T-cell proliferation could be observed in the peripheral tissues at d28 after treatment initiation, as ART+IL-15 could greatly enhance the expression of Ki-67 on memory CD4+ T cells in the LN compared with ART alone, even though this effect was not sufficient to boost the CD4+ T-cell recovery. It remains to be determined why the promotion of T-cell proliferation by IL-15 could not be achieved at mucosal surfaces.
As we saw for total T cells, we observed that IL-15 was not able to increase the frequency of antigen-specific CD4+ and CD8+ T-cells for different antigens, despite an increased, but transient, frequency of proliferating CD8+. Moreover, ART and ART+IL-15 groups did differ in terms of the quality of cytokine production by SIV-specific CD4+ T cells and SIV- or CMV-specific CD8+ T cells, further demonstrating the inefficacy of the treatment in chronic SIV infection, even under complete virus control.
Why could no effects be seen on CD4+ T cells despite transient changes in the CD8+ T-cell compartment? Increased baseline levels of IL-15 in the plasma in chronically SIV-infected monkeys compared with healthy macaques did not influence the capability of T cells to respond to the cytokine therapy. Picker et al reported the ability of IL-15+ART treatment in chronically SIV infected rhesus macaques to induce CD4+ T-cell proliferation, albeit at a lower level than that seen in uninfected macaques.17 Partial suppression of viral replication is a prerequisite of IL-15–induced expansion of CD4+ T cells in SIV-infected animals,17 as the same cells fail to expand with ongoing viral replication15,17 (Figure 1C). Similar to our findings, no significant increase in Ki-67+ CD4+ T-cell proliferation was observed in another study with ART-suppressed rhesus macaques treated with IL-15.30 Could the complete viral suppression, as achieved in this study by potent antiviral therapy, impair the responsiveness to IL-15? Differences in inflammation and soluble IL-15Rα chains may reduce T-cell responsiveness by affecting IL-15 transpresentation31 or the levels of IL-2/15Rβ chain, although we recently excluded a potential role for IL-15Rα on the surface of dendritic cells in mediating IL-15 function in vivo.16 Another important difference is that Picker et al treated with IL-15 animals already virally suppressed with ART,17 whereas in our current study IL-15 treatment was started at the initiation of ART before viral suppression. Such IL-15 treatment before viral replication is suppressed may lead to rapid depletion of CCR5-expressing TEM CD4+ T cells. Indeed animals treated with ART+IL-15 showed increases in numbers of TTM and TEM CD4+ T cells already at d4 and d7 (Figure 2A-B) before viral suppression was achieved.
Furthermore, animals treated with ART+IL-15 showed a delayed time to viral suppression compared with ART alone animals. This may indicate that IL-15 induced CD4+ T-cell proliferation may be providing targets and delaying the effect of ART. Treating animals completely suppressed first with ART before initiating IL-15 treatment may allow for the expansion of TEM CD4+ T cells and avoid their potential elimination by virus. Finally, the frequencies of effector memory CD4+ T cells were low in the ART+IL-15 (2.7% of CD4+ T cells) and ART (3.9%) groups at the start of the treatment and we cannot exclude that such low numbers negatively impact recovery. These percentages of effector memory CD4+ T cells are lower than those in the Picker et al study where effector memory CD4+ T cells were in the range of 10% and ART+IL-15 induced their proliferation.17 Therefore, it is possible that animals with higher CD4+ TEM cells would show better recovery and expansion of CD4+ TEM when treated with ART+IL-15. Future studies need to address how the initial levels of CD4+ TEM cells can impact IL-15–induced recovery of these cells.
Rather than beneficial for immune reconstitution, our data indicate that IL-15 therapy may have deleterious effects. Indeed, after ART interruption, macaques receiving previous IL-15 treatment experienced a faster drop in peripheral CD4+ T-cell counts than those receiving ART alone, but irrespective of the CD4+ T-cell differentiation status, as described in humans.32 We failed to identify the cause of this effect as animals from both groups did not differ in virologic and immunologic parameters, such as in viral load at d46 and d59 or in the count of CD4+ T-cell subpopulations and their activation status before treatment interruption.
In conclusion, our data indicate that IL-15 together with highly active ART does not improve reconstitution of CD4+ T cells in multiple tissues during the course of chronic SIV-infection. Further studies might elucidate the mechanisms by which CD4+ T cells remained unresponsive in this setting and why CD8+ T cells only responded transiently to cytokine therapy. Exhaustion of TEM-phenotype T cells or T-cell precursors in advanced disease may limit their expansion capability, and thus, T-cell recovery. For this reason, combination of multiple therapies simultaneously targeting T-cell proliferation and blockade of inhibitory pathways could potentially improve CD4+ T-cell recovery and antigen-specific immune functions.33
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Drs Joanne Yu and Pratip Chattopadhyay for assistance with antibody conjugation and qualification; Kathy Foulds, Shing-Fen Kao, and Mitzi Donaldson for help with sample processing; and Dr Kenneth Rogers and Jianzhong Zhou for production of recombinant mamu (rhesus macaque MHC) IL-15.
This work has been supported in part by grants National Institutes of Health R01 AI046719 and R21 AI082680 to P.D.K., R24RR016988 to F.V. and by the intramural research program of the NIAID, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: E.L., Y.M.M., P.D.K., and M.R. conceived the study; E.L. and Y.M.M. performed experiments; F.V. provided the simian IL-15, M.G.L. provided technical support; and E.L., Y.M.M., P.D.K., and M.R. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter D. Katsikis MD, PhD, Dept of Microbiology and Immunology, Drexel University College of Medicine, 2900 Queen Ln, Philadelphia, PA; e-mail: peter.katsikis@drexelmed.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal