Abstract
Detection of human Ag-specific T cells is limited by sensitivity and blood requirements. As dendritic cells (DCs) can potently stimulate T cells, we hypothesized that their induction in PBMCs in situ could link Ag processing and presentation to Ag-specific T-cell activation. To this end, unfractionated PBMCs (fresh or frozen) or whole blood were incubated for 48 hours with protein or peptide Ag together with different DC-activating agents to rapidly and sequentially induce, pulse, and mature DCs. DC activation was therefore lined up with Ag recognition by neighboring T cells, thus telescoping the sequential steps of T-cell activation. Efficient processing of protein Ags made prior knowledge of epitopes and HLA restrictions dispensable. While reducing stimulation time, manipulation and blood requirements, in situ DC induction specifically amplified Ag-specific T-cell responses (cytokine secretion, proliferation, CD137/CD154 up-regulation, and binding of peptide-HLA multimers). IL-1β, although released by DCs, was also secreted in an Ag-specific fashion, thus providing an indirect biomarker of T-cell responses. These accelerated cocultured DC (acDC) assays offered a sensitive means with which to evaluate T-cell responses to viral and melanoma Ag vaccination, and may therefore find application for immune monitoring in viral, tumor, autoimmune, and transplantation settings.
Introduction
Despite the central role of T cells in immune responses to foreign or self-Ags, routine immune diagnosis and monitoring relies largely, if not exclusively, on the measurement of Abs. However, Abs do not always mediate or reflect the underlying pathology and may be less informative when the immune process is predominantly T-cell mediated. The sole clinical application of Ag-specific T-cell assays to date has been in the diagnosis of Mycobacterium tuberculosis infection.1 Moreover, T-cell monitoring is required to evaluate immune modulation therapies aimed at boosting viral or tumor-specific immunity,2 or at quenching immunity against self-3,4 or transplanted5 tissues. T-cell–screening tools to assess the immunogenic potential of vaccines6 or of replacement proteins (eg, coagulation factors)7 are also required.
The lack of routine human T-cell assays is mainly because of the very low frequency (0.1%-0.001%)8 of T cells specific for a given Ag in blood. Although these cells are sometimes detectable ex vivo, their rarity challenges the sensitivity of technologies such as ELISPOT and flow cytometry. The frequency of these cells may be augmented by preliminary expansion steps,9 but these require additional time and manipulation.
Epitope peptides that bind to HLA molecules for presentation and recognition by the T-cell receptor are frequently used to elicit T-cell responses because they do not require processing by APCs. While bypassing this initial limiting step for T-cell activation, epitopes nevertheless first need to be identified as binding to specific HLA molecules, and they stimulate a limited repertoire of T-cell responses against selected Ag sequences.10
Dendritic cells (DCs) are specialized APCs endowed with unrivaled Ag processing and stimulatory properties.11 These features make them attractive for boosting T-cell activation, thus enhancing their detection in vitro. To this end, DCs are routinely differentiated from monocytes with GM-CSF and IL-412 for 6 days, and subsequently matured with proinflammatory stimuli to achieve full stimulatory capacity.12,13 These time requirements are not compatible with clinical laboratory practice. Although shorter protocols have been described,14 they still require preliminary monocyte isolation, thus greatly increasing blood requirements, labor, and cost.
To overcome these limitations, we hypothesized that DCs could be induced and matured to promote Ag presentation and T-cell activation in situ in PBMCs or whole blood. The advantage of this approach would be 2-fold. First, it would reduce time, purification steps, and blood needs. Second, it would keep lymphocytes in contact with differentiating DCs, thus stimulating T cells as Ag processing, presentation, and DC maturation occurred. In the present study, because DCs were induced and Ag pulsed within 48 hours while surrounded by cognate T lymphocytes and other blood cells, the corresponding T-cell assays are referred to as accelerated cocultured DC (acDC) assays. Following this strategy, T-cell responses were potently stimulated in an Ag-specific fashion.
Methods
Antigens
Tetanus toxoid (TTX, a gift from Dr R. Rappuoli, Novartis Vaccines), M tuberculosis purified protein derivative (PPD; Tubertest; Sanofi Pasteur), hexavalent vaccine (Infanrix hexa; GlaxoSmithKline), keyhole limpet hemocyanin (KLH; Sigma-Aldrich) were used. Ag purity was confirmed by SDS-PAGE. Endotoxin concentrations were < 0.035 EU/μg by Limulus lysate assay (Lonza). The peptides Flu matrix protein58-66 (MP58-66), Flu hemagglutinin306-318 (HA306-318), melanoma Ag 3247-258 (MAGE247-258), TTX830-844, and influenza protein A/Brisbane 2007 HA (n = 68) and H1N1 nucleoprotein (NP; n = 49) 18-mer peptide pools with 8-amino acid overlaps were > 85% pure (GL Biochem).
acDC stimulation on human PBMCs
The study was approved by the ethics committees of all participating institutions and all subjects gave written informed consent in accordance with the Declaration of Helsinki. PBMCs were isolated and used fresh or frozen-thawed as described previously.15,16 On day 0, PBMCs were plated (106/100 μL/well) in 96-well flat-bottomed plates in AIM-V medium (Invitrogen) supplemented with 1000 U/mL of GM-CSF, 500 U/mL of IL-4 (R&D Systems) and containing protein Ags (0.1-10 μg/mL) titrated according to the responses of each donor. After 24 hours (day 1), maturation stimuli were added, comprising the following reagents in different combinations (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article): TNF-α (1000 U/mL), IL-1β (10 ng/mL), and IFN-γ (1000 U/mL, all from R&D Systems); prostaglandin E2 (PGE2; 1μM; Merck Calbiochem); CpG ODN2216 (5 μg/mL; Cell Sciences), polyinosinic:polycytidylic acid (polyI:C, 20 μg/mL; Cayla/InvivoGen); lipopolysaccharide (LPS, 100 ng/mL; from E coli 055:B5; Sigma-Aldrich); and anti-CD40 mAb (1 μg/mL; clone G28.5) and IFN-α-2a (1000 U/mL; Roferon-A; Roche). Maturation cocktails used in each experiment are detailed in the figure legends. IL-7 (0.5 ng/mL; R&D Systems) was added along with maturation stimuli.17 When used, peptide Ags (0.1-10μM) were added on day 1 with maturation stimuli. On day 2 (48 hours after start of culture), nonadherent cells were collected, washed, and analyzed.
acDC stimulation on human blood
Fresh, undiluted heparinized blood (250 μL) was dispensed in 1.5-mL tubes, cytokines and Ags were added as for PBMC stimulation, and lysed blood and/or plasma supernatants were analyzed after 48 hours.
DC characterization
To generate monocyte-derived DCs (moDCs), monocytes were cultured with GM-CSF/IL-4 for 6 days and matured with TNF-α/PGE2/IL-1β for an additional 24 hours. Phenotypes of acDCs and 7-day moDCs were determined by mAbs specific for HLA-DR, CD14, CD80, CD86, and CD11c (BD Biosciences). Endocytic activity was assessed by FITC-labeled dextran uptake. Flow cytometry experiments were performed on a FACSAria (BD Biosciences) equipped with 488-, 633-, and 405-nm lasers.
ELISPOT assays
After 48 hours of stimulation, nonadherent cells were washed, resuspended in fresh AIM-V, and assayed for 6 hours as described previously.15,16 Spots were counted on a Bioreader 5000 Pro-SF (BioSys), and means of 3-6 replicates were determined. ELISPOT readouts are expressed as spot-forming cells (SFCs)/106 PBMCs. Spontaneous (background) responses in the presence of BSA or no Ag (which were identical in all cases) are either shown or otherwise subtracted from Ag-specific responses.15,17
CFSE assays and T-cell cloning
PBMCs were stained with 0.1μM CFSE (Invitrogen/Molecular Probes) and used for acDC cultures as described in “aDC stimulation on human PBMCs.” After 2 days, nonadherent cells were washed, transferred to ELISPOT plates for 6 hours, and then replated in 96-well U-bottom plates. After 6-8 days of culture, cells were stained and single CD4+ CFSEdim cells were sorted into each well of a 96-well U-bottom plate. Each well contained IL-2 (20 U/mL; R&D Systems), IL-4 (5 ng/mL), anti-CD3 mAb (OKT3, 30 ng/mL), and 2 × 105 irradiated PBMCs from 2 unrelated donors.18 Cells were fed every 7 days with fresh cytokines. Growing clones were tested after ∼ 3 weeks by intracellular IFN-γ staining after incubation with Ag-pulsed or unpulsed moDCs.
Cytokine, CD137, and CD154 staining
For intracellular cytokines, PBMCs were recovered from 48-hour acDC cultures and incubated for 6 hours with brefeldin A before staining. IFN-γ surface capture was performed using an IFN-γ-allophycocyanin kit (Miltenyi Biotec). CD154 was stained with allophycocyanin-labeled TRAP-1 mAb during a 6 hours of incubation with 2μM monensin19 and CD137 with PE-labeled 4B4 mAb (BD Biosciences).20
HLA multimer assays
PE-labeled HLA-DR0401 tetramers (TMrs) loaded with Flu HA306-318 or control peptide (kindly provided by Dr E. James, Benaroya Research Institute, Seattle, WA), and HLA-A0201 pentamers (ProImmune) loaded with Flu MP58-66 or control peptide were used at 10 μg/mL. Stainings were carried out for 3 hours at 37°C21 and for 10 minutes at room temperature, respectively, in the presence of 50nM Lck inhibitor II (Merck Calbiochem).22
acDC stimulation on mouse blood
Balb/c mice were immunized with 50 μg of KLH in complete Freund adjuvant subcutaneously at the base of the tail. After 14 days, blood cells were harvested and, after lysis of red cells, the remaining cells were plated in 48-well plates (2 × 106 cells/well). Mouse GM-CSF and IL-4 (R&D Systems) were added as for human acDCs with or without KLH (0.1 μg/mL); LPS (10 ng/mL) was added at day 1 and ELISPOT assays performed on day 2, as described previously.15,17
Cytokine multiplex assays
Supernatants from 48-hour acDC cultures were analyzed on a Luminex platform (Bio-Plex 200; Bio-Rad) using a Milliplex panel (Millipore/Abacus) comprising the following cytokines and chemokines: G-CSF, GM-CSF, IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-13, IL-17, macrophage inflammatory protein (MIP)-1α, MIP-1β, and TNF-α.
Immune monitoring of smallpox, flu, and melanoma vaccination trials
Statistical analyses
All analyses were 2-tailed and performed according to variable distribution and sample size using Prism 5 software (GraphPad).
Results
GM-CSF/IL-4 and maturation factors enhance Ag-specific T-cell stimulation and induce DCs in unfractionated PBMCs
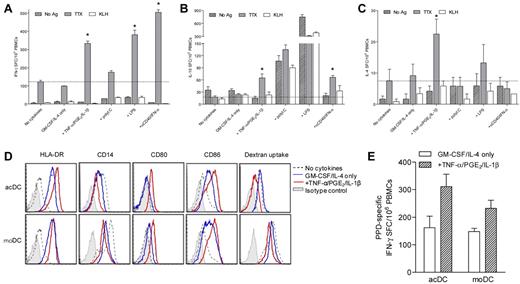
We first investigated whether Ag-specific T-cell responses are enhanced in PBMCs incubated with GM-CSF and IL-4 for 24 hours followed by maturation stimuli for another 24 hours (Figure 1). Protein Ags (TTX, M tuberculosis PPD, hexavalent vaccine, or no Ag) were added from the start of culture. Nonadherent cells were subsequently transferred into IFN-γ ELISPOT plates for 6 hours without additional Ag or cytokine supplementation. IFN-γ ELISPOT responses to TTX were higher in PBMCs treated with different DC-activating agents than in PBMCs left without cytokines (supplemental Figure 1B-C) or stimulated with an anti-CD28 mAb (supplemental Figure 1D). The most effective maturation stimuli were TNF-α/PGE2/IL-1β (2.68- ± 2.88-fold increase over PBMCs alone; P = .039), LPS (3.10- ± 1.78-fold increase, P = .008), anti-CD40/IFN-α (3.05- ± 1.49-increase, P = .008), and, although not significantly, polyI:C (1.95- ± 1.61-fold increase; P = .148). All of these protocols enhanced Ag-specific IFN-γ signals but not background (supplemental Figure 1A), thus ruling out nonspecific T-cell activation by cytokines. Lack of up-regulation of activation markers (ie, CD69, CD25, CD137, and CD154) on T cells from Ag-unpulsed PBMCs exposed to cytokines further excluded nonspecific activation (see Figure 6B-C for CD137 and supplemental Figure 4B for CD154). Other maturation cocktails were excluded either because they did not amplify Ag-specific signals (TNF-α/PGE2, CpG, polyI:C/PGE2, anti-CD40, and anti-CD40/IFN-γ) or because they did so together with significant increases in background (anti-CD40/IL-1β; supplemental Figure 1A).
Schematic of acDC-based T-cell stimulation. Either unfractionated PBMCs (fresh or frozen) or undiluted heparinized whole blood were incubated with GM-CSF and IL-4 for 48 hours in the presence of protein Ags. Maturation stimuli were then added during the last 24 hours, after which amplified T-cell responses were measured by a variety of readouts. Top panels list T-cell readouts with PBMCs; bottom panels show readouts with whole blood. The acDC amplification technique is also compatible with peptide Ags, which were added at 24 hours along with maturation stimuli.
Schematic of acDC-based T-cell stimulation. Either unfractionated PBMCs (fresh or frozen) or undiluted heparinized whole blood were incubated with GM-CSF and IL-4 for 48 hours in the presence of protein Ags. Maturation stimuli were then added during the last 24 hours, after which amplified T-cell responses were measured by a variety of readouts. Top panels list T-cell readouts with PBMCs; bottom panels show readouts with whole blood. The acDC amplification technique is also compatible with peptide Ags, which were added at 24 hours along with maturation stimuli.
We also amplified other Ag-specific cytokine responses (Figure 2A-C). Whereas anti-CD40/IFN-α maturation amplified IFN-γ responses the most (4.1-fold), TNF-α/PGE2/IL-1β was the only combination that boosted all cytokine responses tested (2.7-, 3.7-, and 3.0-fold for IFN-γ, IL-10, and IL-4, respectively). These 2 maturation cocktails were therefore retained. IL-17 responses were not amplified by any of these protocols (data not shown). Moreover, only TTX memory responses, not naive responses to the KLH neo-Ag, were above background (Figure 2A-C).
GM-CSF/IL-4 and maturation factors enhance Ag-specific T-cell stimulation and induce DCs in unfractionated PBMCs. (A-C) IFN-γ (A), IL-10 (B), and IL-4 (C) ELISPOT responses to TTX, KLH, or control Ag obtained by treating PBMCs with selected stimuli (see “Methods” and Figure 1 for details). Dotted lines indicate TTX-specific cytokine signals obtained in the absence of cytokines. *P < .04 for comparison with the “no cytokines” condition. One representative experiment of 3 is shown. (D) Phenotype comparison between acDCs (top) and moDCs (bottom). acDCs were obtained by culturing unfractionated PBMCs for 48 hours with GM-CSF/IL-4 alone (blue profiles) or in combination with TNF-α/PGE2/IL-1β (added during the last 24 hours; red profiles). moDCs were generated by culturing purified monocytes for 7 days with the same cytokine cocktails (TNF-α/PGE2/IL-1β added during the last 24 hours). Comparisons with cultures in the absence of cytokines (dotted profiles) and with isotype control staining (shaded profile) are shown (cells gated as CD19−CD8−CD4lowCD11chigh). (E) Stimulatory potency in IFN-γ ELISPOT assays of acDCs and moDCs matured with TNF-α/PGE2/IL-1β or left immature (GM-CSF/IL-4 only). For acDCs, whole PBMCs (1 × 106/well) were cultured as before in the presence or absence of M tuberculosis PPD for 48 hours. To obtain moDCs, autologous monocytes were isolated by PBMC adherence (1 × 106/well) and stimulated as above for 7 days. Fresh autologous PBMCs (1 × 106/well) were then added onto moDCs with or without PPD for 48 hours. Nonadherent cells were subsequently recovered and subjected to IFN-γ ELISPOT. PPD-specific IFN-γ SFC frequencies were background-subtracted. For panels D and E, similar results were obtained in 3 separate experiments by maturing acDCs and moDCs with anti-CD40/IFN-α (not shown).
GM-CSF/IL-4 and maturation factors enhance Ag-specific T-cell stimulation and induce DCs in unfractionated PBMCs. (A-C) IFN-γ (A), IL-10 (B), and IL-4 (C) ELISPOT responses to TTX, KLH, or control Ag obtained by treating PBMCs with selected stimuli (see “Methods” and Figure 1 for details). Dotted lines indicate TTX-specific cytokine signals obtained in the absence of cytokines. *P < .04 for comparison with the “no cytokines” condition. One representative experiment of 3 is shown. (D) Phenotype comparison between acDCs (top) and moDCs (bottom). acDCs were obtained by culturing unfractionated PBMCs for 48 hours with GM-CSF/IL-4 alone (blue profiles) or in combination with TNF-α/PGE2/IL-1β (added during the last 24 hours; red profiles). moDCs were generated by culturing purified monocytes for 7 days with the same cytokine cocktails (TNF-α/PGE2/IL-1β added during the last 24 hours). Comparisons with cultures in the absence of cytokines (dotted profiles) and with isotype control staining (shaded profile) are shown (cells gated as CD19−CD8−CD4lowCD11chigh). (E) Stimulatory potency in IFN-γ ELISPOT assays of acDCs and moDCs matured with TNF-α/PGE2/IL-1β or left immature (GM-CSF/IL-4 only). For acDCs, whole PBMCs (1 × 106/well) were cultured as before in the presence or absence of M tuberculosis PPD for 48 hours. To obtain moDCs, autologous monocytes were isolated by PBMC adherence (1 × 106/well) and stimulated as above for 7 days. Fresh autologous PBMCs (1 × 106/well) were then added onto moDCs with or without PPD for 48 hours. Nonadherent cells were subsequently recovered and subjected to IFN-γ ELISPOT. PPD-specific IFN-γ SFC frequencies were background-subtracted. For panels D and E, similar results were obtained in 3 separate experiments by maturing acDCs and moDCs with anti-CD40/IFN-α (not shown).
Amplification of Ag-specific T-cell responses in PBMCs treated with GM-CSF/IL-4 and maturation factors was accompanied by in situ induction and maturation of DC-like cells within 48 hours (referred to as acDCs). acDCs showed a phenotype identical to DCs differentiated and matured in 7 days from purified monocytes (moDCs) using the same stimuli (Figure 2D). Up-regulation of HLA-DR and costimulatory molecules was paralleled by CD14 down-regulation, whereas dextran uptake, although more efficient in moDCs, decreased on maturation. acDCs were also at least as efficient as moDCs at eliciting Ag-specific T-cell responses, and more so when matured (Figure 2E).
Amplification of T-cell responses was also obtained with mouse PBMCs treated with GM-CSF/IL-4 followed by LPS. Cells from KLH-immunized mice displayed higher KLH-specific IFN-γ recall responses in the presence of acDC-stimulating factors (supplemental Figure 2).
Reproducibility of acDC-amplified IFN-γ ELISPOT assays within thawed aliquots of PBMCs frozen at the same time and within PBMCs derived from separate blood draws from the same individual yielded variations < 10% (supplemental Figure 3A-B). Variability between fresh and frozen-thawed samples was 6.9% (supplemental Figure 3C).
T cells stimulated by acDCs undergo proliferation and retain Ag specificity
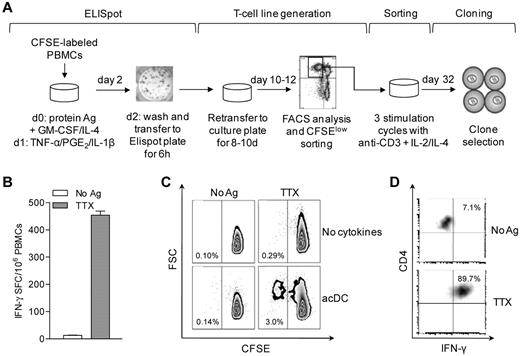
We next investigated whether acDCs could sustain the expansion of Ag-specific T cells. Dilution of the cell-bound dye CFSE was used to assess proliferation18 (Figure 3A). PBMCs were first labeled with CFSE, then assayed by acDC-amplified ELISPOT, and finally recovered and cultured for 8-10 days without further manipulation. Proliferating (CFSElow) cells were then sorted into single cells and expanded.18 A representative example is shown in Figure 3B-D. The initial TTX-specific IFN-γ response detected at 48 hours (441 IFN-γ SFC/106 PBMCs, 0.044%; Figure 3B) gave rise to a TTX-specific CFSElow fraction of 3.0% after 10 days (ie, a 68.2-fold expansion). The acDC condition was superior to conventional expansion in the absence of cytokines, which yielded 10-fold fewer TTX-specific cells (0.29%, P < .001) and comparable background proliferation (0.10%-0.14%; Figure 3C). Sorted TTX-specific CFSElow cells generated TTX-specific CD4+ T-cell clones (Figure 3D).
acDC stimulation supports Ag-specific T-cell expansion. (A) Schematic of the acDC ELISPOT and T-cell clone-generation procedure. acDCs were induced by adding GM-CSF and IL-4 along with protein Ag to CFSE-labeled PBMCs on day 0, followed on day 1 by TNF-α/PGE2/IL-1β. On day 2, nonadherent cells were transferred into ELISPOT wells for 6 hours to quantify Ag-specific responses. Cells were subsequently recovered and recultured without further Ag or cytokines. On day 10-12, proliferating (CFSElow) cells were single-cell-sorted, expanded through 3 stimulation cycles with anti-CD3 + IL-2/IL-4, and tested for Ag specificity at day 32. (B) Representative acDC IFN-γ ELISPOT after TTX or control stimulation for 48 hours. (C) Proliferation of CFSE-labeled PBMCs recovered from ELISPOT wells and cultured for another 8 days. A comparison of standard versus acDC-driven expansion is shown. The TTX-specific CFSElow fraction was sorted and cloned. (D) Recall assay of one clone on TTX- and control-pulsed DCs followed by intracellular IFN-γ staining is shown.
acDC stimulation supports Ag-specific T-cell expansion. (A) Schematic of the acDC ELISPOT and T-cell clone-generation procedure. acDCs were induced by adding GM-CSF and IL-4 along with protein Ag to CFSE-labeled PBMCs on day 0, followed on day 1 by TNF-α/PGE2/IL-1β. On day 2, nonadherent cells were transferred into ELISPOT wells for 6 hours to quantify Ag-specific responses. Cells were subsequently recovered and recultured without further Ag or cytokines. On day 10-12, proliferating (CFSElow) cells were single-cell-sorted, expanded through 3 stimulation cycles with anti-CD3 + IL-2/IL-4, and tested for Ag specificity at day 32. (B) Representative acDC IFN-γ ELISPOT after TTX or control stimulation for 48 hours. (C) Proliferation of CFSE-labeled PBMCs recovered from ELISPOT wells and cultured for another 8 days. A comparison of standard versus acDC-driven expansion is shown. The TTX-specific CFSElow fraction was sorted and cloned. (D) Recall assay of one clone on TTX- and control-pulsed DCs followed by intracellular IFN-γ staining is shown.
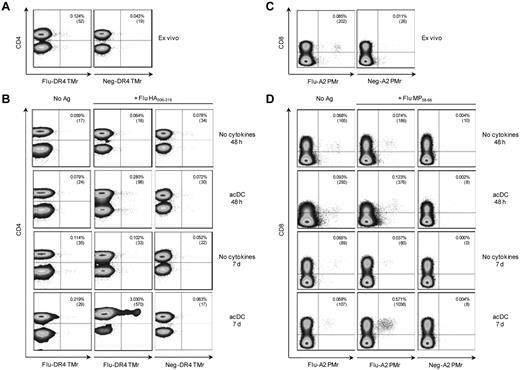
Similar results were obtained after stimulation with peptides and T-cell detection with HLA multimers (Figure 4). Fresh PBMCs were stained ex vivo, and then 48 hours and 7 days after stimulation. After 48 hours, HLA-DR4–restricted, Flu HA306-318–specific CD4+ T cells detected ex vivo (0.124%; Figure 4A) were expanded 2.3-fold (0.280%) by acDCs compared with 0.5-fold (0.064%) in the absence of cytokines (Figure 4B). On replating in the absence of any further stimuli and after an additional 5 days, HA-specific CD4+ T cells further expanded only in acDC-stimulated cultures (24.4-fold or 3.030% expanded vs 0.8-fold or 0.102% expanded; Figure 4B). Similar results were obtained for Flu MP58-66–specific, HLA-A2-restricted CD8+ T cells. Frequencies of peptide-specific CD8+ T cells ex vivo (0.085%; Figure 4C) increased on peptide-specific expansion only in the acDC condition, both at 48 hours (1.4-fold or 0.123% expanded vs 0.9-fold or 0.074% expanded without cytokine) and at 7 days (6.7-fold or 0.571% expanded vs 0.4-fold or 0.037% expanded without cytokines; Figure 4D). Using this multimer-based approach, acDC stimulation of PBMCs from 11 different donors generated 382 CD4+ T-cell clones specific for DR*04:01- or DR*03:01-restricted Flu and tetanus toxoid epitopes. In this large series, expansion was obtained in 10.9% ± 6.2% of single cells seeded; 84.7% ± 24.0% of these growing wells were Ag-specific, as determined by TMr staining.
acDC stimulation drives expansion of peptide-specific T cells. (A) Ex vivo detection of Flu HA306-318–specific CD4+ T cells using Flu or control (Neg) peptide–loaded HLA-DR4 (DR*04:01) TMrs. (B) In vitro expansion of Flu HA306-318–specific CD4+ T cells detected by TMrs. PBMCs were cultured without (first column) or with Flu HA306-318 peptide (second and third columns) for 48 hours or 7 days with or without the acDC cocktail, as indicated. Flu-specific CD4+ T cells were identified with the corresponding HLA-DR4 TMrs (first and second columns), and background staining determined with control TMr (third column). (C-D) The same experiment was performed to detect Flu MP58-66–specific CD8+ T cells using Flu or control peptide–loaded HLA-A2 (A*02:01) pentamers (PMrs). Numbers in the upper right corner of each dot plot indicate the percentage and absolute numbers of CD4+TMr+ or CD8+PMr+ cells. acDC maturation was induced with anti-CD40/IFN-α and dot plots are gated on CD14/CD19− viable cells. Results are representative of 3 independent experiments.
acDC stimulation drives expansion of peptide-specific T cells. (A) Ex vivo detection of Flu HA306-318–specific CD4+ T cells using Flu or control (Neg) peptide–loaded HLA-DR4 (DR*04:01) TMrs. (B) In vitro expansion of Flu HA306-318–specific CD4+ T cells detected by TMrs. PBMCs were cultured without (first column) or with Flu HA306-318 peptide (second and third columns) for 48 hours or 7 days with or without the acDC cocktail, as indicated. Flu-specific CD4+ T cells were identified with the corresponding HLA-DR4 TMrs (first and second columns), and background staining determined with control TMr (third column). (C-D) The same experiment was performed to detect Flu MP58-66–specific CD8+ T cells using Flu or control peptide–loaded HLA-A2 (A*02:01) pentamers (PMrs). Numbers in the upper right corner of each dot plot indicate the percentage and absolute numbers of CD4+TMr+ or CD8+PMr+ cells. acDC maturation was induced with anti-CD40/IFN-α and dot plots are gated on CD14/CD19− viable cells. Results are representative of 3 independent experiments.
These data show that acDC cultures enhance the expansion of Ag-specific T cells and that acDC-amplified T-cell responses are Ag specific.
Protein and peptide Ags and different stimulation periods trigger different T-cell responses
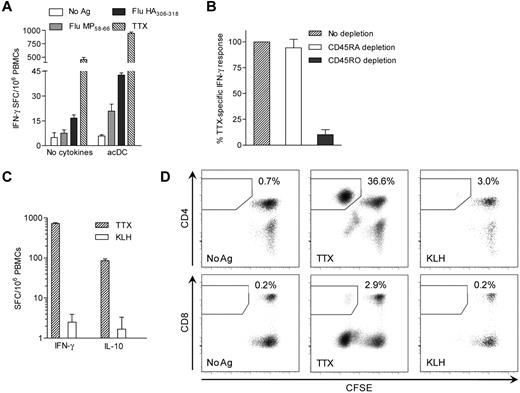
As shown in Figure 4, when HLA class II- or class I–restricted peptide epitopes were used instead of protein Ags, CD4+ and CD8+ T-cell responses were triggered, respectively. Peptide-pulsed acDCs also amplified CD4+ and CD8+ T-cell IFN-γ responses compared with conventional PBMC assays (3.2- and 6.3-fold, respectively, P < .05; Figure 5A). This was confirmed by CD4 and CD8 depletion (not shown). Ag-specific T cells detected with acDCs were predominantly memory cells, because CD45RO (but not CD45RA) depletion reduced the response (89.9% and 5.8% decrease, respectively, P < .05; Figure 5B). In agreement with these findings, KLH did not elicit significant IFN-γ or IL-10 responses during the 48 hours of acDC stimulation (Figure 2A-C and Figure 5C). However, when these cells (which had first been CFSE-labeled) were cultured for another week, low-grade KLH-specific CD4+ responses were detected, consistent with priming in vitro (Figure 5D). Moreover, TTX-specific CD8+ T cells were also detected, showing that cross-presentation also occurred, although with lower efficiency.
Protein/peptide Ags and different stimulation periods trigger different T-cell responses. (A) IFN-γ ELISPOT performed on PBMCs cultured with or without the acDC cocktail. PBMCs from a HLA-A2+ (A*02:01) and -DR4+ (DR*04:01) subject were stimulated with BSA control or no Ag, HLA-A2–restricted Flu MP58-66 peptide, DR4-restricted Flu HA306-318 peptide, or TTX, as indicated. Peptides were added after the first 24 hours of culture, whereas TTX was introduced ab initio. (B) TTX-specific IFN-γ ELISPOT responses in acDC stimulations performed on PBMCs magnetically depleted of CD45RA+ or CD45RO+ cells or left undepleted. Results are expressed as relative IFN-γ responses normalized to undepleted PBMCs. (C) IFN-γ and IL-10 ELISPOT responses to TTX and KLH (background-subtracted) on acDC-stimulated PBMCs labeled with CFSE. (D) CFSE-labeled PBMCs were recovered from the assay wells of panel C and cultured for an additional 10 days in the absence of further stimuli and cytokines. CFSE proliferation of CD4+ and CD8+ T cells to different Ags is shown. Results in panels A-D were obtained after maturing acDCs with TNF-α/PGE2/IL-1β and are representative of 3 independent experiments, with the exception of panel B, in which the means ± SEM of 3 separate experiments are shown.
Protein/peptide Ags and different stimulation periods trigger different T-cell responses. (A) IFN-γ ELISPOT performed on PBMCs cultured with or without the acDC cocktail. PBMCs from a HLA-A2+ (A*02:01) and -DR4+ (DR*04:01) subject were stimulated with BSA control or no Ag, HLA-A2–restricted Flu MP58-66 peptide, DR4-restricted Flu HA306-318 peptide, or TTX, as indicated. Peptides were added after the first 24 hours of culture, whereas TTX was introduced ab initio. (B) TTX-specific IFN-γ ELISPOT responses in acDC stimulations performed on PBMCs magnetically depleted of CD45RA+ or CD45RO+ cells or left undepleted. Results are expressed as relative IFN-γ responses normalized to undepleted PBMCs. (C) IFN-γ and IL-10 ELISPOT responses to TTX and KLH (background-subtracted) on acDC-stimulated PBMCs labeled with CFSE. (D) CFSE-labeled PBMCs were recovered from the assay wells of panel C and cultured for an additional 10 days in the absence of further stimuli and cytokines. CFSE proliferation of CD4+ and CD8+ T cells to different Ags is shown. Results in panels A-D were obtained after maturing acDCs with TNF-α/PGE2/IL-1β and are representative of 3 independent experiments, with the exception of panel B, in which the means ± SEM of 3 separate experiments are shown.
acDC stimulation enhances different T-cell responses in either PBMCs or whole blood
Given the efficiency of acDCs at amplifying IFN-γ and proliferative T-cell responses, other functional T-cell readouts were further investigated using purified PBMCs. No significant TTX-specific IFN-γ or TNF-α intracellular staining was detected without the acDC cocktail (Figure 6A and supplemental Figure 4A). However, these cytokines were detected in both CD4+ and CD8+ T cells after acDC stimulation, with no increase in background. Similar results were obtained, although with lower sensitivity, with IFN-γ capture assays (data not shown) and with the activation markers CD13725 (Figure 6B) and, for CD4+ T cells, CD15419 (supplemental Figure 4B).
Ag-specific cytokine production and CD137 up-regulation are acDC-amplified in PBMCs and whole blood. (A) Intracellular IFN-γ/TNF-α staining performed on PBMCs in the absence (top row) or presence (bottom row) of the acDC cocktail. After 48 hours of incubation, nonadherent cells were stained for intracellular cytokines accumulated during a final 6 hours of incubation with brefeldin A. Percentages indicate cytokine-positive events among CD4+ (left) or CD8+ (right) T cells (absolute cell numbers are shown in brackets). (B) CD137 up-regulation detected on PBMCs with (bottom row) or without (top row) acDC amplification during a 48 hours of culture, as above. (C) Whole blood from the same draw was assayed in parallel by adding Ags with (bottom) or without (top) acDC cytokines. Dot plots were gated on CD4+ (left) or CD8+ (right) T cells to allow comparison between PBMCs and whole blood. Percentages indicate the CD137+ fraction among CD4+ or CD8+ T cells (absolute cell numbers are shown in brackets). (D) Whole blood (250 μL) was cultured for 48 hours with or without TTX in the presence (squares) or absence (circles) of acDC cocktail. Cytokines secreted in plasma supernatants were measured; only those significantly increased in response to Ag are shown. Results are shown as TTX-stimulated cytokine concentrations (filled symbols) and basal values in the absence of Ag (open symbols). Coefficients of variation among triplicate samples were 6.1%-16.2% (not shown). (E) Ag-specific IL-1β production by acDCs. After a 48-hour acDC stimulation, total PBMCs were collected and treated with brefeldin A for 6 hours. Intracellular IL-1β expression in CD11c+ cells (gated on CD14−CD19−CD8−CD4lowHLA-DR+ events) and CD4+ cells (gated on CD14−CD19−CD8−CD11clow events) is shown. Results refer to representative experiments of 10 or more performed using the anti-CD40/IFN-α maturation cocktail.
Ag-specific cytokine production and CD137 up-regulation are acDC-amplified in PBMCs and whole blood. (A) Intracellular IFN-γ/TNF-α staining performed on PBMCs in the absence (top row) or presence (bottom row) of the acDC cocktail. After 48 hours of incubation, nonadherent cells were stained for intracellular cytokines accumulated during a final 6 hours of incubation with brefeldin A. Percentages indicate cytokine-positive events among CD4+ (left) or CD8+ (right) T cells (absolute cell numbers are shown in brackets). (B) CD137 up-regulation detected on PBMCs with (bottom row) or without (top row) acDC amplification during a 48 hours of culture, as above. (C) Whole blood from the same draw was assayed in parallel by adding Ags with (bottom) or without (top) acDC cytokines. Dot plots were gated on CD4+ (left) or CD8+ (right) T cells to allow comparison between PBMCs and whole blood. Percentages indicate the CD137+ fraction among CD4+ or CD8+ T cells (absolute cell numbers are shown in brackets). (D) Whole blood (250 μL) was cultured for 48 hours with or without TTX in the presence (squares) or absence (circles) of acDC cocktail. Cytokines secreted in plasma supernatants were measured; only those significantly increased in response to Ag are shown. Results are shown as TTX-stimulated cytokine concentrations (filled symbols) and basal values in the absence of Ag (open symbols). Coefficients of variation among triplicate samples were 6.1%-16.2% (not shown). (E) Ag-specific IL-1β production by acDCs. After a 48-hour acDC stimulation, total PBMCs were collected and treated with brefeldin A for 6 hours. Intracellular IL-1β expression in CD11c+ cells (gated on CD14−CD19−CD8−CD4lowHLA-DR+ events) and CD4+ cells (gated on CD14−CD19−CD8−CD11clow events) is shown. Results refer to representative experiments of 10 or more performed using the anti-CD40/IFN-α maturation cocktail.
We also investigated whether acDCs could amplify Ag-specific T-cell responses in whole blood. These experiments were performed on the same blood draw used for purifying PBMCs shown in Figure 6B by adding the acDC cocktail directly to undiluted heparinized blood (250 μL) along with Ag. After 48 hours, cells in lysed blood were stained for CD137, revealing that acDC stimulation amplified detection of CD4+ but not CD8+ T cells (Figure 6C).
acDC stimulation amplifies Ag-stimulated cytokine secretion in whole blood
Because acDC stimulation enhanced CD137 up-regulation in blood CD4+ T cells, we investigated whether bulk cytokine secretion in plasma collected after whole-blood Ag stimulation was also amplified. To this end, heparinized whole blood was incubated with acDC cocktail and Ag, and plasma was recovered after 48 hours for cytokine measurements. Except for MIP-1α, Ag-specific signals were higher with acDC (median amplification, 6.1-fold; range, 3.6- to 41.9-fold, P < .001; Figure 6D). Basal secretion did not increase upon acDC exposure, which is consistent with Ag-specific amplification. Comparing whole blood and PBMCs, sensitivity of cytokine detection was greater with PBMCs, with median concentrations being ∼ 4-fold higher than with whole blood (range, 1.0-34.0, P < .001; supplemental Figure 5A). However, this difference disappeared once corrected for the cell concentration in blood and PBMCs (∼ 1 × 103 and 5 × 103 cells/μL, respectively). Surprisingly, some cytokines not usually considered to be secreted by T cells (G-CSF and IL-1β) also behaved as markers of Ag-specific activation. Intracellular staining further showed that Ag-specific IL-1β secretion derived from acDCs and not from CD4+ T cells (Figure 6E) or CD8+ T cells (not shown). IL-1β blocking by mAbs did not reduce Ag-specific T-cell responses (supplemental Figure 5B), suggesting that DC-secreted IL-1β was not responsible for acDC amplification, but instead behaved as an indirect marker of Ag-specific T-cell activation.
acDC stimulation reveals increased T-cell responses after Ag vaccination
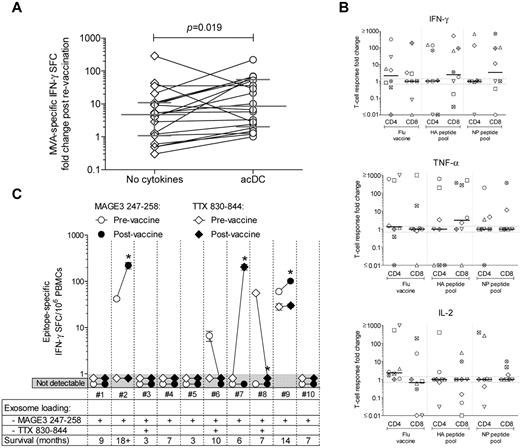
Because acDC stimulation improved the detection of Ag-specific T-cell responses in vitro, we finally evaluated whether it could reveal immune changes induced by vaccination. First, we assayed frozen-thawed PBMCs from healthy subjects before and after smallpox revaccination (Figure 7A). The increase in frequency of modified vaccinia Ankara (MVA)-specific IFN-γ–producing cells after revaccination was higher in acDC than conventional assays (median increase, 8.4-fold; range, 2.3-53.4 vs 4.9-fold; range, 1.2-10.5; P = .019). The inverse correlation between the frequency of MVA-specific IFN-γ responses before revaccination and size of the skin lesion, previously reported in a larger cohort,23 was also confirmed with acDC-amplified (Spearman r = −0.52; P = .03), but not for nonamplified (r = −0.39; P = .10) responses.
acDC stimulation reveals boosting of T-cell responses after Ag vaccination. (A) IFN-γ ELISPOT responses against MVA (0.1 PFUs/cell) were analyzed in frozen-thawed PBMCs from 21 healthy subjects before and after smallpox revaccination, using either nonamplified (diamonds) or acDC-amplified (circles) stimulation. The -fold changes in frequencies of MVA-specific IFN-γ SFCs before and after vaccinations are shown. Bars indicate the median and interquartile range of each distribution, whereas paired samples tested with or without the acDC cocktail are connected by lines. P = .019 for paired comparison between acDC and the “no cytokines” condition. (B) IFN-γ-, TNF-α-, and IL-2–producing CD4+ and CD8+ responses elicited by acDC stimulation in vitro with Flu vaccine or the HA and NP peptide pools analyzed by intracellular staining in frozen-thawed PBMCs from flu-vaccinated healthy subjects. The -fold changes in responses after vaccination are displayed, with each symbol representing a single individual and bars showing median values for each distribution. Dotted lines identify the 0.7- to 1.3-fold interval (ie, no -fold change). (C) acDC-amplified IFN-γ ELISPOT responses against DP4-restricted MAGE-3247-258 (circles) or pan-DR-restricted TTX830-844 (diamonds) epitopes were analyzed in frozen-thawed PBMCs from 10 stage IIIb/IV melanoma patients before (open symbols) and after (filled symbols) vaccination with exosomes loaded with MAGE-3247-258, either alone or in combination with TTX830-844, as indicated. Background-subtracted frequencies of IFN-γ SFC/106 PBMCs (means ± SEM of triplicate wells) are shown before and after vaccination. Counts < 3 SD above background were scored as nondetectable (gray-shaded area). *P < .04.
acDC stimulation reveals boosting of T-cell responses after Ag vaccination. (A) IFN-γ ELISPOT responses against MVA (0.1 PFUs/cell) were analyzed in frozen-thawed PBMCs from 21 healthy subjects before and after smallpox revaccination, using either nonamplified (diamonds) or acDC-amplified (circles) stimulation. The -fold changes in frequencies of MVA-specific IFN-γ SFCs before and after vaccinations are shown. Bars indicate the median and interquartile range of each distribution, whereas paired samples tested with or without the acDC cocktail are connected by lines. P = .019 for paired comparison between acDC and the “no cytokines” condition. (B) IFN-γ-, TNF-α-, and IL-2–producing CD4+ and CD8+ responses elicited by acDC stimulation in vitro with Flu vaccine or the HA and NP peptide pools analyzed by intracellular staining in frozen-thawed PBMCs from flu-vaccinated healthy subjects. The -fold changes in responses after vaccination are displayed, with each symbol representing a single individual and bars showing median values for each distribution. Dotted lines identify the 0.7- to 1.3-fold interval (ie, no -fold change). (C) acDC-amplified IFN-γ ELISPOT responses against DP4-restricted MAGE-3247-258 (circles) or pan-DR-restricted TTX830-844 (diamonds) epitopes were analyzed in frozen-thawed PBMCs from 10 stage IIIb/IV melanoma patients before (open symbols) and after (filled symbols) vaccination with exosomes loaded with MAGE-3247-258, either alone or in combination with TTX830-844, as indicated. Background-subtracted frequencies of IFN-γ SFC/106 PBMCs (means ± SEM of triplicate wells) are shown before and after vaccination. Counts < 3 SD above background were scored as nondetectable (gray-shaded area). *P < .04.
Frozen-thawed PBMCs from healthy subjects before and after intramuscular flu vaccination (Figure 7B) also exhibited increased CD4+ responses to the whole vaccine by acDC stimulation, producing IFN-γ, TNF-α, and IL-2. Although the limited sample size precluded statistical analysis, CD8+ responses that had previously been elusive by conventional techniques26,27 were detected to pooled HA and NP peptides. When considering IFN-γ–producing CD8+ T cells, subjects displaying HA-specific responses also harbored NP-specific responses, reflecting a broad response to vaccine both in terms of effector cytokines and epitopes targeted.
We then used the acDC stimulation to analyze frozen-thawed PBMCs from stage IIIb/IV melanoma patients before and after vaccination with autologous DC-derived exosomes loaded with a DP4-restricted MAGE247-258 peptide, either alone or with a pan-DR TTX830-844 peptide24 (Figure 7C). Although no MAGE-specific responses had been previously detected with the conventional proliferation assays ELISPOT and TMr,24 2 of 10 patients (2 and 9) tested by acDC-amplified ELISPOT assays showed responses (42 and 60 IFN-γ SFC/106 PBMCs, respectively) that increased 5.3- and 1.7-fold after vaccination, respectively (P < .04). No changes in TTX-specific responses were detected, except in one patient (7) not receiving TTX-loaded exosomes, possibly because of an improvement in his general immune status. Patients 2 and 9, whose MAGE-specific responses were boosted after vaccination, were also the ones with the longer survival times (18+ and 14 months; 7-month median survival for all patients). Patient 2 remained nonprogressive for 6 years.
Discussion
The therapeutic potential of DCs is being explored to induce immunogenic or tolerogenic T-cell responses to disease-related Ags.28,29 However, despite their potent Ag-processing and -presenting properties, DCs have not been exploited for T-cell diagnostics because of the low frequency of circulating DCs and the large blood volumes required to obtain DC-type APCs from monocytes and other cells. The acDC strategy fills this gap by amplifying Ag-specific T-cell responses in situ in a format amenable to routine laboratory application. Comparison with reference T-cell assay methods (stimulation with moDCs, standard IFN-γ ELISPOT, intracellular cytokine staining, T-cell proliferation, and ex vivo HLA multimer staining) documented superior sensitivity.
In bulk cultures, induction and Ag pulsing of acDCs is coupled with stimulation—in an Ag-specific fashion—of T cells, thus lining up the 3 critical steps leading to a T-cell response: DC activation, Ag presentation, and T-cell activation. A potential problem with acDC stimulation was nonspecific T-cell activation by cytokines used to induce acDCs, but this was shown not to occur. Responses detected in acDC-based assays were bona fide Ag specific, because T cells expanded in vitro retained their Ag specificity and stained with peptide-HLA multimers.
Stimuli used to mature acDCs were critical. Anti-CD40/IFN-α13 more efficiently amplified IFN-γ–producing responses, whereas TNF-α/PGE2/IL-1β30 allowed for better detection of Ag-specific IL-4 and IL-10 secretion. Comparison of acDCs and moDCs revealed striking similarities, both in terms of phenotype and of stimulatory potency. The notable advantage of acDCs is that they are induced in situ within 48 hours in unfractionated PBMCs or whole blood. Moreover, blood and cytokine requirements are minimal, with only 106 PBMCs or 250 μL of whole blood stimulated in 200- to 250-μL cultures, thus limiting sample volumes and cost. Reproducibility between fresh and frozen samples offers further convenience. These are critical considerations in longitudinal monitoring of T-cell responses, especially in children, and a decided advantage in screening peptide libraries.
acDC-driven T-cell responses are triggered by both whole-protein Ags and peptide epitopes. Protein Ags eliminate the need for epitope identification and donor selection based on HLA type, and they allow T-cell exposure to the whole repertoire of potential epitopes. acDC assays in which cellular material such as donor, tumor, or autoimmune-targeted tissues are used as Ag sources could also be envisioned.
acDC stimulation is compatible with a range of T-cell readouts, some of which can be complemented with downstream sorting of Ag-specific cells. The versatility of the approach was exemplified by single-cell and bulk assays, the sensitivity of which will need to be evaluated for each application. Single-cell assays are often preferred because they provide information about the frequency and phenotype of responding T cells and are usually more sensitive than bulk assays. Nonetheless, bulk assays are easier to perform in routine settings, and their sensitivity may be sufficient for many applications, as in the case of IFN-γ assays for M tuberculosis.31 Moreover, assays using whole blood avoid PBMC purification and maintain cells in a more physiologic milieu.
An interesting observation from bulk acDC assays was the stimulated secretion of G-CSF and IL-1β, which are not considered to be derived from T cells. Indeed, IL-1β was found to be produced by DCs. A positive feedback loop between Ag-presenting DCs and responding T cells may further activate DCs, inducing them to secrete signature cytokines in an Ag-specific fashion. This is reminiscent of IL-12p70 secretion, which is enhanced in mouse and human DCs when matured in the presence of Ag and Ag-specific T cells.32,33 APC-derived cytokines may thus constitute valuable indirect biomarkers of T-cell responses.
acDC-based T-cell monitoring of vaccination trials further invites translation into the clinic. Whereas acDC amplification of strong viral T-cell responses yielded a marginal yet significant detection edge, this was critical for detecting T-cell responses to flu (for CD8+ T cells) and MAGE-3 (for CD4+ T cells) vaccination, which could not be detected previously without amplification.23,25,26 The MELADEX trial results exemplify a requirement of many clinical trials to demonstrate via immune surrogate markers whether a biologic effect of the intervention has been obtained. This is particularly critical in phase 1 trials, in which enrollment of few subjects with advanced disease aims to provide data about feasibility and safety, but not necessarily efficacy. Knowing whether Ag-specific T-cell responses are modified and in which individuals may provide key mechanistic information to plan further trials and modify therapeutic and enrollment strategies accordingly. The same challenge presents in autoimmune diseases, in which agents tested in patients with established disease may provide little or no clinical benefit, but could have potential therapeutic effects revealed by surrogate immune markers.3,4 In this regard, we have recently documented insulin-specific tolerance restoration by acDC-based monitoring of adults with type 1 diabetes treated with intranasal insulin.34
A limitation of the present study is that the nature of acDC precursor(s) has not been addressed. Building on our recent characterization of human DC subsets,35 this is the aim of ongoing investigations. In addition to CD14+ monocytes, 4 DC subsets circulate in human blood: plasmacytoid DCs (pDCs), which are CD11c−CD304+ (BDCA4+) and CD123+ (IL-3 receptor α chain+), and conventional DCs (cDCs), which are subdivided into CD1b/c+ (BDCA1+), CD141+ (BDCA3+), and CD16+ (also termed inflammatory monocytes).35 Their weak stimulatory capacity makes pDCs unlikely candidates, whereas different cDC subsets were all found to be stimulatory, to secrete IL12p70, and to cross-present Ag.35 Moreover, CD14+ and/or CD16+ cells can spontaneously differentiate into DCs in vitro when not isolated from PBMCs,36 similar to our bulk culture condition, a process that could be potentiated by acDC cocktails. Circulating DC precursors (pre-cDCs and MHC class II− FMS-like tyrosine kinase 3 [Flt3+]) have also been described in the mouse,37 but their human counterparts (if any) have not been identified. It is possible that cross-talk between Ag-presenting acDCs and responding T lymphocytes and other cells (eg, natural killer cells) further synergizes to amplify Ag-specific responses. Skewing differentiation of acDC precursors in vitro may offer ways of inducing Ag-specific T cells with different properties. Characterization of the acDC precursor(s) may therefore lead to strategies for the targeted induction of T cells for therapeutic purposes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Mannering for help in setting up the T-cell cloning protocols and for helpful comments; M. Scotto for help with blood drawing; and Dr S. Caillat-Zucman, N.C. Schloot, and members of the Mallone laboratory for critical reading of the manuscript.
This study was supported by grants from the Juvenile Diabetes Research Foundation (JDRF grant 1-2008-106), the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk European Program in Type 1 Diabetes Research 2007), the Inserm Program National de Recherche sur le Diabète 2007, the Fondation Recherche Médicale (grant Installation Nouvelle Equipe), and the Ile-de-France CODDIM (grant Soutien aux Jeunes Equipes) to R.M. and by program (516700) and infrastructure (361646) grants from the National Health and Medical Research Council (NHMRC) of Australia, a Victorian State Government Operational Infrastructure Support Grant, and a grant from the Diabetes Vaccine Development Center, Australia, to L.C.H. R.M. is an Inserm Avenir Investigator and L.C.H. is a Senior Principal Research Fellow of the NHMRC. E.M. was the recipient of a postdoctoral fellowship from the Fondation Recherche Médicale and of an EFSD AlbertRenold travel fellowship.
Authorship
Contribution: E.M., G.A., D.M., B.C., C.B., L.C.H., and R.M. designed the experiments; E.M., G.A., M.-C.G., G.N., D.M., B.C., L.C.H., and R.M. performed the experiments and/or participated in data analysis; B.C., N.C., and L.Z. provided patient samples; and C.B., L.C.H., and R.M. wrote the manuscript.
Conflict-of-interest disclosure: The acDC technology is covered by a patent filed by Inserm-Transfert.
Correspondence: Roberto Mallone, MD PhD, Inserm U986, DeAR Lab Avenir, Saint Vincent de Paul Hospital, 82 avenue Denfert Rochereau, 75674 Paris cedex 14, France; e-mail: roberto.mallone@inserm.fr.