Abstract
Angiopoietin-1 (Ang1) plays a crucial role in vascular and hematopoietic development, mainly through its cognate receptor Tie2. However, little is known about the precise role of Ang1 in embryonic stem cell (ESC) differentiation. In the present study, we used COMP-Ang1 (a soluble and potent variant of Ang1) to explore the effect of Ang1 on endothelial and hematopoietic differentiation of mouse ESCs in an OP9 coculture system and found that Ang1 promoted endothelial cell (EC) differentiation from Flk-1+ mesodermal precursors. This effect mainly occurred through Tie2 signaling and was altered in the presence of soluble Tie2-Fc. We accounted for this Ang1-induced expansion of ECs as enhanced proliferation and survival. Ang1 also had an effect on CD41+ cells, transient precursors that can differentiate into both endothelial and hematopoietic lineages. Intriguingly, Ang1 induced the preferential differentiation of CD41+ cells toward ECs instead of hematopoietic cells. This EC expansion promoted by Ang1 was also recapitulated in induced pluripotent stem cells (iPSCs) and human ESCs. We successfully achieved in vivo neovascularization in mice by transplantation of ECs obtained from Ang1-stimulated ESCs. We conclude that Ang1/Tie2 signaling has a pivotal role in ESC-EC differentiation and that this effect can be exploited to expand EC populations.
Introduction
Embryonic stem cells (ESCs) have been used frequently not only for research in the field of developmental biology, but also in cell-based regenerative medicine.1 Many scientists in the field of vascular biology have studied the endothelial cell (EC) differentiation of ESCs in an attempt to develop treatments for the associated pathologies, and therefore our knowledge of EC differentiation has significantly expanded.2,3 However, the pluripotency of ESCs capable of generating various cell types and the overwhelming complexity of the multiple signaling pathways involved in the process of differentiation make it extremely challenging to control EC differentiation in a precise and efficient manner. Our knowledge of ESC-EC differentiation still remains far from satisfactory, and the possibility of successfully applying stem cells in vascular regenerative medicine remains merely optimistic.
Several recent studies have provided evidence supporting the presence of an intimate linkage between ECs and hematopoietic cells (HCs) during early embryonic development.4,5 It has been suggested that both the endothelial and hematopoietic lineages arise from a common progenitor, the hemangioblasts, which express Flk-1 during the early stage of differentiation.6-8 Moreover, several studies have suggested that the Flk-1+ cells at this stage are actually mesodermal precursor cells (MPCs), based on the fact that they can give rise to not only ECs and HSCs but also to vascular smooth muscle cells (VSMCs) and even cardiomyocytes.9,10 Recent studies have also demonstrated that some HSCs are derived from a subset of ECs called the hemogenic endothelium during the differentiation from ESCs.11-13 Other studies revealed that the initial definitive hematopoietic cells could originate from hemogenic endothelium that resides in the aorta during early embryonic development.14-16 However, the underlying link between ECs and HSCs still remains obscure and is the subject of controversy.
During the early stages of life, various growth factors influence endothelial and hematopoietic development. Among them, Ang1, a cognate ligand for the Tie2 receptor, is one of the key molecules known to regulate the development of the vasculature and the hematopoietic system.17,18 The fact that Ang1-deficient mouse embryos die between embryonic day 10.5 (E10.5) and E12.5 due to failure of vascular remodeling, impaired definitive hematopoiesis, and endocardial defect17 and the fact that Tie2 expression is present in both ECs and HSCs18,19 attracted our attention. Based on these results, we decided to pursue the idea that Ang1/Tie2 signaling might have a pivotal role in endothelial and hematopoietic differentiation from ESCs.
The primary goal of the present study was to investigate the role of the Ang1/Tie2 signaling pathway and its underlying mechanisms in ESC-EC differentiation. In addition, we explored whether Ang1 could be used to maximize the expansion of the endothelial population as a result of controlled ESC-EC differentiation to investigate the possibility of applying Ang1 in therapeutic protocols that require EC production.
Methods
Cell culture
A mouse ESC line (R1) was obtained from the American Type Culture Collection. OP9 cells were gifted from J.K. Yamashita (Kyoto University, Japan). Mouse induced pluripotent stem cells (iPSCs) were prepared as described previously.20 Flk-1+ MPC induction and OP9 coculture for endothelial and hematopoietic differentiation were performed as described previously.10,21 Briefly, for Flk-1+ MPC induction, ESCs were cultured on 0.1% gelatin-coated cell-culture plates at a density of 1-2 × 103 cells/cm2 for 96-108 hours in the differentiation medium (α-MEM [Invitrogen] supplemented with 10% selected FBS [Welgene]) without leukemia inhibitory factor. For endothelial differentiation, Flk-1+ MPCs were purified at E4.5 with a FACSAria II (BD Biosciences) or an AutoMACS Pro Separator (Miltenyi Biotec) combined with allophycocyanin (APC)–conjugated anti–mouse Flk-1 antibody (clone AVAS12a1; eBioscience) and/or anti-APC MicroBeads (Miltenyi Biotec), plated onto the confluent mitomycin-C (Sigma-Aldrich)–treated OP9 cells at a density of 1-2 × 104 cells/cm2, and cultured in the differentiation medium. Endothelial differentiation of human ESCs (CHA4-hES)22,23 was also performed as described previously.23 Briefly, undifferentiated hESCs were cultured for 3 days in ESC medium (ESCM; DMEM/F12 medium supplemented with 20% knockout serum replacement, 1% nonessential amino acids, 0.1mM β-mercaptoethanol; all from Invitrogen) with MEK/ERK inhibitor (50μM, PD98059; Calbiochem) and BMP4 (20 ng/mL; Peprotech), and cultured for another 6 days in ESCM with VEGF-A (100 ng/mL) and b-FGF (100 ng/mL; R&D Systems). CD34+ progenitor cells were purified by MACS using anti–human CD34 microbeads, replated, and cultured in EC basal medium-2 with growth supplements (EGM-2; Clonetics). hESC-derived ECs were induced from CD34+ progenitor cells and analyzed upon EC differentiation 9 days later. Various recombinant proteins and other reagents, including COMP-Ang124 (hereafter abbreviated as Ang1), Ang3, Ang4, sTie2-Fc, VEGF-A, and VEGF-Trap, were prepared as described previously.25,26 RGD peptides (GRADSP and GRGDSP) were purchased from Calbiochem.
Immunofluorescence staining
Cells were fixed with 2% paraformaldehyde and blocked with 5% goat (or donkey) serum in PBST (0.3% Triton X-100 in PBS) for 1 hour at room temperature. The cells were incubated overnight at 4°C with the following primary antibodies: anti–mouse CD144 antibody (clone 11D4.1; BD Pharmingen), anti–mouse CD31 antibody (clone 2H8; Chemicon), anti–mouse phospho-histone H3 (Ser10) antibody (rabbit polyclonal; Upstate Biotechnology), anti–mouse Flk-1 antibody (rabbit polyclonal; gifted from Dr Rolf Brekken, University of Texas-Southwestern Medical Center, Dallas,, TX), FITC-conjugated anti-CD41 antibody (clone MWReg30; eBioscience). After washing in PBST 6 times, the cells were incubated for 3 hours at room temperature with the following secondary antibodies: Cy3-conjugated anti–hamster IgG antibody (Jackson ImmunoResearch), Cy3-conjugated anti–rabbit IgG antibody (Jackson ImmunoResearch), and FITC-conjugated anti–rat antibody (Jackson ImmunoResearch). The cells were then mounted in fluorescent mounting medium (DAKO). Nuclei were stained with Hoechst 33258 (Invitrogen). Immunofluorescent images were acquired using an LSM510 confocal fluorescence microscope (Carl Zeiss).
Flow cytometry and cell sorting
Cells were harvested with 0.25% trypsin-EDTA or dissociation buffer (Invitrogen) and resuspended in Hank buffered salt solution/2% FBS at 1 × 106 cells per 100 μL. The cells were incubated with antibodies described below for 20 minutes, washed twice, and resuspended. Analyses and sorting were performed with a FACS LSRII (BD Biosciences) or FACSAria II (BD Biosciences). The antibodies were anti–mouse Flk-1-APC antibody (clone AVAS12a1; eBioscience), anti–mouse CD31-phycoerythrin (PE) or APC antibody (clone MEC13.3; eBioscience), anti–mouse CD144-Alexa Fluor 647 antibody (clone BV13; eBioscience). anti–mouse CD45-APC antibody (clone 30-F11; eBioscience), anti–mouse CD41-PE or APC antibody (clone MWReg30; BD Biosciences), anti-mouse CD117-APC antibody (clone 2B8; eBioscience), anti–mouse Sca-1–APC antibody (clone D7; eBioscience), anti–mouse Tie2-PE antibody (clone TEK4; eBioscience), anti–mouse Ter119-APC antibody (clone Ter119; eBioscience), and anti–mouse CD34-FITC antibody (clone RAM34; eBioscience). Dead cells were excluded by 7-aminoactinomycin D (Invitrogen). Data were analyzed using FlowJo Version 7.2.5 software (TreeStar). Cell purity of > 95% was confirmed after cell sorting.
Apoptosis assay
After 3 days of differentiation of purified Flk-1+ MPCs on OP9 cells, the cells were quickly trypsinized with 0.25% trypsin-EDTA and stained with anti–mouse Flk-1–APC antibody. Annexin V and propidium iodide staining was performed using the annexin V-FLUO staining kit (Roche) for 10 minutes at room temperature according to the manufacturer's instructions, and then flow cytometric analysis was performed.
In vitro tube-forming assay
For cell preparation, Flk-1+ MPCs differentiated from ESCs were purified and cultured for 3 days on OP9 cells. Ang1 (200 ng/mL) was given on days 0 and 2. CD31+/CD144+ cells were sorted by FACS on day 3 and used as Ang1-induced ESC-derived ECs (Ang1-EC-ESCs) for the in vitro tube-forming assay. Matrigel (300 μL, growth factor reduced; BD Biosciences) was spread on a 24-well polystyrene plate and allowed to solidify at 37°C. Ang1-EC-ESCs were plated on Matrigel-covered plates in EGM-2 at a density of ∼ 20 000-40 000 cells/cm2. After 24 hours, the tube structure of the plated cells was observed using an Axiovert25 microscope (Carl Zeiss). Phase-contrast images were taken using an Infinity X digital camera and DpxView LE software (DeltaPix). HUVECs were prepared as described previously27 and were used as a positive control.
Matrigel plug assay
Six- to 8-week-old CD31-knockout mice were gifted from Dr Tak W. Mak (University of Toronto, Toronto, Ontario, Canada) and used for the Ang1-EC-ESC transplantation experiment. The mice were immunosuppressed with cyclosporine A. From 2 days before to 3 days after transplantation, 30 mg/kg/d of cyclosporine A was administered intraperitoneally and then the dosage was reduced to 15 mg/kg/d. Animal care and experimental procedures were performed with the approval of the animal care committee of KAIST. Ang1-EC-ESCs (0.5 × 106 cells) were mixed with 500 ng/mL of VEGF165 and 200 ng/mL of Ang1 in a total volume of 100 μL. Matrigel was implanted subcutaneously into the dorsal side of the immunosuppressed CD31-deficient mice. After 2 weeks, 100 μg of FITC-lectin (Sigma-Aldrich) was injected into the tail vein 20 minutes before the mice were killed and examined to assess blood perfusion. The implanted Matrigel was fixed by systemic vascular perfusion with 1% paraformaldehyde in PBS, harvested, and whole-mounted for immunofluorescence staining.
Statistics
Data are presented as means ± SD. Significant differences between means were determined by ANOVA followed by the Student-Newman-Keuls test. Significance was set at P < .05.
Results
Ang1 promotes endothelial differentiation via Tie2 signaling
According to previous studies,11,28-30 the formation of CD31+/CD144+ EC colonies originating from ESC-derived Flk-1+ MPCs is regarded as EC differentiation. In agreement with previous studies,11,21,28,30 the Flk-1+ MPCs in our OP9 coculture system successfully gave rise to EC colonies (Figure 1A). In this setting, we examined the effect of various growth factors, including VEGF-A, VEGF-C, Ang1, Ang2, Ang3, Ang4, bFGF, EGF, TGF-β, and PDGF-α, on EC differentiation. Of these, Ang1 exerted the strongest effect on the expansion of the EC colony (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), so our study was focused on the role of Ang1 in ESC-EC differentiation. We used COMP-Ang1, a soluble and potent Ang1 variant protein, for Ang1 supplementation.24 Compared with controls, additional Ang1 (200 ng/mL on days 0 and 2) increased the number and size of the CD144+ EC colony and subsequently promoted the formation of larger EC colonies by fusion on day 3 (Figure 1B-C and supplemental Videos 1 and 2). Flow cytometric analyses revealed a positive correlation between the frequency of generated ECs and the concentration of Ang1 treatment within the range of ∼ 25-200 ng/mL; however, the effect of Ang1 reached a plateau beyond 200 ng/mL (Figure 1D). We chose 200 ng/mL of Ang1 for the following experiments based on its maximum effect on EC differentiation.
Ang1 promotes EC differentiation via Tie2 signaling. Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells. Control buffer (Control), Ang1 (200 ng/mL), sTie2-Fc (sT2, 25μg/mL), RGD peptide (GRGDSP, 25μg/mL), and control peptide (GRADSP, 25μg/mL) were administered alone or together on days 0 and 2, and analyses were performed on day 3. (A) Treatment scheme of Ang1 or inhibitor. (B) Comparison of cell number of each EC colony on day 3 (n = 4). *P < .05 compared with control. (C) Images showing CD144+ EC colonies on day 3. Nuclei were stained with Hoechst. Scale bar represents 200 μm. (D) Dose dependency of Ang1 on the percentage of CD31+/CD144+ ECs from purified Flk-1+ cells on day 3 (n = 3). *P < .05 compared with control. (E,G) Representative FACS analyses of the population of CD31+/CD144+ ECs. Numbers indicate the percentage of CD31+/CD144+ ECs. (F,H) Comparison percentages of CD31+/CD144+ ECs (n = 4). *P < .05 compared with control, GRGDSP, or GRADSP; #P < .01 compared with Ang1.
Ang1 promotes EC differentiation via Tie2 signaling. Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells. Control buffer (Control), Ang1 (200 ng/mL), sTie2-Fc (sT2, 25μg/mL), RGD peptide (GRGDSP, 25μg/mL), and control peptide (GRADSP, 25μg/mL) were administered alone or together on days 0 and 2, and analyses were performed on day 3. (A) Treatment scheme of Ang1 or inhibitor. (B) Comparison of cell number of each EC colony on day 3 (n = 4). *P < .05 compared with control. (C) Images showing CD144+ EC colonies on day 3. Nuclei were stained with Hoechst. Scale bar represents 200 μm. (D) Dose dependency of Ang1 on the percentage of CD31+/CD144+ ECs from purified Flk-1+ cells on day 3 (n = 3). *P < .05 compared with control. (E,G) Representative FACS analyses of the population of CD31+/CD144+ ECs. Numbers indicate the percentage of CD31+/CD144+ ECs. (F,H) Comparison percentages of CD31+/CD144+ ECs (n = 4). *P < .05 compared with control, GRGDSP, or GRADSP; #P < .01 compared with Ang1.
Pretreatment with an excess amount (25 μg/mL) of soluble Tie2-Fc (sT2), a selective blocker of Ang1, completely abrogated the Ang1-induced promotion of CD31+/CD144+ EC frequency on day 3 (Figure 1E-F). Treatment of sTie2 also significantly suppressed (∼ 2.0-fold) the percentage of CD31+/CD144+ ECs in the controls, suggesting the possibility that angiopoietins secreted from either differentiating ESCs or OP9 feeder cells may have served to constitutionally maintain endothelial differentiation. In fact, both CD31+/CD144+ ECs and non-ECs (including OP9 cells) expressed Ang1, whereas only CD31+/CD144+ ECs expressed Ang2 (supplemental Figure 1B). However, the addition of Ang2 (25-800 ng/mL) did not alter the profile of CD31+/CD144+ EC production (supplemental Figure 1C). In addition, because our RT-PCR and FACS analyses revealed that the OP9 cells did not express Tie2 (supplemental Figure 1B and D), Ang1 would not have had a significant effect on the OP9 cells. Therefore, we believe that Ang1, but not Ang2, acted to increase the EC population in a paracrine manner during differentiation. Because recent studies have indicated that Ang1 can also exert its cellular actions through integrin,31,32 we attempted to exclude the possibility of the increased endothelial populations being the result of Ang1 stimulation via integrin. The pan-integrin inhibitor GRGDSP and its control GRADSP did not alter the basal- and Ang1-induced promotion of CD31+/CD144+ EC percentages (Figure 1G-H). Moreover, GRGDSP and GRADSP had no measurable effect on EC adhesion (supplemental Figure 2). These data indicate that Tie2 is the corresponding receptor that mediated signal transduction of Ang1 to increase endothelial differentiation.
We also analyzed the expression pattern of Tie2 during differentiation. Although the Flk-1+ cells derived from ESCs at E4.5 did not express Tie2 immediately after isolation (supplemental Figure 3A), 12.4% of the cells began to express Tie2 as early as 12 hours after being replated on OP9 cells (supplemental Figure 3B). At days 1 and 3, approximately 20%-25% of the cells derived from the Flk-1+ cells expressed Tie2 (supplemental Figure 3B-D). The addition of Ang1 further increased the Tie2+ population up to 38.5% on day 3 (supplemental Figure 3C-D). FACS analysis revealed that most Tie2+ cells were CD31+/CD144+ ECs not CD45+ HSCs (supplemental Figure 3E); therefore, Ang1 increased the percentage of the Tie2+ EC population by ∼ 2 fold compared with control. In addition, Ang1 successfully activated Tie2 in the differentiating cells derived from ESCs (supplemental Figure 4A-B), and pretreatment with the PI3K inhibitor (LY294002, 20μM) almost completely inhibited Ang1-induced CD31+/CD144+ EC expansion, whereas the MEK/ERK inhibitor (PD98059, 50μM) did not. Moreover, Ang1 significantly increased phosphorylation of Akt at serine 473 in the CD144+ ECs (supplemental Figure 4C). These data imply that Ang1 promotes EC differentiation through the activation of Tie2-PI3K-Akt in Flk-1+ MPCs.
Ang1 induces differentiation of ESCs toward ECs
Flk-1+ MPCs are known to give rise to 3 major cell types: ECs, HSCs, and VSMCs.10,33 To assess EC, HSC, and VSMC differentiation from the Flk-1+ MPCs, we analyzed the frequencies of CD31+/CD144+ ECs, CD45+ HSCs, and α-SMA+ VSMCs on days 1.5, 3, and 6 (Figure 2). In the absence of additional Ang1, the percentage of CD31+/CD144+ ECs was observed to gradually decrease, whereas the percentage of CD45+ HSCs rapidly increased over time (Figure 2). FACS analyses revealed that the CD45+ cells were mainly CD11b+ myeloid cells (supplemental Figure 5A). However, when Ang1 was administered, the percentage of ECs continued to increase but the increase rate of CD45+ HSCs was significantly blunted (Figure 2). The suppressive effect of Ang1 on hematopoietic expansion was also observed at the hematopoietic progenitor level, as shown by the reduced hematopoietic colony formation (supplemental Figure 5B-C). The population of α-SMA+ VSMCs was found to be 10%-15% at day 1.5 and dramatically increased over time in the absence of Ang1. However, the rate of increase in VSMCs was markedly slowed down in the presence of Ang1 (Figure 2). These data indicate that Ang1 preferentially drives EC differentiation from Flk-1+ MPCs during differentiation of ESCs.
Ang1 promotes the EC population but reduces the HSC and VSMC populations during Flk-1+ MPC differentiation. Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells. Control or Ang1 was administered on days 0, 2, and 4, and analyses were performed on days 1.5, 3, and 6. (A,C,E) Representative FACS analyses of the populations of CD31+/CD144+ ECs, CD45+ HSCs, and α-SMA+ VSMCs. Numbers indicate percentages of ECs, HSCs and VSMCs. (B,D,F) Comparison of percentages of CD31+/CD144+ ECs, CD45+ HSCs, and α-SMA+ VSMCs over time (n = 5). *P < .01 compared with control.
Ang1 promotes the EC population but reduces the HSC and VSMC populations during Flk-1+ MPC differentiation. Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells. Control or Ang1 was administered on days 0, 2, and 4, and analyses were performed on days 1.5, 3, and 6. (A,C,E) Representative FACS analyses of the populations of CD31+/CD144+ ECs, CD45+ HSCs, and α-SMA+ VSMCs. Numbers indicate percentages of ECs, HSCs and VSMCs. (B,D,F) Comparison of percentages of CD31+/CD144+ ECs, CD45+ HSCs, and α-SMA+ VSMCs over time (n = 5). *P < .01 compared with control.
Ang1 promotes proliferation and suppresses apoptosis of ECs during Flk-1+ MPC differentiation
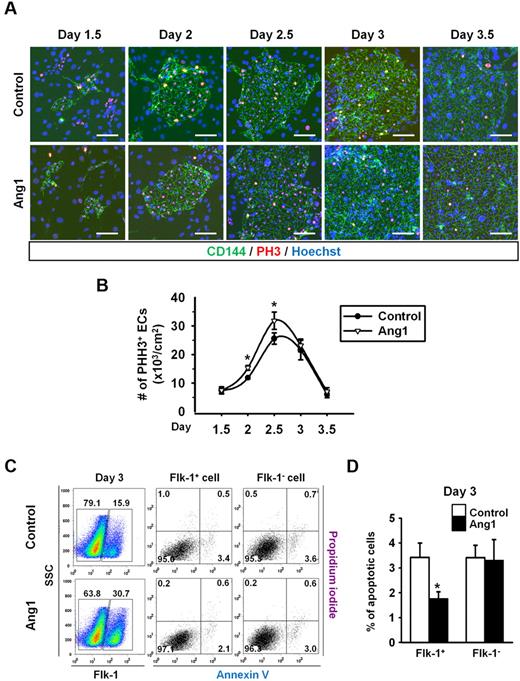
We next examined the underlying mechanism through which Ang1 promotes endothelial differentiation. The proliferative activity measured by phospho-histone H3 immunostaining revealed that Ang1 enhanced the proliferative activities of CD144+ ECs by 1.5-fold and 1.3-fold at days 2 and 2.5, respectively, compared with controls (Figure 3A-B). Furthermore, Ang1 inhibited cell apoptosis in the Flk-1+ cells but not in Flk-1− cells, as measured by costaining with annexin V and propidium iodide (Figure 3C-D). These data indicate that Ang1 enhanced the proliferation and extended the survival of ECs, which could be the cause of the increases in ESC-derived EC populations.
Ang1 increases EC proliferation but suppresses EC apoptosis during Flk-1+ MPC differentiation. Control or Ang1 was administered into Flk-1+ MPCs on days 0, 2, and 4 during OP9 coculture. (A) Images showing PHH3+ cells in CD144+ EC colonies on days 1.5, 2, 2.5, 3, and 3.5. Nuclei were stained with Hoechst. Scale bar represents 100 μm. (B) Comparison of number of PHH3+ ECs over time (n = 5). *P < .05 compared with control. (C) Representative FACS analyses of the populations of annexin+ apoptotic Flk-1+ cells and Flk-1− cells on day 3. Numbers indicate percentages of each population. (D) Comparison of percentage of annexin+ apoptotic Flk-1+ cells and Flk-1− cells on day 3 (n = 3). *P < .05 compared with control.
Ang1 increases EC proliferation but suppresses EC apoptosis during Flk-1+ MPC differentiation. Control or Ang1 was administered into Flk-1+ MPCs on days 0, 2, and 4 during OP9 coculture. (A) Images showing PHH3+ cells in CD144+ EC colonies on days 1.5, 2, 2.5, 3, and 3.5. Nuclei were stained with Hoechst. Scale bar represents 100 μm. (B) Comparison of number of PHH3+ ECs over time (n = 5). *P < .05 compared with control. (C) Representative FACS analyses of the populations of annexin+ apoptotic Flk-1+ cells and Flk-1− cells on day 3. Numbers indicate percentages of each population. (D) Comparison of percentage of annexin+ apoptotic Flk-1+ cells and Flk-1− cells on day 3 (n = 3). *P < .05 compared with control.
Ang1 drives CD41+ cells preferentially toward ECs rather than HSCs
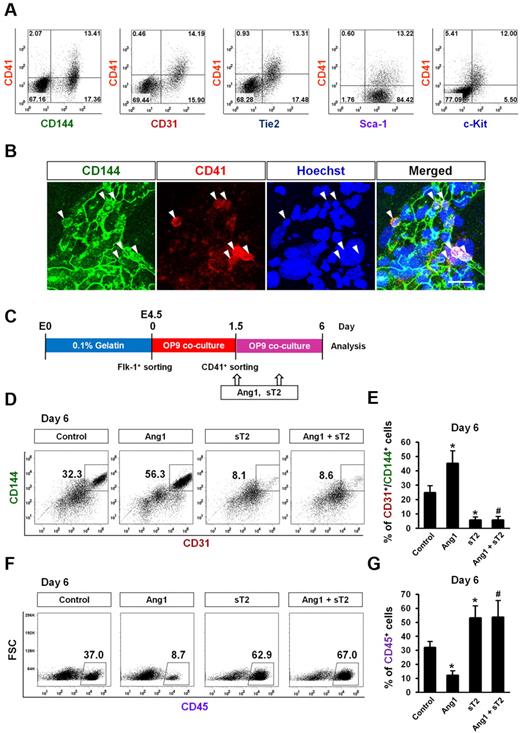
CD41 is expressed in cells that are involved in the early stage of hematopoiesis, and its expression is associated with hematopoietic commitment of hemogenic endothelium in the early murine yolk sac.34-36 It was recently reported that certain hemogenic ECs transiently express CD41 at an early stage of ESC endothelial and hematopoietic differentiation.12 Consistent with these previous findings, our data revealed that ∼ 10%-20% of Flk-1+ MPC–derived cells were CD41+ on day 1.5, and this number gradually decreased over time (Figure 2C). Interestingly, flow cytometric analyses revealed that CD41+ cells on day 1.5 concurrently expressed both endothelial and primitive hematopoietic markers (CD144, CD31, Tie2, Sca-1, and c-Kit; Figure 4A). Immunostaining revealed that there were CD144+/CD41+ cells in the EC colonies (Figure 4B).
Ang1 drives CD41+ cells to ECs rather than HSCs. Expression pattern and differentiation potential of CD41+ cells derived from Flk-1+ MPCs were characterized. (A) Representative FACS analyses showing surface expression patterns of CD144, CD31, Tie2, Sca-1, and c-Kit, each compared with CD41 on Flk-1+ MPC–derived cells on day 1.5. (B) Images showing CD144+/CD41+ cells in endothelial colonies on day 1.5. Nuclei were stained with Hoechst. Scale bar represents 20 μm. (C) Experimental scheme for the differentiation potential of CD41+ cells. Sorted CD41+ cells on day 1.5 were replated on the freshly prepared OP9 cells, treated with Control, Ang1, sT2, or Ang1 + sT2 on days 1.5, and 3.5, and analyses were performed on day 6. (D,F) Representative FACS analyses of the populations of CD31+/CD144+ ECs and CD45+ HSCs from the sorted CD41+ cells. Numbers indicate percentages of each population. (E,G) Comparison of percentages of CD31+/CD144+ ECs and CD45+ HSCs from the sorted CD41+ cells (n = 3). *P < .05 compared with control; #P < .05 compared with Ang1.
Ang1 drives CD41+ cells to ECs rather than HSCs. Expression pattern and differentiation potential of CD41+ cells derived from Flk-1+ MPCs were characterized. (A) Representative FACS analyses showing surface expression patterns of CD144, CD31, Tie2, Sca-1, and c-Kit, each compared with CD41 on Flk-1+ MPC–derived cells on day 1.5. (B) Images showing CD144+/CD41+ cells in endothelial colonies on day 1.5. Nuclei were stained with Hoechst. Scale bar represents 20 μm. (C) Experimental scheme for the differentiation potential of CD41+ cells. Sorted CD41+ cells on day 1.5 were replated on the freshly prepared OP9 cells, treated with Control, Ang1, sT2, or Ang1 + sT2 on days 1.5, and 3.5, and analyses were performed on day 6. (D,F) Representative FACS analyses of the populations of CD31+/CD144+ ECs and CD45+ HSCs from the sorted CD41+ cells. Numbers indicate percentages of each population. (E,G) Comparison of percentages of CD31+/CD144+ ECs and CD45+ HSCs from the sorted CD41+ cells (n = 3). *P < .05 compared with control; #P < .05 compared with Ang1.
To determine the differentiation potential of these CD41+ cells, we sorted CD41+/CD45− cells on day 1.5, replated them on the freshly prepared OP9 cells, and cultured them for another 4.5 days (Figure 4C). On day 6 after plating, ∼ 25% of the cells were CD144+/CD31+ ECs and ∼ 30% were CD45+ HSCs. Compared with controls, Ang1 increased the CD144+/CD31+ EC population by ∼ 2-fold, whereas Ang1 suppressed the CD45+ HSC population by ∼ 3-fold. We also performed a limiting dilution analysis with CD41+/CD45− cells to determine whether they gave rise to the EC colony (supplemental Figure 6A). Even after dilution to 10 cells, the CD41+/CD45− cells were still able to generate the CD144+ EC colony, but after dilution to < 10 CD41+/CD45− cells, they were not (supplemental Figure 6B), suggesting that > 10 CD41+ cells is necessary for the generation of EC colony by an as-yet-unidentified mechanism. To confirm the effect of Ang1 on the suppression of HSC differentiation from the CD41+/CD45− cells, we performed a CFU assay of CD41+/CD45− cells that were obtained from Flk1+ MPCs on OP9 culture at day 1.5 according to the original experimental scheme shown in Figure 4C and supplemental Figure 6C. The CFU assay revealed that the CD41+/CD45− cells gave rise to hematopoietic colonies including B/CFU-E, CFU-M/GM, and, rarely, CFU-GEMM at day 10. FACS analyses revealed that the Ter119+ erythroid cell population in hematopoietic colonies peaked to 30% at day 5 and decreased thereafter, whereas the CD45+ myeloid cell population gradually increased up to 30% at day 10 (supplemental Figure 6D). The addition of Ang1 (1 μg/mL) to the medium suppressed the populations of CD45+ myeloid cells, but had little effect on the population of Ter119+ erythroid cells (supplemental Figure 6D). Likewise, Ang1 significantly reduced the number of CFU-G/GM cells, whereas Ang1 had no significant effect on B/CFU-E and CFU-GEMM cells (supplemental Figure 6E). Based on these findings, we conclude that Ang1 suppressed HSC differentiation from CD41+/CD45− cells.
sTie2 markedly suppressed the CD144+/CD31+ EC population by 5-fold, but it also significantly increased the CD45+ HSC population compared with control. The addition of Ang1 failed to reverse the sTie2-induced changes in EC and HSC differentiation (Figure 4D-G). These data imply that the endogenous Ang1 secreted from differentiating ESC cells and/or OP9 feeder cells may contribute to EC differentiation. It can also be inferred that Ang1 drives the differentiation of CD41+ cells preferentially toward ECs rather than HSCs.
Ang1 promotes endothelial differentiation from mouse iPSCs and human ESCs
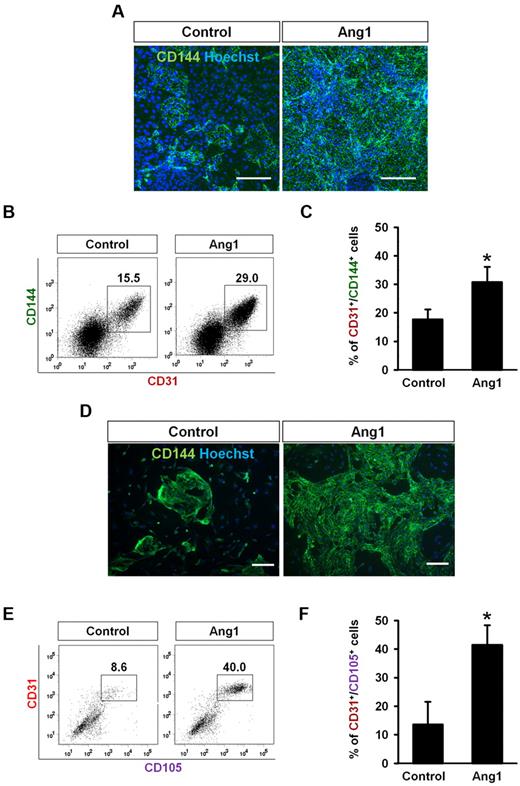
To determine the effect of Ang1 on EC differentiation in another type of pluripotent cell, we applied the same differentiation protocol to mouse iPSCs.37 Similar to mouse ESCs, the presence of Ang1 promoted the size of each colony and the number of CD144+ ECs that it contained (Figure 5A). FACS analyses revealed that Ang1 significantly increased the percentage of CD31+/CD144+ ECs on day 3 compared with controls (Figure 5B-C). We also tested the effect of Ang1 in human ESC differentiation. Purified human CD34+ cells were cultured in EGM-2 medium with or without Ang1 (500 ng/mL) after CD34+ cells were induced from human ESCs. Ang1 promoted the CD144+ EC number and size in each colony (Figure 5D). FACS analyses revealed that Ang1 significantly increased the percentage of CD31+/CD105+ ECs compared with controls (Figure 5E). In summary, our data indicated that Ang1 commonly promoted EC differentiation in both iPSCs and ESCs.
Ang1 increases EC differentiation from mouse iPSCs and human ESCs. (A-C) iPSC-derived Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells with control buffer (Control) or Ang1. Analyses were performed on day 3. (A) Images showing CD144+ EC colonies. Nuclei were stained with Hoechst. Scale bar represents 200 μm. (B) Representative FACS analyses of the population of CD31+/CD144+ ECs. Numbers indicate percentages of ECs from Flk-1+ MPCs during differentiation. (C) Comparison percentages of CD31+/CD144+ ECs from purified Flk-1+ MPCs (n = 3). *P < .05 compared with control. (D-E) hESC-derived CD34+ cells were purified and cultured with Control or Ang1 and analyses were performed 9 days later. (D) Images showing CD144+ EC colonies. Nuclei were stained with Hoechst. Scale bars represent 200 μm. (E) Representative FACS analyses of the population of CD31+/CD105+ ECs. Numbers indicate percentages of ECs from CD34+ cells during differentiation. (F) Comparison percentages of CD31+/CD105+ ECs from purified CD34+ cells (n = 3). *P < .05 compared with control.
Ang1 increases EC differentiation from mouse iPSCs and human ESCs. (A-C) iPSC-derived Flk-1+ MPCs were purified on E4.5 and cultured on OP9 cells with control buffer (Control) or Ang1. Analyses were performed on day 3. (A) Images showing CD144+ EC colonies. Nuclei were stained with Hoechst. Scale bar represents 200 μm. (B) Representative FACS analyses of the population of CD31+/CD144+ ECs. Numbers indicate percentages of ECs from Flk-1+ MPCs during differentiation. (C) Comparison percentages of CD31+/CD144+ ECs from purified Flk-1+ MPCs (n = 3). *P < .05 compared with control. (D-E) hESC-derived CD34+ cells were purified and cultured with Control or Ang1 and analyses were performed 9 days later. (D) Images showing CD144+ EC colonies. Nuclei were stained with Hoechst. Scale bars represent 200 μm. (E) Representative FACS analyses of the population of CD31+/CD105+ ECs. Numbers indicate percentages of ECs from CD34+ cells during differentiation. (F) Comparison percentages of CD31+/CD105+ ECs from purified CD34+ cells (n = 3). *P < .05 compared with control.
Ang1 promotes EC differentiation in the OP9 cell-free system
To examine whether Ang1 also promotes EC differentiation in the OP9 cell-free system, Flk-1+ MPCs were purified at E4.5, plated onto Matrigel-coated plates, and cultured in the differentiation medium containing Ang1 (supplemental Figure 7A). Ang1 also profoundly promoted EC differentiation in the Matrigel-coated system (supplemental Figure 7B-D), indicating that it has a direct effect on EC differentiation from the Flk-1+ MPCs.
Ang1-induced ECs have a neovasculogenic potential in vitro and in vivo
To explore whether the ECs induced by Ang1 have vasculogenic potential, we purified CD31+/CD144+ ECs cultured with Ang1 supplements using FACS on day 3 and refer to them as “Ang1-EC-ESCs.” FACS analysis revealed that some mesenchymal stem cell (MSC) markers (CD105, Sca-1, and c-Kit) were positive in Ang1-EC-ESCs, whereas another MSC marker (CD106) was negative (supplemental Figure 8A). Recently, Kopher et al demonstrated that hESC-derived CD34+ endothelial precursors have phenotypic and functional features similar to MSCs.38 Therefore, Ang1-EC-ESCs may contain MSC components. When Ang1-EC-ESCs were plated on a 0.1% gelatin-coated dish in EGM-2 medium, they showed a typical cobblestone-like appearance as early as 6 hours from the start of incubation (Figure 6A). Typical endothelial adhesion molecules such as CD144 and CD31 were abundantly localized on the cell-cell junctions of the Ang1-EC-ESCs (Figure 6B). Therefore, Ang1-EC-ESCs were morphologically compatible with typical ECs. An in vitro tube-forming assay was performed to characterize the functional properties of Ang1-EC-ESCs. When Ang1-EC-ESCs were seeded on Matrigel-covered plates and incubated in EGM-2 medium, although relatively abundant cell clusters were observed, obvious vessel-like network structures similar to those of HUVECs were formed as early as 12 hours from the start of incubation (Figure 6C), indicating that Ang1-EC-ESCs are capable of achieving in vitro neovascularization. Our additional immunostaining analysis revealed that the vascular network structure formed with Ang1-EC-ESCs in the in vitro tube-forming assay consisted of mostly CD144+ cells and some CD144− adjacent cells (supplemental Figure 8B), suggesting that the Ang1-EC-ESCs in our differentiation system could also have functionally and phenotypically different characteristics from mature ECs.
Ang1-induced CD31+/CD144+ ECs have a neovasculogenic potential in vitro and in vivo. Purified Flk-1+ MPCs differentiated from mouse ESCs were cultured for 3 days on OP9 cells. Ang1 was administered on days 0 and 2. CD31+/CD144+ cells were sorted by FACS on day 3 and used as Ang1-EC-ESCs for further experiments. (A) Phase contrast images showing Ang1-EC-ESCs. Scale bar represents 100 μm. (B) Immunostaining images showing Ang1-EC-ESCs. Nuclei were stained with Hoechst. Scale bar represents 100 μm. (C) Images showing network formations of Ang1-EC-ESCs and HUVECs. Ang1-EC-ESCs and HUVECs were plated on Matrigel-covered plates and incubated in EGM-2. The tube structures were observed after 24 hours. Scale bar represents 200 μm. (D-E) Ang1-mESC-ECs were injected subcutaneously into the dorsal skin of cyclosporine A–treated CD31-deficient mice in Matrigel. The implanted Matrigel was harvested 14 days after injection of FITC-lectin 20 minutes before the mice were killed; The presence of CD31+ vessel-like structures containing FITC-lectin can be identified in regions 1 and 2, indicating that effective perfusion had taken place in the implanted Ang1-EC-ESCs. The bottom 2 panels are the magnified views of indicated regions. Scale bars represent 50 μm.
Ang1-induced CD31+/CD144+ ECs have a neovasculogenic potential in vitro and in vivo. Purified Flk-1+ MPCs differentiated from mouse ESCs were cultured for 3 days on OP9 cells. Ang1 was administered on days 0 and 2. CD31+/CD144+ cells were sorted by FACS on day 3 and used as Ang1-EC-ESCs for further experiments. (A) Phase contrast images showing Ang1-EC-ESCs. Scale bar represents 100 μm. (B) Immunostaining images showing Ang1-EC-ESCs. Nuclei were stained with Hoechst. Scale bar represents 100 μm. (C) Images showing network formations of Ang1-EC-ESCs and HUVECs. Ang1-EC-ESCs and HUVECs were plated on Matrigel-covered plates and incubated in EGM-2. The tube structures were observed after 24 hours. Scale bar represents 200 μm. (D-E) Ang1-mESC-ECs were injected subcutaneously into the dorsal skin of cyclosporine A–treated CD31-deficient mice in Matrigel. The implanted Matrigel was harvested 14 days after injection of FITC-lectin 20 minutes before the mice were killed; The presence of CD31+ vessel-like structures containing FITC-lectin can be identified in regions 1 and 2, indicating that effective perfusion had taken place in the implanted Ang1-EC-ESCs. The bottom 2 panels are the magnified views of indicated regions. Scale bars represent 50 μm.
To determine the in vivo vasculogenic potential of Ang1-EC-ESCs, the cells were mixed with 500 ng/mL of VEGF165 and 200 ng/mL of Ang1 in 100 μL of Matrigel and implanted subcutaneously into the dorsal flank of CD31-deficient mice. Before implantation of the Ang1-EC-ESC mixture, the CD31-deficient mice were pretreated with cyclosporine A to prevent allograft immune rejection (Figure 6D). At 14 days after implantation, FITC-lectin was injected 20 minutes before the implanted Matrigel was harvested to assess blood perfusion. Immunohistochemistry of the implants revealed the presence of CD31+ vessel structures that colocalized with FITC-lectin signals, showing that Ang1-EC-ESCs are capable of establishing blood-perfused neovasculatures in vivo (Figure 6E). We conclude that CD31+/CD144+ ECs stimulated by Ang1 have neovasculogenic potential not only in vitro but also in vivo.
Discussion
The Ang1/Tie2 axis is well known for its general effect on adulthood vessels, causing vascular remodeling and maturation.39 During the quiescent phase of adult vessels, the Ang1/Tie2 pathway acts not only to retain vascular integrity but also to induce the circumferential proliferation of ECs and consequently achieve vascular enlargement.40 During more active periods of adult angiogenesis, Ang1/Tie2 signaling is involved in promoting non-leaky and inflammation-free angiogenesis.39,40 In contrast, during embryonic development, the Ang1/Tie2 axis is thought to induce EC differentiation and pericyte recruitment, and therefore is involved in vessel maturation and stabilization.17 Little is known about the role of the Ang1/Tie2 pathway during early development, mainly due to the lack of a technically suitable system that can platform a valid experimental design for ECs of this period. Several established methods are used to achieve EC differentiation from Flk-1+ MPCs.10,11,28,30,41 Some studies have reported that ECs and VSMCs are selectively induced by culturing purified Flk-1+ MPCs on type-IV collagen-coated dishes with VEGF-A stimulation.10,11,28 Flk-1+ MPCs cultivated on OP9 feeder cells (the OP9 coculture system) also successfully induced ECs, along with HSCs, VSMCs, and cardiomyocytes.11,28,30,41 It has been reported that hemogenic endothelial colonies that are capable of hematopoietic differentiation can be produced using the OP9 coculture system.12 In the present study, we adopted the OP9 coculture system for the comprehensive analysis of ESC-EC differentiation.
Our results show that during the early developmental periods of ESCs, Tie2 is highly expressed in differentiating ECs, whereas Ang1 is expressed not only in differentiating ECs, but also in non-ECs—including OP9 cells. We believe that the proper expression and stimulation of the Ang1/Tie2/PI3-kinase/Akt signaling pathway is essential for EC differentiation because signal interference by treatment with an sTie2 or PI3K inhibitor interfered with this process. We conclude that Ang1 basically acts in a paracrine manner and has a pivotal role in EC differentiation during early development. Conversely, we could not measure any significant effect of Ang2 on EC differentiation and expansion. The interpretation of Ang2 is more complex, because its effect is context dependent and can serve either as an antagonist or agonist of Tie2. However, our preliminary assumption is that Ang2 probably requires additional components that are not provided in differentiating ESCs.
Previous studies have reported that the activation of the Tie2 pathway has diverse effects on hematopoiesis.18,19,42,43 Considering the complexity and combined effects of the signal circuit of Ang1, Ang2 ligands, and their common receptor Tie2,12,14-16,34 we assume that the diverse effect of Tie2 activation on hematopoietic development would be similar to the temporal and spatial profile of emergence of embryonal hematopoiesis; the primitive hematopoiesis begins at blood islands in the yolk sac, but is later reformed into definitive hematopoiesis in several tissues such as the aorta-gonad-mesonephros.44 Recent accumulating evidence supports the presence of hemogenic ECs that generate definitive hematopoiesis12,13 ; however, the identity of these cells remains unclear. In the present study, we demonstrate that CD41+ ECs that are transiently observed during the process of mesodermal precursor cell differentiation have the capacity to give rise to both HSCs and ECs. Therefore, in vitro CD41+ ECs are equivalent to in vivo hemogenic ECs. Our data showed that Tie2 is expressed in these CD41+ ECs, and that stimulation with Ang1 shifted the differentiation toward ECs and away from HSCs. In a similar context, we hypothesize that Ang1/Tie2 signaling might be involved in the emergence of definitive hematopoiesis during embryonic development by controlling the spatiotemporal expression pattern of Tie2 in hemogenic ECs.
The notion that ECs can be obtained and expanded by ex vivo differentiation of stem cells suggested ESCs and/or iPSCs as promising candidate supplies for vascular regenerative therapies. In theory, their pluripotency and proliferative capacity identifies stem cells as ideal resources for therapeutic approaches; however, in reality, the main obstacle is that it is extremely challenging to precisely control differentiation into ECs while at the same time producing sufficient quantities. In this study, we show that Ang1 has a profound role in ESC-EC differentiation mainly through Tie2 signaling (Figure 7) and that it can maximize the efficiency of EC production during this process. Moreover, given that Ang1 also promotes EC expansion in the OP9 cell-free system, it could be a potential supplemental factor to maximize the efficiency of EC production in the cell-free system. Recently, iPSCs have become a conceptual but promising source of pluripotent cells.23,45,46 Therefore, it would be useful to establish a method to generate specific target cells from iPSCs. We have demonstrated herein that the effect of Ang1 supplementation was recapitulated in iPSCs and even in human ESCs. In addition, in vivo transplantation of the ECs derived from ESCs under Ang1 stimulation successfully established vessel structures, indicating that their functionality was retained.
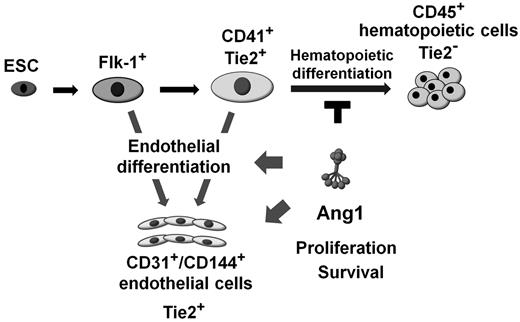
Schematic diagram of the effect of Ang1 on endothelial and hematopoietic differentiation. CD41+ cells derived from Flk-1+ MPCs differentiate further into either the endothelial or the hematopoietic lineage. Ang1 potentiates EC differentiation via Ang1/Tie2 signaling, and its mechanisms are: (1) modulating CD41+ cells to differentiate into the endothelial lineage rather than the hematopoietic lineage, and (2) promoting EC proliferation and survival during differentiation.
Schematic diagram of the effect of Ang1 on endothelial and hematopoietic differentiation. CD41+ cells derived from Flk-1+ MPCs differentiate further into either the endothelial or the hematopoietic lineage. Ang1 potentiates EC differentiation via Ang1/Tie2 signaling, and its mechanisms are: (1) modulating CD41+ cells to differentiate into the endothelial lineage rather than the hematopoietic lineage, and (2) promoting EC proliferation and survival during differentiation.
Because, in the present study, Ang1 supplementation increased the production of ECs during ESC differentiation, we questioned how this effect was achieved. Our data show that Ang1 induced enhanced EC proliferation and extended survival. In fact, Ang1 has been reported as a crucial factor for EC proliferation and survival in fully differentiated ECs in vitro and in vivo, with the proliferation and survival said to be mainly regulated through the ERK1/2 and PI3K-Akt signaling pathways, respectively.24,40,47 Our current results showed that Tie2-PI3K-Akt activation could be the major downstream signaling pathway responsible for Ang1-induced CD31+/CD144+ EC expansion. The spatiotemporal expression profile in our study revealed that the effect of Ang1 supplementation was strongest on proliferation mainly during the earlier periods, whereas the anti-apoptosis effect was continuously observed throughout the entire experimental period. This suggests that continuous supplementation of Ang1 is necessary to maximize the size of the EC population during ESC-EC differentiation. Another explanation of the surplus EC expansion is that CD41+ cells were induced by Ang1/Tie2 signaling to preferentially differentiate into ECs instead of HSCs. On E4.5, the Flk-1+ MPCs, which are considered transient precursors of both ECs and HSCs, expressed neither endothelial (CD31 and CD34) nor hematopoietic (CD41 and CD45) markers. Under normal conditions, Tie2+ cells rarely appeared before E4.5 of mouse ESC differentiation. However, we found that purified Flk-1+ MPCs began to express Tie2 as early as 0.5 days after reseeding ESCs in the OP9 coculture system. On day 1.5 of OP9 coculture, Tie2 was expressed on ∼ 20%-25% of all Flk-1+ MPC–derived cells. Interestingly, > 40% of those Tie2+ cells coexpressed CD41, a surface marker that is temporarily observed in hemogenic ECs undergoing differentiation.12,13,16 Indeed, the fate of these CD41+ cells after differentiation turned out to be bidirectional, producing both ECs and HSCs. Our study demonstrated that Ang1 supplementation increased the production of ECs from CD41+ cells, suggesting that Ang1 tilted the balance of the bidirectional Tie2-expressing CD41+ cells, guiding these indecisive cells toward the endothelial lineage. The downstream signaling pathways responsible for Ang1/Tie2-mediated fate determination in CD41+ cells remain to be further elucidated in subsequent studies.
In conclusion, Ang1 promotes endothelial and suppresses hematopoietic differentiation of CD41+ cells during ESC-EC differentiation. Ang1 also has strong proliferative and antiapoptotic effects on ECs during early development. We believe that these novel effects of Ang1 can be applied to establishing EC protocols that will solidify stem cell–based therapies to introduce new directions in vascular regeneration medicine.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jin Sun Hong, Jongho Jin, and Eun Soon Lee for their technical assistance.
This research was supported by grants from the Stem Cell Research Center of the 21st Century Frontier Research Program (SC-5120 to G.Y.K.) and from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A091345 to H.J.J.).
Authorship
Contribution: H.J.J., H.K., S.-W.P., H.-J.C., and G.Y.K. designed and performed the experiments, analyzed the data, generated the figures, and wrote the manuscript; and H.-S.K., D.-S.L., H.-M.C, I.K., and Y.-M.H. designed the experiments and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh, Graduate School of Medical Science and Engineering, KAIST, 373-1, Guseong-dong, Daejeon, 305-701, Republic of Korea; e-mail:gykoh@kaist.ac.kr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal