Abstract
Corticosteroids and lenalidomide decrease red blood cell transfusion dependence in patients with Diamond-Blackfan anemia (DBA) and myelodysplastic syndrome (MDS), respectively. We explored the effects of dexamethasone and lenalidomide, individually and in combination, on the differentiation of primary human bone marrow progenitor cells in vitro. Both agents promote erythropoiesis, increasing the absolute number of erythroid cells produced from normal CD34+ cells and from CD34+ cells with the types of ribosome dysfunction found in DBA and del(5q) MDS. However, the drugs had distinct effects on the production of erythroid progenitor colonies; dexamethasone selectively increased the number of burst-forming units-erythroid (BFU-E), whereas lenalidomide specifically increased colony-forming unit-erythroid (CFU-E). Use of the drugs in combination demonstrated that their effects are not redundant. In addition, dexamethasone and lenalidomide induced distinct gene-expression profiles. In coculture experiments, we examined the role of the microenvironment in response to both drugs and found that the presence of macrophages, the central cells in erythroblastic islands, accentuated the effects of both agents. Our findings indicate that dexamethasone and lenalidomide promote different stages of erythropoiesis and support the potential clinical utility of combination therapy for patients with bone marrow failure.
Introduction
With the exception of cytokines and hormones, corticosteroids and lenalidomide are the primary pharmacologic agents that directly improve red blood cell production in patients with disorders of erythropoiesis. The molecular basis for this response is not known for either drug, but the determination of their specific effects on erythropoiesis is critical to inform the use of these agents, individually or in combination, in congenital and acquired bone marrow failure syndromes.
Lenalidomide, a derivative of thalidomide, causes both hematologic and cytogenetic responses in patients with anemia due to myelodysplastic syndrome (MDS). In a phase 2 trial in low-risk MDS patients with deletions of chromosome 5q, lenalidomide treatment decreased red blood cell transfusion requirements in 76% of patients, and 61% of patients had a complete cytogenetic response.1 A separate phase 2 trial demonstrated that lenalidomide also decreased transfusion requirements in 25% of low- or intermediate-1–risk MDS patients who lacked the del(5q) abnormality without inducing cytogenetic responses.2 A gene-expression signature indicating impaired erythroid differentiation predicts response to lenalidomide in non-del(5q) MDS.3 Both lenalidomide and pomalidomide (a next-generation thalidomide derivative) have been shown to promote erythropoiesis and induce fetal hemoglobin in vitro.3,4 These findings suggest that the therapeutic efficacy of lenalidomide in some cases of MDS may be in part attributable to the promotion of erythroid differentiation.3,4
Corticosteroids are the mainstay of therapy for patients with Diamond-Blackfan anemia (DBA), a rare congenital disorder characterized by anemia, macrocytosis, reticulocytopenia, and a selective decrease or absence of erythroid precursors in otherwise normocellular bone marrow.5 Approximately 80% of patients with DBA respond to steroids, although there is currently no reliable way to select the patients who will benefit from therapy.6 Corticosteroids increase the proliferation of erythroid progenitor cells in vitro and improve burst-forming units-erythroid (BFU-E) colony formation.7,8 Dexamethasone has been shown to induce the expression of genes found in immature erythroid cells while decreasing the expression of genes specific to non-erythroid hematopoietic differentiation.8
Defective ribosome biogenesis due to heterozygous mutations or deletions of ribosomal genes has been implicated in the pathophysiology of the macrocytic anemia that is characteristic of both DBA and del(5q) MDS.5 The efficacy of corticosteroids and lenalidomide in these molecularly linked disorders, and the erythropoietic effects of both agents, have raised the possibility that lenalidomide might have therapeutic utility in DBA, or that combinations of these drugs might be efficacious in either DBA or MDS. Ribosome dysfunction in DBA is congenital and present in all cells, in contrast to the acquired lesion in a del(5q) clone that causes MDS, raising the potential of aplasia in DBA when treated with drugs that have an unknown mechanism of action. In the present study, we explored the activity of dexamethasone and lenalidomide, alone and in combination, in stimulating erythropoiesis in primary human bone marrow progenitor cells. Our findings indicate that the drugs have distinct functional effects and may have therapeutic use in combination.
Methods
Culture of hematopoietic progenitor cells
Cryopreserved human bone marrow CD34+ cells were obtained from Poietics. Erythroid differentiation was induced in vitro using a 2-phase liquid culture system, as described previously.9 Cells were cultured in serum-free expansion medium (StemCell Technologies) supplemented with 100 U/mL penicillin/streptomycin, 2mM glutamine, 40 μg/mL lipids (Sigma-Aldrich), 100 ng/mL SCF (Miltenyi Biotec), 10 ng/mL IL-3 (Miltenyi Biotec), and 0.5 U/mL erythropoietin (EPO; Amgen).8 Dexamethasone, lenalidomide, or a DMSO control was added to the culture 24 hours after thawing and with medium changes on days 4 and 7 of culture. The concentration of EPO was increased on day 7 to 3 U/mL. Cells were harvested for flow cytometry after 10 days of liquid culture.
Lentiviral vectors and infection
Target sequences of shRNAs against RPS14 and RPS19 and the control shRNA are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The lentiviral backbone vector (pLKO.1) and packaging plasmids were transfected into 293T cells and the viral supernatant was harvested as described previously.10 Cryopreserved, primary hematopoietic cells were infected with lentivirus 1 day after thawing in the presence of 2 μg/mL Polybrene (Sigma-Aldrich) and selected 24 hours later with 2 μg/mL puromycin (Sigma-Aldrich).
Flow cytometry
Erythroid differentiation was evaluated by flow cytometry using a phycoerythrin-Cy5–conjugated CD71 antibody (lot 42267; BD Pharmingen) to identify immature erythroid cells and phycoerythrin-conjugated glycophorin A antibody (CD235a, lot 37628; BD Pharmingen) to identify terminally differentiated erythroid cells. Apoptosis was evaluated by flow cytometry using an antibody against annexin V (BD Pharmingen). Macrophages were assessed using goat polyclonal CD68 antibody (Santa Cruz Biotechnology). Flow cytometry for CD68 was performed after fixation and permeabilization with 2% paraformaldehyde and FITC-conjugated secondary antibody (Invitrogen).
Methylcellulose colony assays
Hematopoietic progenitor cells were plated in methylcellulose containing EPO, SCF, GM-CSF, and IL-3 (H4434; StemCell Technologies). Cells were treated with drug or vehicle for 72 hours before plating; no drug was added to the methylcellulose. Colony formation was assessed after 14-16 days of culture at 37°C in a humidified atmosphere with 5% CO2 as per the manufacturer's instructions. Scoring of colonies was performed by an individual blinded to the experimental conditions.
Compounds
Dexamethasone (Sigma-Aldrich) was resuspended in 1× PBS and stored as a 10mM stock solution. Lenalidomide was obtained from Toronto Research Chemicals, dissolved in DMSO, and stored as a 1mM stock solution. Wells marked untreated had the same percentage of DMSO as experimental conditions.
Gene expression
Human CD34+ cells were cultured for 48 hours in the erythroid differentiation medium described in “Culture of hematopoietic progenitor cells” with the addition of 40 ng/mL FMS-like tyrosine kinase (flt-3; Miltenyi Biotec) and 15 ng/mL G-CSF (Amgen). Cells were cultured in the presence of the drugs of interest for 24 hours. RNA was purified using TRIzol reagent (Invitrogen). RNA was amplified and labeled by in vitro transcription and hybridized to U133A microarrays (Affymetrix). CEL files were normalized by robust multichip average. Differentially expressed genes were ranked by signal-to-noise ratio and analyzed using Gene Set Enrichment Analysis.11,12 All microarray data are available on the Gene Expression Omnibus website under accession number GSE28896.
Coculture with stromal cells
The green fluorescent protein GFP-labeled OP9 cells were a gift from K. Hartwell (Broad Institute). OP9 cells were maintained in MEM-alpha (StemCell Technologies) supplemented with 10% FBS (Omega Scientific), 100 U/mL penicillin/streptomycin, 2mM glutamine, 14.6 mL of sodium bicarbonate 7.5% solution (final percentage, 0.22%; GIBCO), and 5 mL of diluted β-mercaptoethanol (final concentration, 100μM; Sigma-Aldrich). On day 4 of the culture, flow cytometry was performed to sort GFP+ cells from GFP− cells. The GFP− population was subsequently plated on methylcellulose.
Coculture with monocytes/macrophages
Monocytes were isolated from peripheral blood under a Children's Hospital Boston institutional review board–approved protocol by CD14 affinity bead purification (Miltenyi Biotec). Monocytes were cultured in RPMI 1640 medium (GIBCO) with 10% FBS (Omega Scientific). Nonadherent cells were removed by serial rinsing. Macrophage differentiation was stimulated by GM-CSF for 7 days, as described previously.13,14 Macrophages were identified by fluorescence microscopy using antibodies against CD68 (Santa Cruz Biotechnology). Quantitative determination of TNF-α was performed using a chemiluminescent immunoassay (QTAOOB; R&D Systems).
qRT-PCR
RNA was purified from shRNA-infected cells using TRIzol reagent (Invitrogen). First-strand cDNA was reverse transcribed from total RNA using Invitrogen Superscript III. Real-time quantitative RT-PCR (qRT-PCR) was performed using SYBR Green I Master Mix (Applied Biosystems). Expression of test genes was normalized to expression of the endogenous β-actin gene. Changes in expression of the target nucleic acid sequence were analyzed using the comparative CT method (ΔΔCT). Primer sequences are listed in supplemental Table 2.
Western blots
Antibodies against RPS19 (RPS19 WW-4 mouse monoclonal antibody; Santa Cruz Biotechnology) and RPS14 (A01; Abnova) were used at a 1:200 dilution. Beta-actin (C4) mouse monoclonal IgG1 (Santa Cruz Biotechnology) was used as a control at a 1:5000 dilution. The target proteins were analyzed using SuperSignal West Pico Chemiluminescent Substrate for horseradish peroxidase (ThermoScientific).
Statistical analysis
The significance of the experimental results was determined by 2-tailed Student t test when only one comparison was being studied. Experimental results were analyzed using ANOVA techniques in which the dose of dexamethasone and the dose of lenalidomide were identified as fixed effects. P ≤ .05 was considered statistically significant.
Results
Dexamethasone and lenalidomide promote erythropoiesis but have differential effects on erythroid colony formation
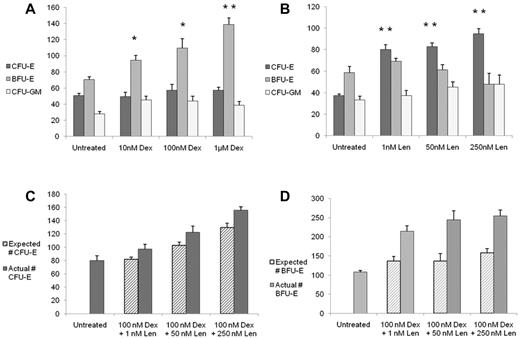
To examine the effects of dexamethasone and lenalidomide on human hematopoietic progenitor cells, we treated primary bone marrow CD34+ cells with drugs in liquid culture for 3 days and assessed the effects on progenitor cells with methylcellulose colony assays. As shown in Figure 1A, dexamethasone caused a dose-dependent and statistically significant increase in BFU-E without significantly altering the numbers of CFU-E or CFU-GM. In addition, we noted that the BFU-E colonies were consistently larger than the colonies treated with vehicle control; representative images are show in supplemental Figure 1. In contrast, as shown in Figure 1B, lenalidomide caused a dose-dependent and statistically significant increase in CFU-E, but did not affect BFU-E or CFU-GM colonies. In this experimental assay, EPO increased the numbers of both BFU-E and CFU-E colonies (supplemental Figure 2). The differential effects of dexamethasone and lenalidomide on colony formation were consistent across different durations of treatment (supplemental Figure 3). These findings indicate that dexamethasone and lenalidomide have distinct functional effects on the production of erythroid progenitor cells.
Dexamethasone increases BFU-E colony formation, whereas lenalidomide increases CFU-E colony formation, and the combination of drugs is additive. After 3 days of treatment with the compounds in liquid culture, cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) are shown in panel A and the response to lenalidomide (Len) is shown in panel B. The effects of combinations of Dex and Len on CFU-E are shown in panel C and the effects on BFU-E in panel D. The expected number of colonies was calculated as follows: expected number of colonies = (Dex-alone colonies − control) + (Len-alone colonies − control) + control. The experiments were performed in triplicate and repeated with similar results. In panels A and B, a 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05. In panels C and D, ANOVA was used and the statistical findings are discussed in the text.
Dexamethasone increases BFU-E colony formation, whereas lenalidomide increases CFU-E colony formation, and the combination of drugs is additive. After 3 days of treatment with the compounds in liquid culture, cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) are shown in panel A and the response to lenalidomide (Len) is shown in panel B. The effects of combinations of Dex and Len on CFU-E are shown in panel C and the effects on BFU-E in panel D. The expected number of colonies was calculated as follows: expected number of colonies = (Dex-alone colonies − control) + (Len-alone colonies − control) + control. The experiments were performed in triplicate and repeated with similar results. In panels A and B, a 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05. In panels C and D, ANOVA was used and the statistical findings are discussed in the text.
We next studied the effects of combinations of dexamethasone and lenalidomide on hematopoietic progenitor cells. As shown in Figure 1C, for the CFU-E colonies, the interaction between dexamethasone and lenalidomide was additive, with dexamethasone (P = .018) and lenalidomide (P < .0001) each contributing significantly. As shown in Figure 1D, for the BFU-E colonies, there remained a significant effect for dexamethasone (P < .0001) and a significant dexamethasone/lenalidomide interaction (P = .006), but there was no main effect for lenalidomide alone, suggesting that the impact of lenalidomide in combination with dexamethasone on BFU-E colony formation is through the effect of dexamethasone.
Having demonstrated the effects of dexamethasone and lenalidomide on erythroid progenitor cells, we next examined the effects of these drugs on the production of terminally differentiated erythroid cells. We treated normal human CD34+ hematopoietic progenitor cells with dexamethasone or lenalidomide and induced erythroid differentiation in a 2-phase liquid culture for 10 days. The total numbers of cells were enumerated and the proportion of these cells that were erythroid was evaluated by flow cytometry. Both dexamethasone and lenalidomide caused a dose-dependent increase in the number of erythroid cells in culture (supplemental Figure 4).
Dexamethasone and lenalidomide rescue erythropoiesis, alone and in combination, in RPS14- and RPS19-deficient cells
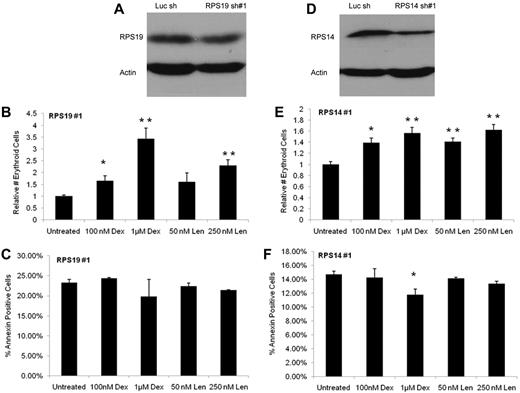
Ribosome dysfunction is the likely etiology of anemia in both DBA and del(5q) MDS,5 raising the possibility that the erythropoietic effects of dexamethasone and lenalidomide might differ in cells with defective ribosome biogenesis. Previous studies have demonstrated that the use of RNA interference to knock down expression of RPS19, the most commonly mutated gene in DBA, and RPS14, a critical gene on chromosome 5q, in hematopoietic progenitor cells recapitulates the erythroid defect and ribosome dysfunction of both disorders.8,9,15,16 In the present study, the lentivirally expressed shRNAs for RPS19 and RPS14 did not completely ablate expression of their target genes, mimicking haploinsufficiency rather than homozygous inactivation (Figure 2A,D, supplemental Figure 5, and supplemental Table 4). We found that both dexamethasone and lenalidomide increase the erythroid-proliferative capacity of CD34+ cells expressing RPS19 or RPS14 shRNAs. As shown in Figure 2B, increasing concentrations of dexamethasone and the higher concentration of lenalidomide resulted in a statistically significant increase in the relative number of erythroid cells derived from CD34+cells infected with RPS19 lentivirus compared with untreated cells. Similar effects were seen in CD34+ cells infected with RPS14 lentivirus (Figure 2E). Comparable results were found with a second shRNA for both RPS19 and RPS14 (supplemental Figure 6). We also found that although erythropoiesis was promoted, there was no concurrent increase in the level of apoptosis. As shown in Figure 2C and F and supplemental Figure 5, there was no increase in annexin V staining noted in cells expressing RPS19 or RPS14 shRNAs and treated with either dexamethasone or lenalidomide compared with vehicle control.
Dexamethasone and lenalidomide increase the production of erythroid cells from CD34+ cells expressing RPS19 or RPS14 shRNAs without increasing apoptosis. In panels A and D, a Western blot shows the decreased level of protein with RPS19 knockdown (56.7% knockdown by qRT-PCR) and with RPS14 knockdown (61.9% knockdown by qRT-PCR). The absolute number of erythroid cells after 10 days in liquid culture was determined by multiplying the number of cells counted per well by the percentage of those cells expressing any erythroid markers (CD71, glycophorin A) as assayed by flow cytometry. Data are presented as relative numbers to eliminate differences caused by cell number and infection rate. The effects of dexamethasone (Dex), lenalidomide (Len), and control on cells infected with a RPS19 shRNA are shown in panel B. The effects of Dex, Len, and control on cells infected with a RPS14 shRNA are shown in panel E. No increase in annexin staining was noted in CD34+ cells expressing RPS19 or RPS14 shRNAs and treated with either Dex or Len compared with vehicle control, as shown in panels C and F. The experiments were performed in triplicate and the entire experiment was repeated with similar results with an independent shRNA (supplemental Figure 5). A 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05.
Dexamethasone and lenalidomide increase the production of erythroid cells from CD34+ cells expressing RPS19 or RPS14 shRNAs without increasing apoptosis. In panels A and D, a Western blot shows the decreased level of protein with RPS19 knockdown (56.7% knockdown by qRT-PCR) and with RPS14 knockdown (61.9% knockdown by qRT-PCR). The absolute number of erythroid cells after 10 days in liquid culture was determined by multiplying the number of cells counted per well by the percentage of those cells expressing any erythroid markers (CD71, glycophorin A) as assayed by flow cytometry. Data are presented as relative numbers to eliminate differences caused by cell number and infection rate. The effects of dexamethasone (Dex), lenalidomide (Len), and control on cells infected with a RPS19 shRNA are shown in panel B. The effects of Dex, Len, and control on cells infected with a RPS14 shRNA are shown in panel E. No increase in annexin staining was noted in CD34+ cells expressing RPS19 or RPS14 shRNAs and treated with either Dex or Len compared with vehicle control, as shown in panels C and F. The experiments were performed in triplicate and the entire experiment was repeated with similar results with an independent shRNA (supplemental Figure 5). A 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05.
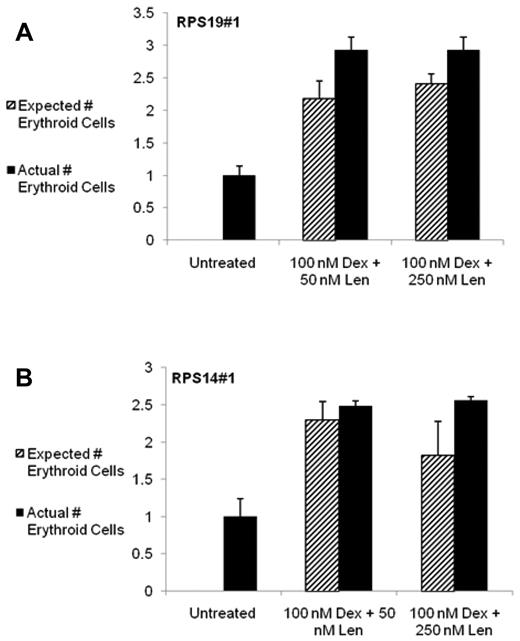
Simultaneous treatment with both drugs was additive in the production of erythroid cells, as shown in Figure 3 and supplemental Figure 6, indicating that the effects of dexamethasone and lenalidomide are likely to be mediated by independent and complementary mechanisms. For each lentivirus, the effect of dexamethasone was consistently the variable with the largest impact (P < .0001 for each). The effect of lenalidomide was associated with a P value < .0001 for RPS19#1, P = .0001 for RPS19#2, and P = .0002 for each of the RPS14 viruses. These data suggest that both drugs are able to improve the erythroid capacity of both normal cells and cells with decreased expression of key ribosomal genes, and that treatment with dexamethasone and lenalidomide in RPS19- or RPS14-deficient cells does not increase apoptosis.
The combination of dexamethasone and lenalidomide has an additive effect on increasing the production of erythroid cells from CD34+ cells expressing RPS19 or RPS14 shRNAs. The effects of combination treatment with dexamethasone (Dex) and lenalidomide (Len) and control on cells infected with a RPS19 shRNA are shown in panel A and with a RPS14 shRNA in panel B. The absolute number of erythroid cells after 10 days in liquid culture was determined by multiplying the number of cells counted per well by the percentage of cells expressing any erythroid markers (CD71, glycophorin A) as assayed by flow cytometry. Data are presented as relative number to eliminate differences caused by cell number and infection rate. The expected number of erythroid cells was calculated as follows: expected number of cells = (absolute number of erythroid cells with Dex treatment alone − control) + (absolute number of erythroid cells with Len treatment alone − control) + control. The experiments were performed in triplicate and the entire experiment was repeated with similar results with an independent shRNA (supplemental Figure 6). ANOVA was used and the statistical findings are discussed in the text.
The combination of dexamethasone and lenalidomide has an additive effect on increasing the production of erythroid cells from CD34+ cells expressing RPS19 or RPS14 shRNAs. The effects of combination treatment with dexamethasone (Dex) and lenalidomide (Len) and control on cells infected with a RPS19 shRNA are shown in panel A and with a RPS14 shRNA in panel B. The absolute number of erythroid cells after 10 days in liquid culture was determined by multiplying the number of cells counted per well by the percentage of cells expressing any erythroid markers (CD71, glycophorin A) as assayed by flow cytometry. Data are presented as relative number to eliminate differences caused by cell number and infection rate. The expected number of erythroid cells was calculated as follows: expected number of cells = (absolute number of erythroid cells with Dex treatment alone − control) + (absolute number of erythroid cells with Len treatment alone − control) + control. The experiments were performed in triplicate and the entire experiment was repeated with similar results with an independent shRNA (supplemental Figure 6). ANOVA was used and the statistical findings are discussed in the text.
Dexamethasone and lenalidomide induce distinct gene-expression profiles
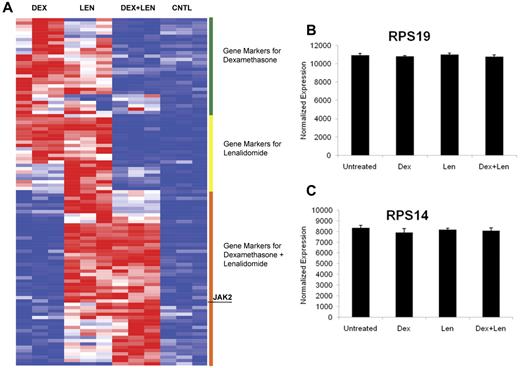
The molecular basis for the erythropoietic effects of lenalidomide and dexamethasone is unknown. We performed gene-expression profiling on primary human CD34+ cells treated with dexamethasone, lenalidomide, and a combination of both drugs. As shown in Figure 4A, dexamethasone and lenalidomide induced distinct but overlapping expression profiles. Consistent with the functional studies, the combination of dexamethasone and lenalidomide induced a signature of erythroid differentiation more powerfully than either agent alone (supplemental Table 3). Several genes known to play a role in erythropoiesis had increased expression after treatment with dexamethasone, lenalidomide, or the combination; these are highlighted in Table 1. Dexamethasone increased the expression of suppressor of cytokine signaling-1 (SOCS-1), and mice lacking SOCS-1 have been shown to have a subnormal hematocrit and an accumulation of immature red blood cells.17 Lenalidomide up-regulates FLT3, and forced expression of FLT3 in CD34+ cells confers a proliferative advantage with increased erythroid differentiation.18 The combination of dexamethasone and lenalidomide increases expression of Janus kinase 2 (JAK2), which is known to play a significant role in erythropoiesis.19,20 The full list of differentially expressed genes is included in supplemental Table 5.
Dexamethasone and lenalidomide induce distinct but overlapping gene-expression profiles. The heat map (panel A) illustrates the top 25 genes induced by dexamethasone (Dex), lenalidomide (Len), and the combination of both drugs. Genes induced by one drug and the combination are labeled as genes induced by the combination. The genes were arranged by hierarchical clustering. High gene expression is portrayed in red and low expression is blue. Expression of RPS19 and RPS14 in response to drug treatment is shown in panels B and C, respectively.
Dexamethasone and lenalidomide induce distinct but overlapping gene-expression profiles. The heat map (panel A) illustrates the top 25 genes induced by dexamethasone (Dex), lenalidomide (Len), and the combination of both drugs. Genes induced by one drug and the combination are labeled as genes induced by the combination. The genes were arranged by hierarchical clustering. High gene expression is portrayed in red and low expression is blue. Expression of RPS19 and RPS14 in response to drug treatment is shown in panels B and C, respectively.
Genes up-regulated by dexamethasone (Dex), lenalidomide (Len), and the combination of the two drugs with published effects on erythropoiesis
| Name . | Description . | Up-regulated . | Role in erythropoiesis . |
|---|---|---|---|
| IL-18R1 | IL-18 receptor 1 | Dex | Expressed on normal erythroid cell lines42 |
| SOCS-1 | Suppressor of cytokine signaling 1 | Dex | Mice lacking SOCS-1 have a subnormal hematocrit and an accumulation of immature red blood cells17 |
| IL-1R2 | IL-1 receptor, type II | Len | Nonsignaling receptor; expressed on hematopoietic stem cells43 |
| FLT3 | fms-related tyrosine kinase 3 | Len | Forced expression of FLT3-ITD in CD34+ cells confers proliferative advantage with increased erythroid differentiation18 |
| CD163 | CD163 molecule | Len | Macrophage adhesion molecule involved in erythroblastic islands44 |
| RHCE | Rh blood group, CcEe antigens | Dex + Len | Rh antigens appear early during erythropoietic differentiation45 |
| JAK2 | Janus kinase 2 (a protein tyrosine kinase) | Dex + Len | Essential role in erythropoiesis, including response to erythropoietin46 |
| AQP3 | Aquaporin 3 (GIL blood group) | Dex + Len | Water and glycerol channel present on human erythrocytes; also encodes GIL blood group system47 |
| Name . | Description . | Up-regulated . | Role in erythropoiesis . |
|---|---|---|---|
| IL-18R1 | IL-18 receptor 1 | Dex | Expressed on normal erythroid cell lines42 |
| SOCS-1 | Suppressor of cytokine signaling 1 | Dex | Mice lacking SOCS-1 have a subnormal hematocrit and an accumulation of immature red blood cells17 |
| IL-1R2 | IL-1 receptor, type II | Len | Nonsignaling receptor; expressed on hematopoietic stem cells43 |
| FLT3 | fms-related tyrosine kinase 3 | Len | Forced expression of FLT3-ITD in CD34+ cells confers proliferative advantage with increased erythroid differentiation18 |
| CD163 | CD163 molecule | Len | Macrophage adhesion molecule involved in erythroblastic islands44 |
| RHCE | Rh blood group, CcEe antigens | Dex + Len | Rh antigens appear early during erythropoietic differentiation45 |
| JAK2 | Janus kinase 2 (a protein tyrosine kinase) | Dex + Len | Essential role in erythropoiesis, including response to erythropoietin46 |
| AQP3 | Aquaporin 3 (GIL blood group) | Dex + Len | Water and glycerol channel present on human erythrocytes; also encodes GIL blood group system47 |
The expression of both RPS19 and RPS14 was unchanged after drug treatment (Figure 4B-C). Similarly, expression of the set of all ribosomal genes was unchanged by drug treatment, as assessed by Gene Set Enrichment Analysis (supplemental Figure 7). These findings further demonstrate that dexamethasone, lenalidomide, and the combination promote erythropoiesis directly, rather than correcting aberrant or insufficient ribosomal gene expression.
Effects of dexamethasone and lenalidomide on erythropoiesis are augmented by the presence of stromal cells
Erythropoiesis occurs in the context of a specific microenvironment within the bone marrow.21 Dexamethasone has long been recognized to have effects on human bone marrow stromal cells,22 and lenalidomide has been shown to exert some of its anti–multiple myeloma effects by modulating the microenvironment.23 We therefore dissected the influences of this microenvironment on erythropoiesis and on drug effects by coculturing erythroid progenitor cells with bone marrow stromal cells or macrophages.
Stromal cells are an important component of the bone marrow, and the extracellular matrix proteins secreted by these cells provide binding sites for hematopoietic stem and progenitor cells.24-26 OP9 cells, derived from mouse bone marrow stroma, support the growth of hematopoietic stem and progenitor cells.27 As shown in Figure 5A, there was a significant effect of lenalidomide (P = .0075) on CFU-E colony formation, but there was no effect from dexamethasone. There was also no effect on CFU-E colony formation after coculture with the OP9 stromal layer. As shown in Figure 5B, there was a significant effect of dexamethasone on BFU-E colony formation (P = .0006) and no effect from lenalidomide. There was an effect on BFU-E colony formation after coculture with OP9 stromal cells (P = .03 for lenalidomide and P = .006 for dexamethasone), but no evidence of an interaction with stromal cells for either drug. Similar results were obtained with human HS5 stromal cells (data not shown).
Stromal cells promote colony formation without altering the effects of dexamethasone and lenalidomide. After 3 days of treatment with the compounds in liquid culture, hematopoietic cells were separated from GFP+ OP9 cells by flow sorting, and the hematopoietic cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) and lenalidomide (Len) on CFU-E colony formation are shown in panel A. The effects of Dex and Len on BFU-E colony formation are shown in panel B. The experiments were performed in triplicate and the entire experiment was repeated on HS5 stromal cells with similar results. ANOVA was used and the statistical findings are discussed in the text.
Stromal cells promote colony formation without altering the effects of dexamethasone and lenalidomide. After 3 days of treatment with the compounds in liquid culture, hematopoietic cells were separated from GFP+ OP9 cells by flow sorting, and the hematopoietic cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) and lenalidomide (Len) on CFU-E colony formation are shown in panel A. The effects of Dex and Len on BFU-E colony formation are shown in panel B. The experiments were performed in triplicate and the entire experiment was repeated on HS5 stromal cells with similar results. ANOVA was used and the statistical findings are discussed in the text.
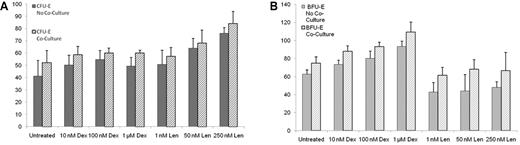
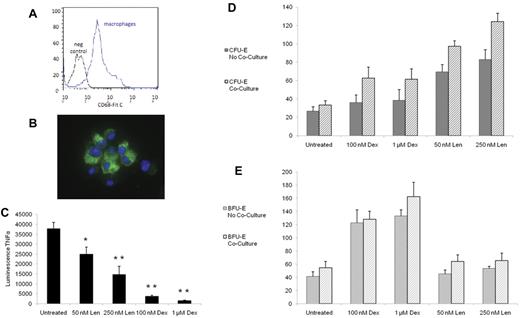
Macrophages are the central cells in bone marrow erythroblastic islands.28 We established culture conditions to recapitulate these interactions in the context of pharmacologic manipulations. We isolated monocytes from the peripheral blood and induced macrophage differentiation with GM-CSF, as described previously.13,14 As shown in Figure 6A, the cells were > 90% positive for the intracellular macrophage marker CD68. Using immunofluorescence, these cells also stained positive for CD68 and DAPI and demonstrated morphology consistent with stromal cells (Figure 6B).
The effects of dexamethasone and lenalidomide on erythropoiesis are influenced by macrophage cocultures. Macrophages were confirmed by presence CD68 by flow cytometry (A) and by immunofluorescence (B), with the green stain representing CD68 and the blue stain representing DAPI. After 3 days of treatment with the compounds in liquid culture, the amount of TNF-α in the supernatant was quantified by a chemiluminescent immunoassay (C). The experiment was performed in triplicate and repeated with similar results. After 3 days of treatment with the compounds in liquid culture, cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) and lenalidomide (Len) on CFU-E colony formation are shown in panel D. The effects of Dex and Len on BFU-E colony formation are shown in panel E. The experiments were performed in triplicate and repeated with similar results. In panel C, a 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05. In panels D and E, ANOVA was used and the statistical findings are discussed in the text.
The effects of dexamethasone and lenalidomide on erythropoiesis are influenced by macrophage cocultures. Macrophages were confirmed by presence CD68 by flow cytometry (A) and by immunofluorescence (B), with the green stain representing CD68 and the blue stain representing DAPI. After 3 days of treatment with the compounds in liquid culture, the amount of TNF-α in the supernatant was quantified by a chemiluminescent immunoassay (C). The experiment was performed in triplicate and repeated with similar results. After 3 days of treatment with the compounds in liquid culture, cells were plated in methylcellulose without drugs. Data are presented as the number of colonies per 3200 cells plated. The effects of dexamethasone (Dex) and lenalidomide (Len) on CFU-E colony formation are shown in panel D. The effects of Dex and Len on BFU-E colony formation are shown in panel E. The experiments were performed in triplicate and repeated with similar results. In panel C, a 2-tailed Student t test was used. **P ≤ .01; *P ≤ .05. In panels D and E, ANOVA was used and the statistical findings are discussed in the text.
Monocytes and macrophages produce TNF-α, a powerful inhibitor of erythropoiesis.28 Inhibition of TNF-α production has been reported previously for dexamethasone29 and lenalidomide,30 and may contribute to their roles in promoting erythropoiesis. As shown in Figure 6C, we found that the addition of either dexamethasone or lenalidomide resulted in a dose-dependent and statistically significant decrease in TNF-α production.
We cocultured human progenitor cells with macrophages. As shown in Figure 6D, for CFU-E colony formation, there were no significant interactions between drug and macrophages, but there was an additive effect of stromal exposure through macrophages (P = .0002). Lenalidomide continued to have a significant effect on CFU-E on its own (P < .0001), whereas dexamethasone had a marginal effect (P = .06) that had not been seen previously. As shown in Figure 6E, for BFU-E colony formation, there were no significant interactions between drug and macrophages. Lenalidomide dosing did not affect the number of BFU-E, whereas dexamethasone again had a strong effect (P < .0001). Macrophages also had a significant impact (P = .04), suggesting an additive effect of the stromal cells and drug treatment. These findings indicate that some of the effects of lenalidomide and dexamethasone on erythropoiesis may be augmented in the presence of macrophages in erythroblastic islands.
Discussion
Our results indicate that dexamethasone and lenalidomide have distinct effects on erythroid progenitor cells. The two drugs (1) have different effects on erythroid colony formation, with selective induction of BFU-E colonies by dexamethasone and CFU-E colonies by lenalidomide; (2) activate powerful but distinct gene-expression programs; and (3) in combination have additive effects on erythropoiesis. The erythropoietic activity of both lenalidomide and dexamethasone was independent of ribosome dysfunction, because the drugs had similar effects in normal, RPS19-deficient, and RPS14-deficient cells and did not affect the expression of ribosomal protein genes. Whereas erythropoietic effects of lenalidomide and dexamethasone have been reported previously, our direct comparisons of the 2 agents demonstrate that the cell-autonomous effects on erythroid progenitor cells are distinct. These findings suggest that lenalidomide and dexamethasone, individually or in combination, have the potential to increase red blood cell production in erythroid disorders of ribosome dysfunction and to improve the anemia of other bone marrow failure syndromes.
Combination therapy with dexamethasone and lenalidomide has been explored with therapeutic success in clinical trials for multiple myeloma.31-33 However, direct effects on erythropoiesis cannot be determined from these studies because a decreased burden of myeloma cells would also be likely to ameliorate anemia. Complications of combination therapy included an increased risk of thromboembolic disease, but the doses of both dexamethasone and lenalidomide used in these trials were significantly higher than the doses used in DBA or MDS.
We investigated the role of the microenvironment in the physiologic response to dexamethasone and lenalidomide by designing coculture systems to better mimic the bone marrow microenvironment, which is known to play a critical role in erythropoiesis. Macrophages not only anchor erythroblasts but also influence erythroid proliferation and differentiation.28 We found that coculture with macrophages had an additive effect, with dexamethasone and lenalidomide, on erythropoiesis. This suggests that the drugs might have both cell-autonomous and non–cell-autonomous effects on erythropoiesis.
DBA is the prototypical congenital disorder of impaired red blood cell production.5 Mutations in genes encoding ribosomal proteins have now been identified in up to 50% of patients with DBA, and no disease-causing mutations have been identified in genes that do not encode ribosomal proteins. Current therapies are limited to steroids, which are complicated by immunosuppression and other side effects of long-term use; chronic transfusions, which are complicated by iron overload; and bone marrow transplantation, which is complicated by that fact that not all patients have suitable donors.34 With existing treatments, the overall survival of patients, as reported by the DBA Registry, is 75.1% at 40 years of age.35 Lenalidomide, alone or in combination with corticosteroids, has the potential to improve red blood cell production and quality of life in these patients.
The 5q− syndrome, a subtype of MDS with an isolated deletion of chromosome 5q, has a similar phenotype to DBA, including severe macrocytic anemia.36 RPS14 deficiency recapitulates the erythroid defect of the 5q− syndrome in human hematopoietic progenitor cells.9,37 A murine model of conditional heterozygous deletion of RPS14 along with 8 adjacent genes recapitulates the macrocytic anemia characteristic of the disease.38 Lenalidomide is thought to selectively induce apoptosis in del(5q) myeloblasts and to cause a preferential cell-cycle arrest through inhibition of 2 phosphatases, Cdc25C and PP2A, which are also located in the common deleted region on chromosome 5.39,40
An important concern about the use of lenalidomide in patients with DBA is the possibility of aplasia, given the selective induction of apoptosis in del(5q) cells by lenalidomide. Our data do not indicate that lenalidomide or the combination of dexamethasone and lenalidomide led to synthetic lethality with ribosomal haploinsufficiency. Instead, we found that both lenalidomide and dexamethasone promoted erythropoiesis in RPS14- and RPS19-deficient cells and that there was no concurrent increase in apoptosis. Moreover, in patients with del(5q) MDS, lenalidomide does not kill hematopoietic stem cells.41 In non-del(5q) MDS patients, lenalidomide decreases transfusion requirements without leading to cytogenetic remission,2 suggesting that it stimulates erythropoiesis in these cells. Overall, these data indicate that lenalidomide is capable of increasing red blood cell production independently of ribosome dysfunction, and that lenalidomide does not kill hematopoietic stem cells in vivo.
Corticosteroids have been a mainstay of therapy for DBA, and lenalidomide is an important new therapy for MDS. We have found that these therapeutic agents promote erythropoiesis in human hematopoietic progenitor cells with distinct functional effects and consequences on gene expression. Moreover, we found that combinations of corticosteroids and lenalidomide have additive or synergistic effects on erythropoiesis. These findings indicate that lenalidomide, corticosteroids, and combinations of the 2 may have therapeutic utility in the treatment of anemia in a variety of bone marrow failure syndromes.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Katherine Lin, Damien Wilpitz, Supriya Gupta, and Robert Onofrio for technical assistance, microarray analysis, and production of virus.
This work was funded by the National Institutes of Health (grants 5R01 HL082945 and P01 CA108631) and by the Burroughs-Wellcome Fund (CAMS) to B.L.E.
National Institutes of Health
Wellcome Trust
Authorship
Contribution: A.N. and B.L.E. conceived the project; A.N., S.D., J.R.M., S.H., and M.M. performed the experiments; F.A.-S. performed the gene-expression analysis; D.N. assisted with the statistical interpretation of data; B.L.E. supervised the experimental work and interpretation of data; A.N. and B.L.E. wrote the manuscript; and S.D., J.R.M., S.H., M.M., F.A.-S., and D.N. reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benjamin L. Ebert, Brigham and Women's Hospital, Karp Research Bldg, CHRB 05.210, 1 Blackfan Cir, Boston, MA 02115; e-mail: bebert@partners.org.