Abstract
Megakaryocytes generate platelets by remodeling their cytoplasm first into proplatelets and then into preplatelets, which undergo fission to generate platelets. Although the functions of microtubules and actin during platelet biogenesis have been defined, the role of the spectrin cytoskeleton is unknown. We investigated the function of the spectrin-based membrane skeleton in proplatelet and platelet production in murine megakaryocytes. Electron microscopy revealed that, like circulating platelets, proplatelets have a dense membrane skeleton, the main fibrous component of which is spectrin. Unlike other cells, megakaryocytes and their progeny express both erythroid and nonerythroid spectrins. Assembly of spectrin into tetramers is required for invaginated membrane system maturation and proplatelet extension, because expression of a spectrin tetramer–disrupting construct in megakaryocytes inhibits both processes. Incorporation of this spectrin-disrupting fragment into a novel permeabilized proplatelet system rapidly destabilizes proplatelets, causing blebbing and swelling. Spectrin tetramers also stabilize the “barbell shapes” of the penultimate stage in platelet production, because addition of the tetramer-disrupting construct converts these barbell shapes to spheres, demonstrating that membrane skeletal continuity maintains the elongated, pre-fission shape. The results of this study provide evidence for a role for spectrin in different steps of megakaryocyte development through its participation in the formation of invaginated membranes and in the maintenance of proplatelet structure.
Introduction
Blood platelets, like erythrocytes, must withstand high shear forces during circulation. Retaining their discoid shape is critical to platelets, because their small size and shape cause them to be propelled by blood flow to the endothelial surface, where they are positioned to readily sense and respond to vascular damage. To provide structural support and prevent gross deformations as they circulate, mature platelets contain a robust membrane skeleton that is formed by spectrin molecules, adducin, and actin filament barbed ends.1-3 Two thousand spectrin tetramers, 200-nm-long head-to-head assemblies of αβ heterodimers, compose the bulk of this 2D network. Although less is known about how the spectrin-actin network forms and connects to the plasma membrane in platelets relative to erythrocytes, certain differences between the 2 membrane skeletons have been recognized. First, spectrin strands comprising the platelet membrane skeleton interconnect on the ends of long actin filaments originating from the cytoplasm instead of short actin oligomers.3-5 Therefore, the platelet spectrin lattice and its associated actin filaments assemble into a continuous ultrastructure. Second, tropomodulins do not appear to have a major role in capping actin filament pointed ends, as occurs in erythrocytes.6,7 Instead, a substantial number of these ends exist free or are capped by Arp2/3 in the resting platelet. Barbed-end capping by adducin also serves the function of targeting barbed ends to the spectrin-based membrane skeleton, because the affinity of adducin-actin complexes for spectrin is greater than that of either actin or adducin alone.8,9 In addition, cortical actin filaments are attached at multiple points along their lengths to the plasma membrane in platelets by numerous Filamin A-GP1bα connections (25 000/platelet). Whereas our view of the membrane skeleton in resting platelets is coming into focus, little information is available concerning when and where these membrane-cytoskeletal linkages form during the megakaryocyte-platelet transition.
Blood platelets release from the ends of proplatelets, which are long, pseudopodial extensions produced by megakaryocytes that transverse through the bone marrow sinusoids into the blood.10 Proplatelet elaboration is preceded by a massive expansion of the megakaryocyte cytoplasmic volume and an internal membrane reservoir, originally called the demarcation membrane system (DMS) and more recently the invaginated membrane system (IMS). This reservoir supplies membrane for proplatelet formation, a process driven by a dramatic reorganization of the megakaryocyte cytoskeleton.11-13 Microtubules and actin filaments have different roles in proplatelet production.14,15 Cortical microtubules line the shafts of proplatelets and are slid by cytoplasmic dynein power sources, thereby elongating the proplatelets.14,16 F-actin, present throughout proplatelets, forms the assemblies required to bend and bifurcate proplatelets to amplify proplatelet ends.14,16
The biogenesis and function of the spectrin cytoskeleton in megakaryocyte maturation and proplatelet extension have not been explored. In the present study, biochemical, morphological, and disruptive approaches were used to understand the function of the membrane skeleton in proplatelet and platelet formation. Our objectives were to determine: (1) whether megakaryocytes have a spectrin-based membrane cytoskeleton and, if so, when is it assembled; (2) the spectrin composition of this membrane skeleton; and (3) whether the spectrin cytoskeleton is required for proplatelet formation and stability. We found that proplatelets have a spectrin cytoskeleton similar in structure to that of the mature platelet. The nonerythroid subunits αII and βII spectrin are predominately expressed in mouse megakaryocytes, proplatelets, and platelets, but erythroid αI and βI spectrin isoforms are also expressed. To assess the importance of the spectrin-based membrane skeleton in platelet biogenesis, we used a high-affinity spectrin-binding construct that disrupts spectrin tetramers. Our data indicate that the formation of spectrin tetramers is critical to the formation of the megakaryocyte IMS, proplatelet elaboration, and the proplatelet → preplatelet → platelet transition.
Methods
In vitro culture of MKs
Murine megakaryocytes (MKs) were harvested from the fetal livers of mouse embryos at gestational day 13.5 and cultured in the presence of thrombopoietin, as described previously.17,18 All animal experiments were performed according to protocols approved by Children's Hospital Institutional Animal Care and Use Committee.
Electron microscopy
Preparation of samples for electron microscopy and immunogold electron microscopy was as described previously.3 Membrane skeletal pore sizes were determined using ImageJ Version 1.37v software by manually outlining the individual fibers composing the pores of the network and measuring the areas inside the outlines. For immunoelectron microscopy, coverslips were covered with 25μL of primary antibodies (B13 anti–β1 spectrin rabbit polyclonal antibody and 10D anti–β2 rabbit polyclonal antibody) at concentrations of 10 μg/mL, incubated for 3 hours at room temperature, and washed 3 times in PBS with BSA. The IMS was quantified within a selected region (143.28 μm2) by measuring the area of the electron lucent zones corresponding to the IMS. The area was determined in pixels using ImageJ software. In each experiment, a total of 10 megakaryocytes each were analyzed for both control and αII spectrin (spα2N1)–treated cells. Experiments were done in triplicate.
Western blot analysis
Purified murine fetal liver cells (FLCs; day 0), MKs (separated by BSA gradient on days 2, 3, and 4), platelets, 3T3 fibroblasts, and erythrocyte ghosts were prepared as described previously.19,20 Cells were lysed with PHEM buffer (60mM PIPES, 25mM HEPES, 10mM EGTA, and 2mM MgCl2) containing 0.1% Triton X-100 for total and cytoskeleton-associated and soluble distribution of spectrin isoforms. Lysates, along with protein ladder, were electrophoresed on 7.5% SDS-PAGE minigels (Lonza). Gels were transferred to nitrocellulose membrane and blocked in TBS-T (Tris-buffered saline with 0.2% Tween-20) with 1% BSA for 1 hour and probed with antibody overnight at 4°C. Primary antibodies used included G5183 anti–αI spectrin, B13 anti–βI spectrin, G5187 anti–spα2N1, and 10D anti–βII spectrin, all rabbit polyclonal antibodies. Blots were washed 3 times in TBS-T and incubated in 1:5000 dilution of HRP-conjugated secondary antibody for 30 minutes. Blots were washed again 3 times in TBS-T and developed using SuperSignal ECL substrates (Pierce Biotechnology). For isolation of the cytoskeleton, FLCs, MKs, and platelets were washed twice in PBS and cells were lysed with 0.1% Triton X-100 in PHEM buffer containing 10μM phallacidin. F-actin associated proteins were collected by centrifugation at 100 000g for 30 minutes at 4°C. Triton X-100–insoluble (pellet) and -soluble (supernatant) fractions were resolved by SDS-PAGE.
Quantitative RT-PCR
Total RNA from MKs or platelets from 3 mice was pooled and extracted using TRIzol reagent (Invitrogen). Residual DNA was removed by treatment with 1U DNAse I (Ambion) for 30 minutes at 37°C. RNA (0.5 μg) was reverse-transcribed with SuperScript III (Invitrogen) using 500 ng of random hexamers. Real-time PCR was performed using SYBR Green Master Mix (Applied Biosystems) in a thermocycler workstation (ABI Prism 9700 Sequence Detection System; Applied Biosystems). Primer sequences are listed in Table 1. Each sample was run in duplicate and each experiment included 2 nontemplate control wells. Results are expressed as means ± SD.
Quantitative RT-PCR primer sequences
| Primer . | Forward sequence . | Reverse sequence . |
|---|---|---|
| α1 spectrin | AGAAATCCAACACCGAAGAGC | TCCAGGTCATCTGCGTCTCTC |
| β1 spectrin | CATCAGCGACCTCTACAAGGA | GAGCCCATGTTTTCCAGGTG |
| α2 spectrin | AGGGAGAACCTCCTAGAAGAGC | CTTCCCGGAACAACATGAACTT |
| β2 spectrin | ACACAGGAGACAAGTTCCGCTTCT | TCAACAGATGACACATCCCGTGGT |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Primer . | Forward sequence . | Reverse sequence . |
|---|---|---|
| α1 spectrin | AGAAATCCAACACCGAAGAGC | TCCAGGTCATCTGCGTCTCTC |
| β1 spectrin | CATCAGCGACCTCTACAAGGA | GAGCCCATGTTTTCCAGGTG |
| α2 spectrin | AGGGAGAACCTCCTAGAAGAGC | CTTCCCGGAACAACATGAACTT |
| β2 spectrin | ACACAGGAGACAAGTTCCGCTTCT | TCAACAGATGACACATCCCGTGGT |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Immunofluorescence microscopy of proplatelets and platelets
Immunofluorescence of MKs or purified platelets was carried out using methods described previously.21,22 For extraction of proplatelets, proplatelet-producing MKs were centrifuged onto poly-L-lysine–coated coverslips. Cells were treated with extraction buffer (PHEM buffer containing 0.1% Triton X-100, 2μM phallacidin, 20μM taxol, and protease inhibitors) for 2 minutes. Cells were then washed with PHEM buffer containing 0.1μM phallacidin and 20μM taxol, and then fixed in PHEM buffer containing 4% formaldehyde for 15 minutes. Cytoskeletons were washed briefly in PHEM buffer, incubated in blocking buffer, and immunostained. For antibody staining, cells were incubated in primary antibody diluted in blocking buffer for 1.5 hours at room temperature or overnight at 4°C, then washed in triplicate with PBS. Rabbit polyclonal anti-spectrin antibodies were used at 1:100 dilutions. All immunofluorescence and electron microscopy studies were evaluated by a blinded observer.
Expression of a dominant-negative spectrin construct in MKs
A cDNA encoding the NH2-terminal 145 amino acids of spα2N1 was originally cloned into BamHI and EcoRI sites of the pWZL retroviral vector containing sequences for green fluorescent protein (GFP) such that spα2N1 was cloned in-frame to the NH2 terminus of GFP. The construct was subcloned into a modified pMSCV retroviral vector using SpeI and XhoI sites. This resultant vector, along with the same vector expressing GFP alone, was used to generate retroviral supernatants. Cotransfection of the retroviral plasmid with plasmids encoding gag-pol and VsvG into 50% confluent 293T cells was performed through calcium phosphate or Fugene 6 (Roche Diagnostics). Six to 8 hours after transfection, the medium was changed; 48-60 hours later, the supernatant was collected, passed through a 0.45-μm syringe filter, aliquoted, and stored at −80°C. MKs were infected on day 1.5-2 of culture, as described previously.16 Infected MKs were identified by fluorescence microscopy based on the expression of GFP. The percentage of proplatelet-producing cells was measured by determining the number of MKs that exhibited at least a single proplatelet (defined as a pseudopodial extension with a teardrop-shaped tip extending from the MK cell body) from the population of GFP-positive cells. A minimum of 50 cells were counted in each of 3 independent experiments. Statistical analysis was done using a paired Student t test.
Thin-section electron microscopy
For correlative light and electron microscopy, we used coverslips with a finder grating that can be recognized by both light and electron microscopy. To prepare culture dishes for correlative microscopy, a circular 18-mm hole was drilled in the bottom of a 35-mm plastic culture dish and a 22 × 22 mm glass coverslip was mounted onto the bottom. The coverslip was previously coated with gold through a locater grid (400 mesh; Ted Pella) to create a visible pattern. After MKs were visualized by fluorescence and differential interference contrast (DIC) microscopy, they were fixed with 1.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, for 8 hours, and processed for electron microscopy. Cells were examined with a Tecnai G2 Spirit BioTWIN transmission electron microscope at an accelerating voltage of 80 kV, and images were recorded with an AMT 2k CCD camera. The internal reference marks on the coverslip facilitated relocalization of the same cells that were visualized by fluorescence and DIC microscopy. For immunogold electron microscopy of cryosections, MKs were processed, stained, and visualized as described previously.23
Introduction of spα2N1 and control peptides into permeabilized proplatelets
Proplatelets were placed in video chambers formed by mounting a glass coverslip coated with 3% BSA. Attached cells were washed with platelet buffer, and then permeabilized using 0.1 vol of n-octyl-b-glucopyranoside (OG; final concentration of 0.4%) in PEM buffer (60mM PIPES, 10mM EGTA, and 2mM MgCl2). Cells were washed with PEM buffer and incubated in PEM buffer containing either 10μM GST-spα2N1 or a GST control. Preparations were maintained at 37°C and examined on a Zeiss Axiovert 200 inverted microscope equipped with a 63× DIC objective (numerical aperture, 1.4). Images were obtained using a Hamamatsu CCD camera and frames were captured at 1-minute intervals. Videos were generated using the Metamorph image analysis program (Universal Imaging Corporation, Molecular Devices). To generate the recombinant spectrin fragment spα2N1, the cDNA encoding the N-terminal region of α2 spectrin comprising residues 1-154 was expressed and purified as described previously.24-26
Results
Structure of the proplatelet membrane skeleton and identification of spectrin as its major component
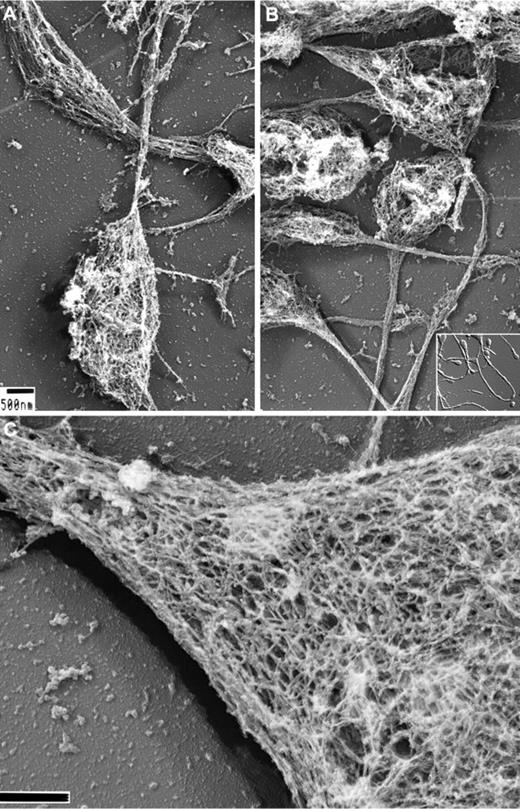
We have previously reported on the structure of the membrane skeleton of resting human platelets and how it is disassembled after treatment with agonists.3,27,28 To study the development of the membrane skeleton in MKs and proplatelets, in the present study, we exposed the cells to detergents in a stabilization buffer containing fixative. Figure 1 shows representative micrographs of Triton X-100–demembranated mouse proplatelets processed for electron microscopy by rapid-freezing, freeze-drying, and metal casting. Figure 1A-B shows that the proplatelet shafts and tips are composed of dense fibrous cytoskeletal networks of ∼100-nm × 5-10-nm strands. At high magnification and in stereo-paired images (Figure 1C), the membrane skeleton appears as a flat network that covers both the tops and bottoms of proplatelet shafts and ends. Quantification of the lattice structure by morphometry shows its pores to have average areas of 6033 + 2772 nm2 (n = 418 pores), similar in size to those found in the human platelet membrane skeleton and the mouse erythrocyte skeleton (supplemental Figure 1, available on the Blood Web site; see the supplemental Materials link at the top of the online article).3
Structure of the proplatelet membrane skeleton. (A-B) Representative electron micrographs of the detergent-insoluble proplatelet cytoskeleton. Proplatelets were permeabilized with 0.75% Triton X-100, 0.1% glutaraldehyde, and 5μM phallacidin in PHEM buffer. Examination of the proplatelet membrane skeleton through electron microscopy reveals an intact membrane skeleton that laminates the underside and extends along the entire length of proplatelets. Inset: DIC image of murine proplatelets. Scale bar indicates 500 nm. (C) High-magnification, 3D electron micrograph of the proplatelet membrane skeleton showing the lattice-like network of elongated filamentous strands, which is similar in nature to the spectrin-based meshwork in erythrocytes and platelets. The membrane skeleton continuously laminates the underside of the proplatelet. A cytoplasmic bridge is shown (left) connecting to a swelling (right). Scale bar indicates 200 nm.
Structure of the proplatelet membrane skeleton. (A-B) Representative electron micrographs of the detergent-insoluble proplatelet cytoskeleton. Proplatelets were permeabilized with 0.75% Triton X-100, 0.1% glutaraldehyde, and 5μM phallacidin in PHEM buffer. Examination of the proplatelet membrane skeleton through electron microscopy reveals an intact membrane skeleton that laminates the underside and extends along the entire length of proplatelets. Inset: DIC image of murine proplatelets. Scale bar indicates 500 nm. (C) High-magnification, 3D electron micrograph of the proplatelet membrane skeleton showing the lattice-like network of elongated filamentous strands, which is similar in nature to the spectrin-based meshwork in erythrocytes and platelets. The membrane skeleton continuously laminates the underside of the proplatelet. A cytoplasmic bridge is shown (left) connecting to a swelling (right). Scale bar indicates 200 nm.
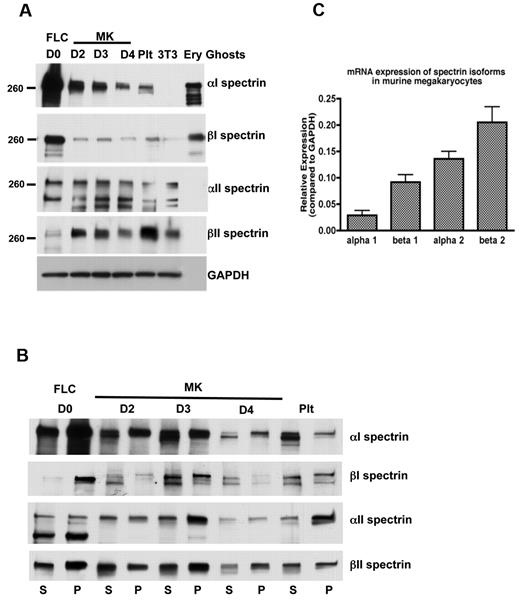
Elongated spectrin strands cross-linked by actin filaments form the platelet and erythrocyte membrane skeleton. To begin to understand the assembly of the membrane skeleton during megakaryocyte development and thrombopoiesis, we first examined spectrin isotype expression in cultured MKs. Figure 2A depicts immunoblots of MKs at different states of maturity (days in culture) and platelets stained for erythroid (α1 and β1) and nonerythroid spectrin (α2 and β2) isotypes. These cultures start with FLCs containing both MK and erythroid lineages. By day 2, the large size of maturing MKs allowed them to be enriched and collected by centrifugation. Proplatelet formation, the next event of consequence, began late on culture day 3. Figure 2A shows β2 spectrin to be highly expressed in MKs relative to the starting cell population, a finding confirmed by real-time PCR (Figure 2C). α2 spectrin is also strongly expressed (Figure 2A,C), although much of the native subunit was processed by proteolytic cleavage, as reported previously in other cells.29,30 Neither nonerythroid spectrin isoform antibody reacted against erythrocyte ghost proteins, indicating specificity for only the nonerythroid forms. Because both platelets and erythrocytes derive from a common myeloid progenitor cell, we also examined proplatelets and platelets for expression of the erythroid α1 and β1 spectrins. Both were abundantly expressed in the initial cell culture, but decreased dramatically once MKs were separated and enriched, although some expression of each remained as the megakaryocytes developed proplatelets and platelets. Both erythroid isoforms were also found in mouse platelets. Furthermore, anti-α1 immunoblots detected a strong band in platelet and MK lysates from wild-type mice that was not present in platelet lysates from mice that lacked α1 spectrin (supplemental Figure 2). These results indicate that MKs, proplatelets, and platelets contain a combination of erythroid and nonerythroid spectrins.
Spectrin isoforms in megakaryocytes and platelets. (A) Immunoblot showing the presence of erythroid and nonerythroid spectrin isoforms in MKs and platelet lysates. Isoform-specific antibodies were used to identify α1, β1, α2, and β2 spectrins in MKs at different stages of maturation and in platelets (Plt). Accordingly, α2 and β2 antibodies failed to recognize the erythroid spectrin isoforms in lysates of erythrocyte ghosts, whereas α1 and β1 antibodies identified the erythroid spectrin isoforms in the erythrocyte ghosts. Murine fibroblasts (3T3 Swiss) were used as a negative control for α1 and β1 spectrins. GAPDH was used as a loading control. Blots show anti-spectrin isoform labeling during different days of megakaryocyte culture: FLCs (day 0) and MKs at different stages: day 2, young MKs; day 3, MKs just before producing proplatelets; day 4, MKs after producing proplatelets. (B) Immunoblot analysis showing the distribution of spectrin isoforms in the pelleted (P) actin cytoskeleton and soluble (S) fractions of FLCs, MKs, and platelets. A higher fraction of αII and βII spectrin isoforms associated with the cytoskeletons in MKs just before making proplatelets (day 3), compared with other MK stages. Western blots from 3 different experiments were quantified by densitometry (supplemental Figure 6). (C) Quantitative PCR. The relative mRNA expression (compared with GAPDH) of spectrin isoforms in MKs determined by quantitative RT-PCR. Nonerythroid spectrins (α2 and β2 spectrins) were expressed at higher levels than erythroid isoforms (α1 and β1 spectrins) in MKs.
Spectrin isoforms in megakaryocytes and platelets. (A) Immunoblot showing the presence of erythroid and nonerythroid spectrin isoforms in MKs and platelet lysates. Isoform-specific antibodies were used to identify α1, β1, α2, and β2 spectrins in MKs at different stages of maturation and in platelets (Plt). Accordingly, α2 and β2 antibodies failed to recognize the erythroid spectrin isoforms in lysates of erythrocyte ghosts, whereas α1 and β1 antibodies identified the erythroid spectrin isoforms in the erythrocyte ghosts. Murine fibroblasts (3T3 Swiss) were used as a negative control for α1 and β1 spectrins. GAPDH was used as a loading control. Blots show anti-spectrin isoform labeling during different days of megakaryocyte culture: FLCs (day 0) and MKs at different stages: day 2, young MKs; day 3, MKs just before producing proplatelets; day 4, MKs after producing proplatelets. (B) Immunoblot analysis showing the distribution of spectrin isoforms in the pelleted (P) actin cytoskeleton and soluble (S) fractions of FLCs, MKs, and platelets. A higher fraction of αII and βII spectrin isoforms associated with the cytoskeletons in MKs just before making proplatelets (day 3), compared with other MK stages. Western blots from 3 different experiments were quantified by densitometry (supplemental Figure 6). (C) Quantitative PCR. The relative mRNA expression (compared with GAPDH) of spectrin isoforms in MKs determined by quantitative RT-PCR. Nonerythroid spectrins (α2 and β2 spectrins) were expressed at higher levels than erythroid isoforms (α1 and β1 spectrins) in MKs.
Spectrin isoforms have distinct distributions during platelet formation
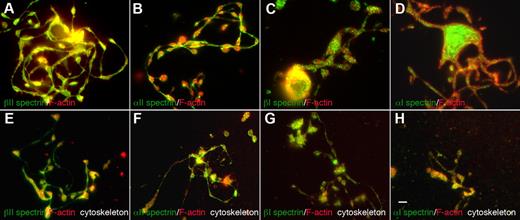
The erythroid and nonerythroid spectrins have different spatial distributions within proplatelets (Figure 3). Both anti-α2 and anti-β2 spectrin antibodies labeled proplatelets and proplatelet cytoskeletons uniformly along their length, including shafts, swellings, and tips, but were excluded from F-actin–enriched protrusions such as filopods and blebs. F-actin was more enriched in proplatelet swellings than in shafts. Anti-α1 and β1 spectrin antibodies, on the other hand, decorated proplatelets in a punctate, speckled pattern, suggestive of vesicular structures (Figure 3A-D). All spectrin isoform–specific antibodies also labeled mature platelets. α2 and β2 anti-spectrin antibodies labeled the platelet cortex and the punctate structures inside them, as reported previously (supplemental Figure 3).27 α1 and β1 spectrin labeling in platelets was also punctate, as in proplatelets (supplemental Figure 3). Therefore, erythroid and nonerythroid spectrins reside in different spatial locations in proplatelets and platelets.
Localization of spectrin isoforms within proplatelets. Micrographs of immunofluorescence studies performed with spectrin antibodies reveal differential localizations for spectrin isoforms within proplatelets. The top panels (A-D) show proplatelet-producing MKs that were fixed before staining. The bottom panels (E-H) denoted “cytoskeleton,” show proplatelets permeabilized in 0.1% Triton-X 100 before fixation to remove soluble and membrane-associated structures. All micrographs are merged images of cells that were double-labeled with spectrin isoform antibodies (green) and F-actin staining by phalloidin (red). The isotype probed and the fluorophore of the secondary antibody are indicated in each panel. (A-D) α1 and β1 spectrins decorate punctate spots distributed throughout the proplatelets. α2 and β2 spectrins localized strongly along proplatelet shafts, proplatelet tips, and swellings. Both α2 and β2 spectrin isoforms colocalized with F-actin. All 4 spectrin isoforms tested were retained in the cytoskeleton of permeabilized cells (E-H) and displayed a similar localization pattern to nonextracted cells. Spectrin 1 isoforms stained in a punctate pattern throughout the proplatelet skeleton, whereas spectrin 2 isoforms displayed a more cytoskeletal localization in permeabilized cells. Scale bar indicates 5 μm.
Localization of spectrin isoforms within proplatelets. Micrographs of immunofluorescence studies performed with spectrin antibodies reveal differential localizations for spectrin isoforms within proplatelets. The top panels (A-D) show proplatelet-producing MKs that were fixed before staining. The bottom panels (E-H) denoted “cytoskeleton,” show proplatelets permeabilized in 0.1% Triton-X 100 before fixation to remove soluble and membrane-associated structures. All micrographs are merged images of cells that were double-labeled with spectrin isoform antibodies (green) and F-actin staining by phalloidin (red). The isotype probed and the fluorophore of the secondary antibody are indicated in each panel. (A-D) α1 and β1 spectrins decorate punctate spots distributed throughout the proplatelets. α2 and β2 spectrins localized strongly along proplatelet shafts, proplatelet tips, and swellings. Both α2 and β2 spectrin isoforms colocalized with F-actin. All 4 spectrin isoforms tested were retained in the cytoskeleton of permeabilized cells (E-H) and displayed a similar localization pattern to nonextracted cells. Spectrin 1 isoforms stained in a punctate pattern throughout the proplatelet skeleton, whereas spectrin 2 isoforms displayed a more cytoskeletal localization in permeabilized cells. Scale bar indicates 5 μm.
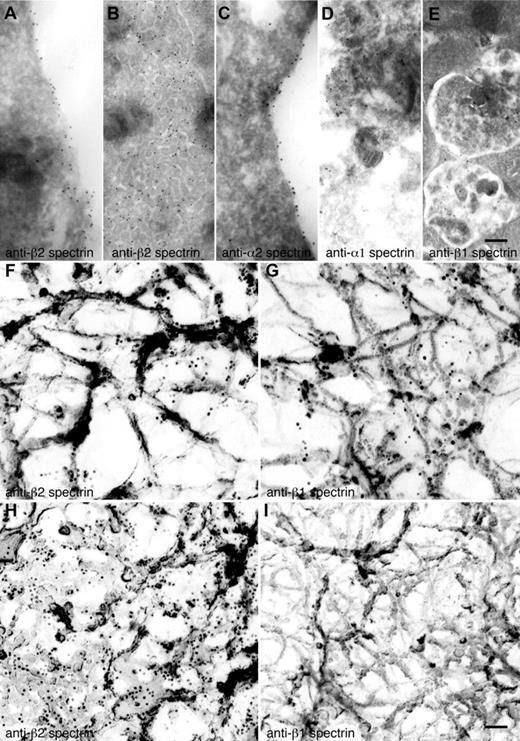
The spectrin isotype staining patterns observed by immunofluorescence microscopy were confirmed by immunogold electron microscopy. Gold particles indicative of anti-α2 and anti-β2 spectrin antibodies preferentially decorate the most cortical regions of MKs and proplatelets (Figure 4A,C) and the IMS (Figure 4B), suggestive of a role for spectrin in organizing and remodeling the IMS. Antibodies against α1 and β1 spectrin were found on internal vesicular structures in MKs and proplatelets (Figure 4D-E).
Localization of spectrin isoforms at high resolution. (A-E) Localization of erythroid and nonerythroid spectrin isoforms in ultrathin sections of mouse MKs. Immunogold labeling of sections was performed with anti–β2 spectrin (A,B), anti–α2 spectrin (C), anti–α1 spectrin (D), and anti–β1 spectrin (E) antibodies. Gold particles (10-nm) recognizing anti–β2 spectrin (A-B) are evident on the plasma membrane and invaginated membranes of MKs. Gold particles recognizing anti–α2 spectrin (C) are also found on MK membranes. Gold particles recognizing anti–α1 spectrin (D) and anti–β1 spectrin (E) stained multivesicular bodies of MKs. Scale bar represents 200 nm. (F-I) Localization of spectrin isoforms in the detergent-insoluble cytoskeletons of proplatelets and platelets. Immunoelectron microscopic studies were used to localize individual spectrin isoforms in the membrane skeletons of proplatelets (F-G) and platelets (H-I). Preparations were incubated with affinity-purified anti-β2 (F,H) and anti–β1 spectrin (G,I) antibodies, followed by 10-nm gold particles coated with secondary antibodies. Scale bar shown in panel I indicates 100 nm. Proplatelets labeled with anti–β2 spectrin (F) and anti–β1 spectrin (G) have similar staining patterns, labeling the strands composing the membrane skeleton. Human platelet cytoskeletons were labeled with anti–β2 spectrin (H) and anti–β1 spectrin (I). β2 spectrin gold labeling was increased, whereas β1 was decreased in the platelet cytoskeleton. The gold particle density per square micrometer of cytoskeleton preparation was 53 ± 10 for anti–β2 spectrin of proplatelets (F), 69 ± 8 for anti–β1 spectrin of proplatelets (G) 232 ± 25 for anti–β2 spectrin of platelets (H), and 4 ± 2 for anti–β1 spectrin of platelets. Data are provided as means ± SE (n = 20). Experiments were carried out in triplicate. Scale bar represents 100 nm.
Localization of spectrin isoforms at high resolution. (A-E) Localization of erythroid and nonerythroid spectrin isoforms in ultrathin sections of mouse MKs. Immunogold labeling of sections was performed with anti–β2 spectrin (A,B), anti–α2 spectrin (C), anti–α1 spectrin (D), and anti–β1 spectrin (E) antibodies. Gold particles (10-nm) recognizing anti–β2 spectrin (A-B) are evident on the plasma membrane and invaginated membranes of MKs. Gold particles recognizing anti–α2 spectrin (C) are also found on MK membranes. Gold particles recognizing anti–α1 spectrin (D) and anti–β1 spectrin (E) stained multivesicular bodies of MKs. Scale bar represents 200 nm. (F-I) Localization of spectrin isoforms in the detergent-insoluble cytoskeletons of proplatelets and platelets. Immunoelectron microscopic studies were used to localize individual spectrin isoforms in the membrane skeletons of proplatelets (F-G) and platelets (H-I). Preparations were incubated with affinity-purified anti-β2 (F,H) and anti–β1 spectrin (G,I) antibodies, followed by 10-nm gold particles coated with secondary antibodies. Scale bar shown in panel I indicates 100 nm. Proplatelets labeled with anti–β2 spectrin (F) and anti–β1 spectrin (G) have similar staining patterns, labeling the strands composing the membrane skeleton. Human platelet cytoskeletons were labeled with anti–β2 spectrin (H) and anti–β1 spectrin (I). β2 spectrin gold labeling was increased, whereas β1 was decreased in the platelet cytoskeleton. The gold particle density per square micrometer of cytoskeleton preparation was 53 ± 10 for anti–β2 spectrin of proplatelets (F), 69 ± 8 for anti–β1 spectrin of proplatelets (G) 232 ± 25 for anti–β2 spectrin of platelets (H), and 4 ± 2 for anti–β1 spectrin of platelets. Data are provided as means ± SE (n = 20). Experiments were carried out in triplicate. Scale bar represents 100 nm.
Given the dynamics of proplatelets, we sought to investigate the nature of the molecular linkages of proteins integrated into the proplatelet membrane skeleton. We examined the localization and distribution of spectrin isoforms between cytoskeletal and soluble cellular fractions. Proplatelets attached to glass coverslips were permeabilized with 0.1% Triton-X 100 to remove the plasma membrane and membrane-bound organelles from the cells. The proplatelets were then washed, fixed, and stained for immunofluorescence microscopy. All 4 spectrin isoforms were retained in the cytoskeleton and localized as described above in fixed cells (Figure 3E-H). α1 and β1 spectrins had punctate appearances in detergent-extracted cells (Figure 3G-H). In contrast, α2 and β2 spectrin isoforms retained their uniform cytoskeletal distribution and colocalized with F-actin (Figure 3E-F). Biochemical evidence also indicates that spectrin isoforms are associated with the cytoskeleton of MKs and platelets. When platelets and MKs permeabilized with Triton X-100 were centrifuged at high centrifugal forces (> 100 000 g), proteins associated with F-actin pellet. As shown in Figure 2B, all 4 spectrin isoforms sedimented in pellets in detergent-lysed MKs and platelets. These results indicate that the spectrin isoforms accumulating within proplatelets were stably linked to the membrane skeleton.
To look further into the roles of the erythroid versus nonerythroid spectrins, we labeled proplatelet and platelet cytoskeletons with anti-β chain antibodies followed by immunogold electron microscopy. Anti-β2 spectrin immunogold labeled the strands composing the planar network component of the cytoskeleton of proplatelets (Figure 4F) and platelets (Figure 4H), although anti-β2 labeling of platelets was more robust than that found in proplatelets, suggesting increased expression and incorporation of β2 in the mature membrane skeleton of the platelet. Anti-β1 immunogold also labeled along the fine strands that compose the proplatelet membrane skeleton (Figure 4G), but did so considerably less well than anti-β2 immunogold. In marked contrast to β2 labeling, β1 did not label the platelet cytoskeleton (Figure 4I), which is indicative of a switch from β1 to β2 in the mature platelet. Labeling was specific for anti-spectrin antibodies; removal of anti-spectrin antibody during the labeling procedure resulted in the absence of gold labeling of membrane skeletal components (data not shown).
Proper assembly of the spectrin-based membrane skeleton is required for proplatelet formation
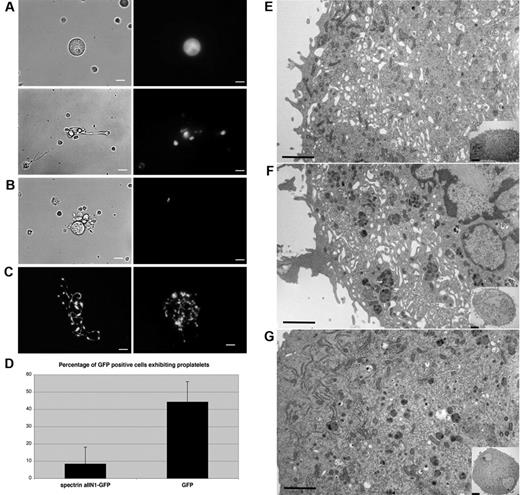
Because spectrin is a prominent component of the proplatelet membrane skeleton, we investigated whether it is required for proplatelet elaboration from MKs. To disrupt spectrin function, we expressed the dominant-negative construct spα2N1 in MKs. This construct is composed of the NH2-terminal 154 residues of the α2 spectrin chain, and is known to exchange into the heterodimer self-association site to dissociate tetramers into dimers in red blood cell ghosts to markedly decrease the mechanical stability of the red blood cell membrane.24-26 Spα2N1 was fused to GFP to facilitate its identification in retrovirally infected megakaryocytes. GFP-positive cells were imaged using fluorescence optics, and the number of MKs producing proplatelets was quantified and compared with cells expressing only the GFP tag. Most spα2N1-GFP–expressing MKs failed to generate proplatelets (Figure 5A top), in marked contrast to the GFP-expressing control cells, in which proplatelet production was not impeded (Figure 5C). Rare proplatelets observed on the spα2N1-GFP–expressing cells were short and poorly branched (compare Figure 5A and C). Quantification revealed that only 8% of spα2N1-GFP–expressing MKs constructed proplatelets, compared with 44% in cells expressing GFP alone (Figure 5D). Infected cells increased in size and ploidy during the culture period, excluding growth inhibition as a basis for the arrested proplatelet development. These results indicate that spectrin tetramer assembly is necessary for proplatelet formation.
Expression of spα2N1 inhibits proplatelet elaboration by MKs and prevents IMS maturation. (A) The introduction of spα2N1-GFP in MKs through retroviral infection inhibited proplatelet formation. Most MKs expressing spα2N1-GFP failed to make proplatelets (A top panel), although a few developed primitive proplatelets (A bottom panel). Left panels show phase contrast images and right panels show fluorescence images. (B) Control uninfected MKs, identified by lack of green fluorescence, form normal proplatelets. (C) Fluorescence images of proplatelet formation by control MKs expressing GFP alone. Scale bars indicate 7.5 μm. (D) Quantitative analysis of proplatelet formation in spα2N1-GFP–expressing and control, GFP-expressing MKs. spα2N1-GFP–expressing MKs show a dramatic reduction in the percentage of proplatelets formed (8% compared with 44% in control cells). Bars represent the standard deviations. (E-G) Representative electron micrographs of a noninfected MK (E), a MK expressing GFP alone (F), and a MK expressing spα2N1-GFP (G). Control MKs (E-F) show an extensive, open IMS that fills the cell cytoplasm, whereas spα2N1-GFP–expressing MKs (G) do not. Insets show low-magnification views of the corresponding cells. Scale bars indicate 4μm.
Expression of spα2N1 inhibits proplatelet elaboration by MKs and prevents IMS maturation. (A) The introduction of spα2N1-GFP in MKs through retroviral infection inhibited proplatelet formation. Most MKs expressing spα2N1-GFP failed to make proplatelets (A top panel), although a few developed primitive proplatelets (A bottom panel). Left panels show phase contrast images and right panels show fluorescence images. (B) Control uninfected MKs, identified by lack of green fluorescence, form normal proplatelets. (C) Fluorescence images of proplatelet formation by control MKs expressing GFP alone. Scale bars indicate 7.5 μm. (D) Quantitative analysis of proplatelet formation in spα2N1-GFP–expressing and control, GFP-expressing MKs. spα2N1-GFP–expressing MKs show a dramatic reduction in the percentage of proplatelets formed (8% compared with 44% in control cells). Bars represent the standard deviations. (E-G) Representative electron micrographs of a noninfected MK (E), a MK expressing GFP alone (F), and a MK expressing spα2N1-GFP (G). Control MKs (E-F) show an extensive, open IMS that fills the cell cytoplasm, whereas spα2N1-GFP–expressing MKs (G) do not. Insets show low-magnification views of the corresponding cells. Scale bars indicate 4μm.
Disruption of spectrin tetramers severely diminishes the maturation of the IMS
To further explore how the disruption of spectrin tetramers affects proplatelet production, spα2N1-GFP–expressing MKs were studied in the electron microscope. Uninfected MKs (Figure 5E) or those expressing GFP alone (Figure 5F) developed an extensive, open IMS that penetrated the entire cytoplasm. Although MKs expressing spα2N1-GFP experienced cytoplasmic expansion and contained normal numbers and distribution of granules, they displayed a clear defect in the maturation of the IMS (Figure 5G). These cells lacked the high degree of IMS invagination and convolution found in control cells: 88% of the control cells displayed a well-developed IMS, whereas only 3% of the cells expressing spα2N1-GFP displayed an IMS. Image analysis was used to measure the percent area of the IMS and to determine the amount of internal membrane. The internal membranes were found to be 3.8 times smaller by area in spα2N1-expressing cells compared with control cells (supplemental Figure 4). These results indicate that spectrin tetramer assembly is required to form a normal IMS, a conclusion supported by our observation that spectrin associates with the DMS.
Membrane skeletal integrity stabilizes proplatelet architecture
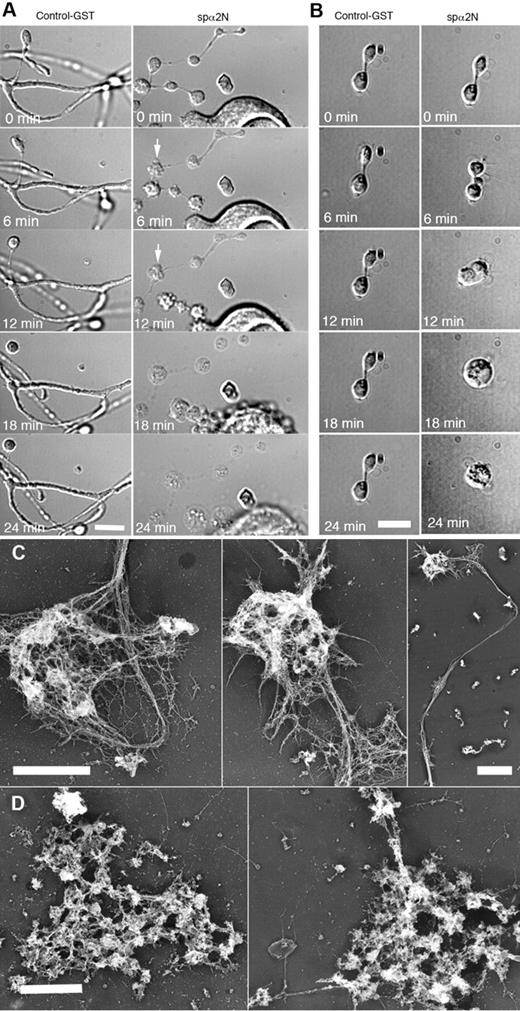
Because MK expression of spα2N1 prevented proplatelet elaboration, we were unable to study the role of the spectrin membrane skeleton in proplatelet morphogenesis. To circumvent this limitation, MKs were treated with 0.4% of the detergent OG in PHEM buffer, an approach that delivers the impermeant spα2N1 construct into the cytoplasm.21 Treatment with 0.4% OG did not alter the general shape and structure of proplatelets, but did generate small holes 20-100 nm in diameter at the edge of proplatelets (supplemental Figure 5). Platelet-sized swellings, cytoplasmic bridges, and branches were maintained for > 1 hour after OG treatments (data not shown). Permeabilized proplatelets treated with a control GST peptide maintained their overall structure, having clearly defined swellings, bulbous tips, cytoplasmic bridges, and branches (Figure 6A left panel and supplemental Video 1). However, in 11 independent experiments, delivery of the spα2N1 peptide into proplatelets grossly disrupted their morphology, initially causing blebs to appear on the proplatelets, followed by a massive swelling of their overall structure by ∼ 18 minutes (Figure 6A right panel). Time-lapse video microscopy reveals the details of this disruptive process (supplemental Video 2). Treatment of unpermeabilized proplatelets with spα2N1 had no effect on proplatelet structure (data not shown). Disruption of the membrane skeleton affected all stages of proplatelet development, including the final step of platelet production, when preplatelets, anucleate discoid particles 3-10 μm in diameter, convert to barbell-shaped structures that undergo fission to generate 2 platelets.31 Figure 6B shows the effect of spα2N1 on this process. Permeabilization alone or permeabilization plus treatment with a control GST-tagged polypeptide did not alter the barbell morphology (Figure 6B left panel and supplemental Video 3). After the addition of the spα2N1 peptide, blebs appeared at multiple points, including the cytoplasmic bridge, before the structure swelled into a spherical shape (Figure 6B right panel and supplemental Video 4). The effect of OG and spα2N1 peptide treatments on the cytoskeleton of proplatelets was further evaluated by electron microscopy. Figure 6C shows that the cytoskeleton of OG-permeabilized proplatelets remained intact when exposed to a GST control peptide. However, spα2N1 exposure caused disruption and a massive aggregation of cytoskeletal elements (Figure 6D). Therefore, the spectrin-based membrane skeleton is crucial for stabilizing the extended structure of proplatelets and for preplatelet fission events.
Effect of spα2N1 on proplatelets. (A-B) Time-lapse DIC micrographs of permeabilized (0.4% OG) proplatelets treated with GST control polypeptide (left panels) and the spectrin-disrupting polypeptide spα2N1 (right panels) show that proplatelets treated with control GST maintain their “beads-on-a-string” structure and branches. In contrast, platelet-sized beads on proplatelets treated with spα2N1 blebbed and then underwent extensive swelling. After treatment with spα2N1, barbell-shaped proplatelets first blebbed and then fused their 2-platelet-sized swellings, forming a preplatelet-sized spheroid. (C-D) Electron micrographs of representative cytoskeletons from permeabilized proplatelets treated with either GST-control (C) or spα2N1 peptide (D). Scale bars indicate 5 μm. The cytoskeletons of GST-treated cells remain intact, whereas the cytoskeletons of spα2N1-treated cells are disrupted and aggregated.
Effect of spα2N1 on proplatelets. (A-B) Time-lapse DIC micrographs of permeabilized (0.4% OG) proplatelets treated with GST control polypeptide (left panels) and the spectrin-disrupting polypeptide spα2N1 (right panels) show that proplatelets treated with control GST maintain their “beads-on-a-string” structure and branches. In contrast, platelet-sized beads on proplatelets treated with spα2N1 blebbed and then underwent extensive swelling. After treatment with spα2N1, barbell-shaped proplatelets first blebbed and then fused their 2-platelet-sized swellings, forming a preplatelet-sized spheroid. (C-D) Electron micrographs of representative cytoskeletons from permeabilized proplatelets treated with either GST-control (C) or spα2N1 peptide (D). Scale bars indicate 5 μm. The cytoskeletons of GST-treated cells remain intact, whereas the cytoskeletons of spα2N1-treated cells are disrupted and aggregated.
Discussion
To assemble and release platelets, MKs follow a maturation program that converts the bulk of their cytoplasm into multiple long proplatelets. Central in this morphogenesis is the remodeling and evagination of MK membranes to form large pseudopodia that subsequently elongate, thin, bend, and branch. These proplatelet extensions can detach from the MK body and generate platelet-size particles at their ends that are linked by thin cytoplasmic bridges. Remodeling of the microtubule and actin cytoskeletons drives these shape changes. In the present study, we investigated the role of the spectrin cytoskeleton in this morphogenetic process. We first characterized the spectrin-based membrane skeleton of MKs and proplatelets. Both erythroid and nonerythroid spectrin assembled into a 2D lattice that underlay and stabilized the IMS in MKs, and the MK and proplatelet plasma membranes. Second, we demonstrated that the spectrin membrane skeleton is critical in establishing and maintaining proplatelets during platelet biogenesis.
Both erythroid and nonerythroid spectrin isoforms accumulate in MKs and platelets. To date, α1 spectrin has remained unique to erythrocytes, whereas β1 spectrin variants have been found in muscle and in certain subsets of neurons.32-34 α2 and β2 spectrins, which are absent from the erythrocyte membrane skeleton, are the major spectrin isoforms of nonerythroid cells and are abundantly expressed in MKs and platelets.3 Maintenance of erythroid spectrins in MKs and platelets is therefore surprising. In erythrocytes, the affinity of α1-β1 tetramerization site confers a dynamic element to the membrane skeleton, because membranes are subjected to shear forces in the blood. In contrast, the high affinity of α2-β2 tetramerization in nonerythroid cells promotes stable complexes. It is tempting to speculate that the mixture of erythroid and nonerythroid spectrins in MKs and proplatelets contributes to the membrane elasticity required for proplatelet production.
The distinct localizations of the different spectrins within MKs suggest unique roles in platelet formation. The erythroid isoforms exhibit a more punctate localization in proplatelets and MKs, whereas the nonerythroid isoforms have a cytoskeletal localization that is enriched with F-actin at the cell periphery. In immunoelectron studies, both erythroid (β1) and nonerythroid (β2) spectrin antibodies colabeled the proplatelet membrane skeleton. β1 spectrin antibody labeling of the platelet membrane skeleton, however, was reduced compared with proplatelets, whereas the labeling of β2 spectrin was robust. Given these results, we favor a model in which both pairs of spectrin isoforms incorporate into the MK and proplatelet membrane skeleton, resulting, at least temporally, in a hybrid α1β1-α2β2 spectrin skeleton. As the maturation process continues, nonerythroid spectrin is likely to assume the dominant role in stabilizing the plasma membrane of platelets. The reduction of erythroid spectrin staining in mature platelets versus proplatelets suggests that spectrin isoform interactions and dynamics are important in forming platelets, but that once platelets are released, nonerythroid spectrins are sufficient to maintain their structure in circulation. In addition to the spectrin isoforms examined in these studies, 3 other β spectrin variants (βIII, βIV, and βV) have been identified in mice and humans. Whether these isoforms are expressed within MKs and platelets and their functions in these cells remain to be determined.
Our data indicate that not only is spectrin expressed in proplatelets, but its assembly into tetramers is also required for proplatelet elaboration. Proplatelet production was markedly reduced when MKs expressed the dominant-negative spectrin peptide fused to GFP (spα2N1-GFP). spα2N1-GFP–expressing MKs had > 5-fold fewer proplatelets compared with control cells, and the few residual proplatelets found on these MKs were short and unbranched. Spα2N1 consists of the N-terminal 154 amino acids of α2 spectrin, including the spectrin self-association domain.24,35 This region of α spectrin generates the head-to-head interaction between αβ spectrin dimers that form tetramers.36 During tetramer formation, the N-terminal end of α spectrin forms a helix that interacts with 2 helices from the C-terminus of β spectrin, resulting in a triple helix that links the dimers. The spα2N1-GFP polypeptide binds to β spectrin, preventing tetramer formation.
Proplatelet and therefore platelet formation is dependent on the generation of a large membrane reservoir connected to the plasma membrane (the IMS) in MKs.11 Despite the importance of the IMS in MK development, very little is known about the mechanisms that control its assembly and reorganization during platelet production. Initially, it was thought that the DMS defined platelet units within the MK, which were released after a generalized fragmentation of MKs along DMS fracture lines. Recent studies, however, indicate that this IMS is more likely a membrane reservoir that regurgitates to cover the surface of the many proplatelets extended by a single MK.12,13 Platelet formation was severely restricted in MKs expressing spα2N1-GFP. Expression of spα2N1-GFP in MKs blunted IMS development, suggesting that the membrane skeleton is intimately involved in either the assembly or stabilization of this extensive and highly dynamic membrane system. Therefore, disruption of spectrin tetramers results in an underdeveloped IMS with insufficient membrane to form proplatelets. A link between an underdeveloped IMS and platelet production has also been suggested in studies of the morphology of MKs from genetically engineered mouse strains such as NF-E2–deficient, GATA1-deficient, and GPIbα-deficient mice.37-40
In addition to contributing to the formation and stabilization of the IMS, the membrane skeleton acquires additional roles during the later stages of thrombopoiesis. MKs and proplatelets have a 2D membrane skeletal lattice composed of spectrin strands that localizes just beneath the plasma membrane and appears to be continuous from the MK cell body throughout the proplatelet length.2,3 Proplatelet elongation from MKs occurs at a rate of approximately 1μm/min primarily using a cytoplasmic dynein-driven microtubule-sliding mechanism.41 Given this speed of elongation and the finding that the membrane skeleton is intact along proplatelets, we favor a model that first coats the IMS membranes and then flows outward as the proplatelets extend. To assess spectrin function in proplatelets, we treated OG-permeabilized proplatelets with spα2N1, which resulted in rapid and striking blebbing, followed by swelling and rounding of proplatelet processes. It has been demonstrated previously that this fragment of α2 spectrin interacts with α1β1 heterodimers with high affinity, disrupting tetramerization and destabilizing the erythrocyte membrane.24,25 Whether this disruptive interaction occurs only with the erythroid spectrins in platelets, or if it also occurs with the nonerythroid spectrins, remains to be determined.
Our findings also suggest an essential function for spectrin in the final stages of platelet production.31 We recently identified a new intermediate stage in platelet production called the preplatelet. Preplatelets, which are abundantly present in megakaryocyte cultures, are anucleate discoid particles 3-10 μm across that convert reversibly into barbell structures by twisting, microtubule-based forces. Barbell proplatelets undergo fission to release 2 or more individual platelets from their ends. The addition of spα2N1 to permeabilized barbell-shaped proplatelets converts them into spheres, suggesting that the spectrin undercoat stabilizes the cytoplasmic bridge between 2 platelet-sized ends.
In conclusion, our analysis now allows us to incorporate all 3 cytoskeletal systems into a model for platelet formation (Figure 7). Our collective findings bring forth a new role for spectrin in thrombopoiesis. We found a continuous and homogeneous, spectrin-based membrane skeleton spanning from the MK cell body to the nascent platelets assembled within proplatelet tips. Our data also strongly suggest that spectrin assembly is a critical factor in platelet formation, most likely due to the association, reorganization, and stabilization of IMS membranes fated for proplatelets. Although these findings address certain questions regarding the role of spectrin during platelet formation, they also lead to further inquiry, including examination of the precise structure and function of platelets in the face of spectrin defects and insufficiencies. Mutations in spectrins result in hemolytic anemias, including hereditary spherocytosis, hereditary elliptocytosis, and hereditary pyropoikilocytosis.32,42 Defects in erythrocyte form and fragility are common to these hemolytic anemias. In mouse models of spectrin deficiencies, indications of effects that may be tied to abnormal platelet function also exist, including stroke and thrombus formation.43,44 Careful examination of these events may lead to further insights into spectrin function in platelets.
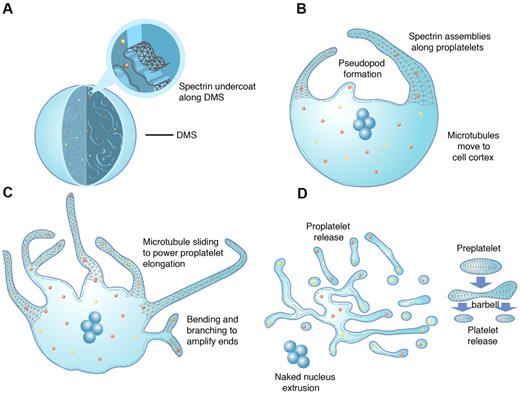
Model of platelet production as suggested by present data and previous studies. As MKs transition from immature cells (A) to released platelets (D), a systematic series of events occurs. (A) MKs develop a highly IMS as they mature. Assembly of the spectrin-based membrane skeleton is involved in the formation of the IMS, providing a membrane reservoir for future formation of proplatelets. (B) Proplatelet production begins with the extension of large pseudopodia that use unique cortical bundles of microtubules to elongate and form thin proplatelet processes with bulbous ends. Proplatelet membranes are lined with a spectrin undercoat. Proplatelet termini contain a bundle of microtubules that loop on themselves. (C) Proplatelet elongation requires the sliding of microtubules past one another, driven by the molecular motor cytoplasmic dynein. As proplatelets elongate, expansion of the membrane surface area requires the outflow of the IMS, a process that likely requires remodeling of the membrane skeleton. Microtubules function as the highways on which mitochondria and granules traffic to the tips of proplatelets. Actin promotes the branching and amplification of proplatelet tips, representing a mechanism to increase the numbers of proplatelet ends, and ultimately, platelets. (D) The entire MK cytoplasm is converted into a mass of proplatelets and preplatelets (anucleate discoid particles 2-10 μm across), which are released from the cell. Preplatelets reversibly convert into barbell proplatelets, a process that is driven by twisting microtubule-based forces. The membrane skeleton stabilizes this barbell form. Platelets release from proplatelet ends after the final fission event. The nucleus is eventually extruded from the mass of proplatelets.
Model of platelet production as suggested by present data and previous studies. As MKs transition from immature cells (A) to released platelets (D), a systematic series of events occurs. (A) MKs develop a highly IMS as they mature. Assembly of the spectrin-based membrane skeleton is involved in the formation of the IMS, providing a membrane reservoir for future formation of proplatelets. (B) Proplatelet production begins with the extension of large pseudopodia that use unique cortical bundles of microtubules to elongate and form thin proplatelet processes with bulbous ends. Proplatelet membranes are lined with a spectrin undercoat. Proplatelet termini contain a bundle of microtubules that loop on themselves. (C) Proplatelet elongation requires the sliding of microtubules past one another, driven by the molecular motor cytoplasmic dynein. As proplatelets elongate, expansion of the membrane surface area requires the outflow of the IMS, a process that likely requires remodeling of the membrane skeleton. Microtubules function as the highways on which mitochondria and granules traffic to the tips of proplatelets. Actin promotes the branching and amplification of proplatelet tips, representing a mechanism to increase the numbers of proplatelet ends, and ultimately, platelets. (D) The entire MK cytoplasm is converted into a mass of proplatelets and preplatelets (anucleate discoid particles 2-10 μm across), which are released from the cell. Preplatelets reversibly convert into barbell proplatelets, a process that is driven by twisting microtubule-based forces. The membrane skeleton stabilizes this barbell form. Platelets release from proplatelet ends after the final fission event. The nucleus is eventually extruded from the mass of proplatelets.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Erik Hett for critical review of the manuscript, Jon Morrow for spectrin antibodies, and Gretchen Jones and Jason Barnett for help in designing the model of platelet production.
This study was supported by National Institutes of Health grant HL068130 to J.E.I. and HL56949 to J.H.H. J.E.I. is an American Society of Hematology Junior Scholar. N.J.W. was supported by American Heart Association grant 0530073N and by the Midwest Athletes Against Childhood Cancer (MACC) Fund.
National Institutes of Health
Authorship
Contribution: S.P.-H., H.W., A.J.B., and J.N.T. designed, performed, and analyzed experiments and results; E.C.A. designed and performed experiments and assisted with manuscript preparation; N.J.W. assisted with experimental design, sample preparation, and analysis; X.A. and N.M. provided reagents and assisted with experimental design and interpretation; J.H.H. designed experiments, interpreted results, and assisted with manuscript preparation and editing; and J.E.I. designed experiments, interpreted results, formulated discussions, and assisted in manuscript preparation and editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph E. Italiano, Jr, Hematology Division, Brigham & Women's Hospital, 1 Blackfan Circle, Karp Bldg, 6th Fl, Rm 214, Boston, MA 02115; e-mail: jitaliano@rics.bwh.harvard.edu.