Abstract
Treatment of chronic myeloid leukemia (CML) with the tyrosine kinase inhibitors (TKIs) imatinib mesylate and nilotinib represents a successful application of molecularly targeted anticancer therapy. However, the effect of TKIs on leukemic stem cells remains incompletely understood. On the basis of a statistical modeling approach that used the 10-year imatinib mesylate treatment response of patients with CML and a patient cohort receiving first-line nilotinib therapy, we found that successful long-term therapy results in a triphasic exponential decline of BCR-ABL1 transcripts in many patients. Within our framework, the first slope of −0.052 ± 0.018 (imatinib mesylate) and −0.042 ± 0.015 (nilotinib) per day represents the turnover rate of leukemic differentiated cells, whereas the second slope of −0.0057 ± 0.0038 (imatinib mesylate) and −0.0019 ± 0.0013 (nilotinib) per day represents the turnover rate of leukemic progenitor cells. The third slope allows an inference of the behavior of immature leukemic cells, potentially stem cells. This third slope is negative in most patients, positive in others, and not observable in some patients. This variability in response may be because of insufficient follow-up, missing data, disease heterogeneity, inconsistent compliance to drug, or acquired resistance. Our approach suggests that long-term TKI therapy may reduce the abundance of leukemic stem cells in some patients.
Introduction
Chronic myeloid leukemia (CML) is the first hematologic malignancy treated with a molecularly targeted small molecule inhibitor, the tyrosine kinase inhibitor (TKI) imatinib mesylate1 (Gleevec). This agent induces clinical, cytogenetic, and molecular remission and prolongs progression-free survival.2,3 The phase 3 multicenter International Randomized Study of Interferon versus STI-571 (IRIS) trial reported the superiority of imatinib mesylate over IFN-α plus cytosine arabinoside in 1106 previously untreated chronic-phase patients. Five years after the initiation of imatinib mesylate therapy, 40% of chronic-phase patients achieved a complete molecular response,4 and estimated overall survival was 89% at 5 years and 85% at 8 years.5 Recently, trials that used the second-generation TKIs nilotinib and dasatinib as first-line therapy were initiated and showed promising results.6,7 However, the question remains whether leukemic stem cells are sensitive to TKI therapy and whether this treatment represents a cure of the disease.8,9
To study the dynamics of the response to imatinib mesylate treatment, we had previously analyzed data from the IRIS trial as well as a phase 2 trial (Therapeutic Intensification in De novo Leukemia [TIDEL]10 ) conducted by the Australasian Leukemia and Lymphoma Group. The TIDEL trial enrolled patients with newly diagnosed chronic-phase CML and used 600 mg of imatinib mesylate per day initially, increasing to 800 mg if specified response criteria were not met. Based on the 12 months of follow-up data of a subset of these patients treated with 600 mg of imatinib mesylate per day, our analysis showed that imatinib mesylate therapy leads to a biphasic exponential decline of the leukemic cell burden.11 The biphasic shape of the treatment response curve was later reconfirmed with data of patients treated with 400 mg per day enrolled in the German cohort of the IRIS trial12 ; therefore, the biphasic nature of the treatment response is apparently not dependent on the dosage of imatinib mesylate used as long as a biologically active concentration is administered.
We then designed a mathematical framework that was based on a 4-compartment model which could explain the kinetics of the molecular response to TKIs in this patient cohort.11,13 On the basis of our framework,11 the 2 slopes were interpreted as representing the decline of differentiated leukemic cells (the slope between the baseline measurement and the 3rd month of treatment) and leukemic progenitor cells (the slope between the 6th and 12th month of treatment). We also analyzed the dynamics of the leukemic cell burden in 3 patients who discontinued imatinib mesylate therapy after 1-3 years of treatment, finding that treatment cessation led to a rapid rebound to levels at or beyond pretreatment baseline. These rebound kinetics led us to hypothesize that the cell population driving the disease, leukemic “stem cells,” were not depleted by a large amount in these 3 patients because otherwise, imatinib mesylate cessation would have led to a rebound to levels significantly below pretreatment baseline. However, further data are necessary to make general conclusions about the effect of imatinib mesylate treatment on leukemic stem cells. Two types of data contain information about the behavior of leukemic stem cells during imatinib mesylate therapy. First, if a large number of patients with CML discontinue imatinib mesylate and their relapse kinetics are closely followed,14-16 then the dynamics of leukemic stem cells during and after imatinib mesylate treatment can be inferred (M.T., Jasmine Foo, M.G., Joëlle Guilhot, François-Xavier Mahon, F.M., unpublished data). Second, information on the response kinetics of patients receiving long-term imatinib mesylate may provide clues about the treatment effect on leukemic stem cells. Here, we used 10-year follow-up data of patients enrolled in the IRIS trial, together with a statistical modeling approach and our mathematical framework,11,13 to investigate the effects of long-term imatinib mesylate therapy on CML stem cells. Furthermore, we used a dataset of patients with CML who were treated with first-line nilotinib6 (400 mg twice a day) to elucidate the treatment response to a second-generation TKI.
Methods
We used data of 103 patients with newly diagnosed disease who were treated with first-line imatinib mesylate for 12 months within the TIDEL trial.10 For each patient, real-time quantitative (RQ)–PCR was used to determine the BCR-ABL1/BCR values at the baseline as well as months 1, 2, 3, 6, 9, and 12. We also used data of 29 patients with newly diagnosed disease who were treated with first-line imatinib mesylate (400 mg per day) within the IRIS trial for ≤ 10 years. The BCR-ABL1 percentage values of these patients were recorded every 3 months. For the long-term imatinib mesylate cohort, values below detection of the RQ-PCR assay (values of zero in the data) were converted to positive BCR-ABL1 values that were probably present in the sample. This conversion was needed for the logarithmic transformation described in supplemental Information (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and justified because several studies have led to the conclusion that undetectable BCR-ABL1 transcripts are not synonymous with a cure of the disease, but that residual leukemic cells frequently remain in those patients.14-16 Furthermore, the limit of detection of each sample varies with the quality of the RNA and the efficiency of the reverse transcription reaction, which can lead to fluctuations in PCR positivity.17 The conversion is based on the BCR control gene transcript value and the standardized baseline value for our BCR-ABL1 detection assay4,8 (see supplemental Information). This converted database was then used to examine the long-term imatinib mesylate treatment response. Our analyses are based on the assumption that the converted values and the true BCR-ABL1 abundances are directly proportional. Finally, we used data of a subcohort of patients (n = 29) with newly diagnosed disease who were treated with first-line nilotinib (400 mg twice daily) at the M. D. Anderson Cancer Center with a follow-up of ≤ 66 months.6 The BCR-ABL1 percentage values of these patients were recorded every 6 months, with an additional time point at 3 months after treatment initiation. Details of the patient selection and statistical and mathematical modeling approaches are provided in the supplemental Information, and the individual BCR-ABL1/BCR percentage plots of the selected patients are shown in supplemental Figures 1 through 3.
Results
To investigate the effects of imatinib mesylate and nilotinib on different subpopulations of leukemic cells, we used a statistical modeling approach to identify the shape of the treatment response curves in the short-term (12 months of follow-up) and long-term (10 years of follow-up) imatinib mesylate patient cohorts, as well as the first-line nilotinib patient cohort (≤ 66 months follow-up). Because no data were recorded between the baseline and the third month of treatment for patients in the long-term imatinib mesylate cohort, we were not able to estimate the first slope of decline (between 0 and ∼ 3 months of treatment) observed in the leukemic cell burden11 in this cohort. We therefore analyzed the short-term imatinib mesylate patient cohort to obtain the kinetics of the initial decline and then used the 10-year follow-up data to infer the dynamics of the long-term response to treatment. Although the imatinib mesylate dose administered differs between the short- and long-term cohorts and therefore the slope of depletion may vary among those patients, the shape of the treatment response curve is apparently independent of the imatinib mesylate dose administered11,12 as long as a biologically active dose is used. The nilotinib patient cohort was analyzed to determine the shape of the nilotinib treatment response curve.
Imatinib mesylate treatment response
We investigated 2 statistical models to identify the one with the best fit with our short-term imatinib mesylate cohort (0-12 months of treatment) and then repeated this approach with the long-term imatinib mesylate cohort (6 months to 10 years of treatment). The 2 statistical models analyzed were (1) an exponential model, which predicts that the leukemic cell burden declines at a single exponential rate, and (2) a biphasic exponential model predicting that the BCR-ABL1/BCR counts decline at 2 exponential slopes with a turning point (supplemental Information). We first fit these models to the short-term imatinib mesylate response data (Figure 1). Table 1 provides summary information for the statistical model fitting to individual patient data, as well as the overall R2 for each model. We found that the biphasic exponential model provides a larger final R2, as well as better patient-specific R2i, than the exponential model. We then showed, with a bootstrapping approach, that the final R2 of the biphasic exponential model is significantly larger than the final R2 of the single exponential curve (P = 0; supplemental Information). A Bayesian Information Criterion (BIC), which penalizes model size, also favors the biphasic model for the entire patient cohort (supplemental Information). We thus chose the biphasic exponential model as the best-fitting statistical model for the short-term imatinib mesylate treatment response data; this result is in agreement with previous findings.11 Supplemental Figure 1 displays the individual patient data with the subject-specific fit of the biphasic model. Besides the analysis of the entire cohort, we also performed individual model fitting to compare, for each individual patient, the fit of the linear and biphasic models. On the basis of the BIC criterion, we obtained a better fit of the biphasic model for all 44 patients. The first slope of depletion for these 44 patients was identified as −0.052 ± 0.018 per day (Table 2); all slopes are given in units per day. The second slope was negative in 35 patients, zero in 1 patient, and positive in 8 patients; the positive slopes were potentially due to the emergence of acquired resistance in these patients. However, we did not use this patient cohort to determine the second slope because the long-term imatinib mesylate cohort contained more information (ie, a longer follow-up); therefore, the second slope could be determined with greater precision with the use of data from the long-term cohort.
Dynamics of short-term imatinib mesylate treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with imatinib mesylate for 1 year after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves). This fitted model shows that there is a biphasic exponential depletion of leukemic cells within the first year of treatment. (E) The panel displays the median (orange circles) and quartiles of the short-term imatinib mesylate response data together with the fitted biphasic exponential model (red curve). See supplemental Figure 1 for all patient plots and model fitting.
Dynamics of short-term imatinib mesylate treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with imatinib mesylate for 1 year after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves). This fitted model shows that there is a biphasic exponential depletion of leukemic cells within the first year of treatment. (E) The panel displays the median (orange circles) and quartiles of the short-term imatinib mesylate response data together with the fitted biphasic exponential model (red curve). See supplemental Figure 1 for all patient plots and model fitting.
Summary of the 2 statistical models for the imatinib response data
| . | Biphasic exponential model . | Exponential model . |
|---|---|---|
| Short-term (12 mo) mesylate imatinib response patient cohort (n = 51) | ||
| Minimum* of Ri2 | 0.91 | 0 |
| First quartile* of Ri2 | 0.98 | 0.76 |
| Median* of Ri2 | 0.99 | 0.83 |
| Mean* of Ri2 | 0.98 | 0.79 |
| Third quartile* of Ri2 | 0.99 | 0.87 |
| Maximum* of Ri2 | 1 | 0.96 |
| †Final R2 | 0.98 | 0.81 |
| ‡Sum of BICs | −62.6 | 28.0 |
| Long-term (10 y) imatinib response patient cohort (n = 22) | ||
| Minimum* of Ri2 | 0.67 | 0.62 |
| First quartile* of Ri2 | 0.85 | 0.69 |
| Median* of Ri2 | 0.88 | 0.77 |
| Mean* of Ri2 | 0.87 | 0.77 |
| Third quartile* of Ri2 | 0.91 | 0.84 |
| Maximum* of Ri2 | 0.96 | 0.93 |
| †Final R2 | 0.89 | 0.80 |
| ‡Sum of BICs | −23.3 | −15.3 |
| . | Biphasic exponential model . | Exponential model . |
|---|---|---|
| Short-term (12 mo) mesylate imatinib response patient cohort (n = 51) | ||
| Minimum* of Ri2 | 0.91 | 0 |
| First quartile* of Ri2 | 0.98 | 0.76 |
| Median* of Ri2 | 0.99 | 0.83 |
| Mean* of Ri2 | 0.98 | 0.79 |
| Third quartile* of Ri2 | 0.99 | 0.87 |
| Maximum* of Ri2 | 1 | 0.96 |
| †Final R2 | 0.98 | 0.81 |
| ‡Sum of BICs | −62.6 | 28.0 |
| Long-term (10 y) imatinib response patient cohort (n = 22) | ||
| Minimum* of Ri2 | 0.67 | 0.62 |
| First quartile* of Ri2 | 0.85 | 0.69 |
| Median* of Ri2 | 0.88 | 0.77 |
| Mean* of Ri2 | 0.87 | 0.77 |
| Third quartile* of Ri2 | 0.91 | 0.84 |
| Maximum* of Ri2 | 0.96 | 0.93 |
| †Final R2 | 0.89 | 0.80 |
| ‡Sum of BICs | −23.3 | −15.3 |
i = 1, …, N, calculated from the corresponding fitted model for each patient, where N is the total number of patients and Ri2 = 1 − SSEi/SSTi.
Final R2, calculated as 1 − Σi SSEi/Σi SSTi, evaluates the overall fit of the corresponding model to the whole time series data with all patients.
Sum of BICs is the sum of BICs over all subjects for each model.
Summary statistics of slopes and turning points for the imatinib mesylate and nilotinib response data
| Slopes and turning points . | Short-term imatinib mesylate . | Long-term imatinib mesylate . | Nilotinib . | |||
|---|---|---|---|---|---|---|
| Mean (SE) . | Median (no. of patients) . | Mean (SE) . | Median (no. of patients) . | Mean (SE) . | Median (no. of patients) . | |
| β1* | −0.0519 (0.0180) | −0.0525 (44) | † | † | −0.0424 (0.0154) | −0.0408 (28) |
| β2* | −0.0041 (0.0061) | −0.0044 (44) | −0.0057 (0.0038) | −0.0042 (14) | −0.0014 (0.0017) | −0.0014 (28) |
| β2 Negative‡ | −0.0064 (0.0038) | −0.0056 (35) | −0.0057 (0.0038) | −0.0042 (14) | −0.0019 (0.0013) | −0.0017 (24) |
| β3* | § | § | −0.0006 (0.0006) | −0.0008 (14) | § | § |
| β3 Negative‡ | § | § | −0.0008 (0.0003) | −0.0008 (13) | § | § |
| τ1, mo* | 4.23 (1.41) | 4.14 (44) | † | † | 4.81 (2.20) | 3.99 (28) |
| τ1 Negative, mo‡ | 4.00 (1.36) | 3.91 (35) | † | † | 4.33 (1.62) | 3.70 (24) |
| τ2,mo* | § | § | 34.5 (22.6) | 28.3 (14) | § | § |
| τ1 Negative, mo‡ | § | § | 29.5 (13.4) | 27.3 (13) | § | § |
| Slopes and turning points . | Short-term imatinib mesylate . | Long-term imatinib mesylate . | Nilotinib . | |||
|---|---|---|---|---|---|---|
| Mean (SE) . | Median (no. of patients) . | Mean (SE) . | Median (no. of patients) . | Mean (SE) . | Median (no. of patients) . | |
| β1* | −0.0519 (0.0180) | −0.0525 (44) | † | † | −0.0424 (0.0154) | −0.0408 (28) |
| β2* | −0.0041 (0.0061) | −0.0044 (44) | −0.0057 (0.0038) | −0.0042 (14) | −0.0014 (0.0017) | −0.0014 (28) |
| β2 Negative‡ | −0.0064 (0.0038) | −0.0056 (35) | −0.0057 (0.0038) | −0.0042 (14) | −0.0019 (0.0013) | −0.0017 (24) |
| β3* | § | § | −0.0006 (0.0006) | −0.0008 (14) | § | § |
| β3 Negative‡ | § | § | −0.0008 (0.0003) | −0.0008 (13) | § | § |
| τ1, mo* | 4.23 (1.41) | 4.14 (44) | † | † | 4.81 (2.20) | 3.99 (28) |
| τ1 Negative, mo‡ | 4.00 (1.36) | 3.91 (35) | † | † | 4.33 (1.62) | 3.70 (24) |
| τ2,mo* | § | § | 34.5 (22.6) | 28.3 (14) | § | § |
| τ1 Negative, mo‡ | § | § | 29.5 (13.4) | 27.3 (13) | § | § |
The table displays the first (β1), second (β2), and third (β3) slopes as well as the first (τ1) and second (τ2) turning points for all patients whose treatment response data displayed a biphasic trend on the basis of the BIC criterion (see supplemental Information), as well as for those whose second and third slopes were negative.
The total number of patients used to calculate mean, median, and SE are given by those who displayed a biphasic trend in their treatment response kinetics (see supplemental Information).
Not observable because of data resolution.
Mean and median are calculated using all individual patients whose response data displayed a biphasic trend on the basis of the BIC criterion and whose data also had negative slopes (see supplemental Information).
Not observable because of insufficient follow-up.
We then analyzed the long-term IRIS trial data (Figure 2). We again fit our 2 statistical models, using data starting from month 6 after the initiation of treatment. This choice was made because there was not sufficient data between months 0 and 6 in this cohort to determine the first slope observed in the short-term cohort, and because, on the basis of the analysis of the short-term data, the second slope starts between months 3 and 6. The summary information of the fitting is displayed in Table 2. We again identified the model with the significantly larger final R2 through a bootstrapping simulation (P = .04; supplemental Information) and also used the BIC criterion to distinguish the models. We used the weighted least squares method to give less weight to the converted data points in the long-term patient cohort (see supplemental Information). With the use of these different approaches, we obtained consistent results: the biphasic exponential model was chosen as the model with a better fit to the long-term imatinib mesylate patient cohort. Supplemental Figure 2 displays the individual patient data with the subject-specific fit of the biphasic model. Besides the analysis over the whole cohort, we also performed individual model fitting to compare, for each individual patient, the 2 statistical models. Based on the BIC criterion, the biphasic model represented the better fit for 14 of 22 patients. The slope starting from the sixth month of treatment, which is equivalent to the second slope starting from the time of treatment initiation on the basis of the short-term cohort, for these 14 subjects was −0.0057 ± 0.0038 (Table 2). This slope was negative in all 14 patients, suggesting that these patients did not harbor acquired resistance during that time. The last slope, which commences at ∼ 34 months after the initiation of imatinib mesylate therapy and represents the third slope after treatment initiation, was negative in 13 patients and positive in 1 (patient 17). This last slope was −0.0006 ± 0.0006 averaged over all 14 patients and −0.0008 ± 0.0003 averaged over those 13 patients who had negative last slopes. The estimated turning point averaged over all 14 subjects was 34.5 ± 22.6 months, whereas the turning point for the patient with a positive last slope was 8.25 years (Table 2).
Dynamics of long-term imatinib mesylate treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with imatinib mesylate for 10 years after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves, starting from month 6 of treatment). Converted data points are shown in green (see “Methods” and supplemental Information). This fitted model shows that there is a biphasic exponential depletion of leukemic cells, starting from 6 months after the initiation of therapy. (E) The panel displays the median (orange circles) and quartiles of the long-term imatinib mesylate response data together with the fitted biphasic exponential model (red curve). See supplemental Figure 2 for all patient plots and model fitting.
Dynamics of long-term imatinib mesylate treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with imatinib mesylate for 10 years after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves, starting from month 6 of treatment). Converted data points are shown in green (see “Methods” and supplemental Information). This fitted model shows that there is a biphasic exponential depletion of leukemic cells, starting from 6 months after the initiation of therapy. (E) The panel displays the median (orange circles) and quartiles of the long-term imatinib mesylate response data together with the fitted biphasic exponential model (red curve). See supplemental Figure 2 for all patient plots and model fitting.
Based on these statistical analyses of both short- and long-term imatinib mesylate treatment responses, we conclude that the imatinib mesylate treatment response of BCR-ABL1 transcripts in the peripheral blood of most patients displays 3 phases: during the first phase (between 0 and ∼ 4 months after the initiation of therapy), the abundance of leukemic cells decreases with a slope of −0.052 ± 0.018 per day; this slope was identified using the short-term patient cohort because only this group contained a sufficient number of early time points such that the slope could be robustly identified. During the second phase (between 4 and ∼ 34 months after the initiation of therapy), the leukemic burden declines with a slope of −0.0057 ± 0.0038 per day; this slope was identified with the long-term cohort because the short-term cohort had a follow-up of only 12 months and might therefore lead to biased estimates of the second slope. During the third phase (starting at ∼ 34 months after treatment initiation), the leukemic cell burden decreased in 13 of 14 patients with an average slope of −0.0008 ± 0.0003, whereas it increased in one patient with a slope of 0.0014. Overall (all 14 patients), the third slope is −0.0006 ± 0.0006 (Table 2).
Nilotinib treatment response
We then analyzed the nilotinib treatment response with the use of our nilotinib patient cohort and fit our 2 statistical models to this database (Figure 3). The summary information of the fitting is displayed in Table 3. We identified the model with a significantly larger final R2 and also used the BIC criterion to distinguish the models (supplemental Information). The biphasic exponential model was again chosen as the model with the better fit to the nilotinib response data. Supplemental Figure 3 displays the individual patient data with the subject-specific fit of the biphasic model. In addition to the analysis over the whole cohort, we also performed individual model fitting to compare, for each individual patient, the performance of the 2 statistical models. On the basis of the BIC criterion, the biphasic model had a better fit for 28 of 29 patients. There were 5 patients (IDs 1-5) with a follow-up of ≥ 60 months. We hypothesized that these patients might have a sufficiently long follow-up such that a triphasic decline may be identified in these patients. We therefore compared the fit of single-phasic, biphasic, and triphasic models for these 5 subjects using the Joinpoint Regression Program.18 Based on the BIC criterion, 3 patients (IDs 1, 2, and 4) were identified as having a triphasic decline of their leukemic cell burden, whereas 2 patients (patients 3 and 5) displayed a biphasic decline (supplemental Table 3). Thus, on the basis of our analysis of the nilotinib cohort containing 29 patients, 1 subject had a single-phasic decline, 25 subjects had a biphasic, and 3 had a triphasic decline. The first slope for the 28 subjects who had > 1 slope was −0.0424 ± 0.0154 (Table 2). This slope was negative in all patients, suggesting that these patients did not harbor acquired resistance during that time. The second slope was negative in 24 patients, zero in 2 patients (IDs 6 and 25), and positive in 2 patients (IDs 26 and 27). The second slope averaged over all 28 subjects was −0.0014 ± 0.0017, whereas it was −0.0019 ± 0.0013 averaged over the 24 patients with negative second slopes. The estimated turning points for all 28 subjects were 4.81 ± 2.20 months, whereas they were 4.33 ± 1.62 months for the 24 patients with negative second slopes (Table 2). The third slopes for patients 1, 2, and 4 were −0.00028, −0.00002, and −0.00697, respectively.
Summary of the 2 statistical models for the nilotinib response data
| . | Biphasic exponential model . | Exponential model . |
|---|---|---|
| Minimum* of Ri2 | 0.74 | 0.31 |
| First quartile* of Ri2 | 0.95 | 0.47 |
| Median* of Ri2 | 0.97 | 0.64 |
| Mean* of Ri2 | 0.96 | 0.61 |
| Third quartile* of Ri2 | 0.99 | 0.73 |
| Maximum* of Ri2 | 1 | 0.89 |
| †Final R2 | 0.97 | 0.61 |
| ‡Sum of BICs | −83.22 | 34.48 |
| . | Biphasic exponential model . | Exponential model . |
|---|---|---|
| Minimum* of Ri2 | 0.74 | 0.31 |
| First quartile* of Ri2 | 0.95 | 0.47 |
| Median* of Ri2 | 0.97 | 0.64 |
| Mean* of Ri2 | 0.96 | 0.61 |
| Third quartile* of Ri2 | 0.99 | 0.73 |
| Maximum* of Ri2 | 1 | 0.89 |
| †Final R2 | 0.97 | 0.61 |
| ‡Sum of BICs | −83.22 | 34.48 |
Information is for the first-line nilotinib response patient cohort (n = 29); i = 1, …, N, calculated from the corresponding fitted model for each patient, where N is the total number of patients and Ri2 = 1 − SSEi/SSTi.
Final R2, calculated as 1 − Σi SSEi / Σi SSTi, evaluates the overall fit of the corresponding model to the whole time series data with all patients.
Sum of BICs is the sum of BICs over all subjects for each model.
Based on these statistical analyses of the nilotinib treatment response, we concluded that a second-generation BCR-ABL inhibitor, nilotinib, elicits very similar treatment responses as the first-generation inhibitor, imatinib mesylate: the BCR-ABL1 transcripts in the peripheral blood of most patients decrease in 2 phases during short-term treatment (Figure 3). During the first phase (between 0 and ∼ 4 months after the initiation of nilotinib therapy), the abundance of leukemic cells decreases with a slope of −0.0424 ± 0.0154 per day. During the second phase, the leukemic burden declines in 24 of 28 patients with an average slope of −0.0019 ± 0.0013 per day, whereas it is zero in 2 patients and increases in 2 patients with slopes of 0.0011 and 0.0030. Overall (all 28 patients), the second slope is −0.0014 ± 0.0017 (Table 2). Of 5 patients with a sufficient follow up, 3 displayed a triphasic trend; the third slopes for these 3 patients were −0.00028, −0.00002, and −0.00697, respectively.
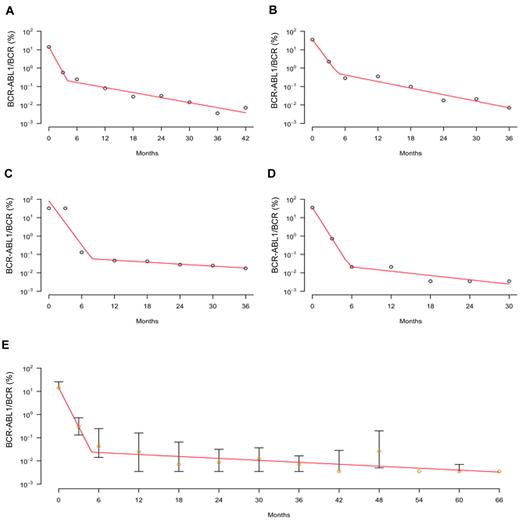
Dynamics of nilotinib treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with first-line nilotinib for ≤ 66 months after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves). This fitted model shows that there is a biphasic exponential depletion of leukemic cells, starting from the initiation of therapy. (E) The panel displays the median (orange circles) and quartiles of the nilotinib response data together with the fitted biphasic exponential model (red curves). See supplemental Figure 3 for all patient plots and model fitting.
Dynamics of nilotinib treatment response. The figure displays the kinetics of the leukemic cell burden in patients treated with first-line nilotinib for ≤ 66 months after diagnosis in early chronic phase. (A-D) The panels show the percentage of cancer cells (BCR-ABL1/BCR as measured by RQ-PCR) of 4 individual patients with CML (circles) and the fitted biphasic exponential model (red curves). This fitted model shows that there is a biphasic exponential depletion of leukemic cells, starting from the initiation of therapy. (E) The panel displays the median (orange circles) and quartiles of the nilotinib response data together with the fitted biphasic exponential model (red curves). See supplemental Figure 3 for all patient plots and model fitting.
Mathematical modeling
We then used our mathematical framework, describing 4 subtypes of leukemic cells,11,13 to predict the dynamics of BCR-ABL1/BCR values in the peripheral blood of patients with newly diagnosed CML treated with a TKI. This model was designed to relate the available data on BCR-ABL1 transcript levels in peripheral blood to the kinetics of other, unobservable differentiation levels of leukemic cells (supplemental Information). In the context of this mathematical framework, the 3 slopes observed in the data are interpreted as a decline in the abundance of differentiated (but not terminally differentiated) cells (first slope), progenitor cells (second slope), and, finally, a depletion (or expansion) of leukemic stem cells (third slope). In addition to these 3 cell types, we postulated the existence of a fourth differentiation level, made up of terminally differentiated cells that have an average lifespan of a day, whose depletion cannot be observed in the data because of limited resolution.11,13 This framework, together with the estimates of the 3 slopes obtained with our statistical model fitting approach, was then used to predict the kinetics of the treatment response (Figure 4). After treatment initiation, the leukemic cell population in peripheral blood declines at the death rate of differentiated cells during therapy (equal to the first slope identified in the data, mean −0.052 ± 0.018 [imatinib mesylate] and −0.042 ± 0.015 [nilotinib]) until the latter reach a steady state with progenitor cells; from this time onward, the kinetics display a shallower decrease, signifying the depletion of progenitor cells during imatinib mesylate treatment (equal to the second slope identified in the data, mean −0.0057 ± 0.0038 [imatinib mesylate] and −0.0019 ± 0.0013 [nilotinib]). Finally, there is a second turning point in the dynamics of leukemic cells, which is caused by the progenitor cells reaching a steady state with their upstream population, potentially stem cells; after that time, the kinetics of treatment response are driven by the behavior of this population. Due to insufficient follow-up or missing data or both, this third slope could not be identified in all patients but was observed in 14 patients treated with imatinib mesylate (mean, −0.0006 ± 0.0003) and 3 patients treated with nilotinib (−0.00028, −0.00002, and −0.00697). It is possible that the population declining with the third slope does not represent leukemic stem cells but an immature population more differentiated than stem cells. In the latter case, we predict that the third slope signifies the decline (or increase) of this immature population and that we would observe a fourth slope, representing the behavior of leukemic stem cells, if the detection limits of PCR assays improved and if even longer follow-up was available.
A mathematical framework accurately predicts the dynamics of TKI treatment responses. (A-D) The panels display the abundance of healthy (black) and leukemic (blue) stem cells (SCs), progenitor cells (PCs), differentiated cells (DCs), and terminally differentiated cells (TCs) over time (years) since the initiation of imatinib mesylate therapy, as predicted by the mathematical framework.10,12 (E-F) The panels display the median (orange circles) and quartiles of the short-term (E) and long-term (F) imatinib mesylate response data together with the results of the mathematical framework. Based on the model presented in the supplemental Information, the mathematical model prediction is given by αy3/(2x3 + y3). Here x3 and y3 denote the abundance of normal and leukemic terminally differentiated cells, and α specifies the average number of copies of BCR-ABL1 per cell times a factor representing batch effects between cohorts. Parameter values are d0 = 0.0008, d1 = 0.0057, d2 = 0.0519, d3 = 1, rx = 0.008, ry = 0.01, px = 9 × 10−6, py = 1.15 × 10−6, ax = 0.57, bx = 5.19, cx = 100, ay = 2ax, by = 2bx, cy = cx, a'y = ay/200, b'y = by/300, c'y = cy, r'y = ry/15, and α = 3 (E) and α = 4 (F) (see supplemental Information). Apart from the dimension-less parameters, all values are given in units per day. Note that these parameter choices represent only one example that can recapitulate the dynamics of the treatment response seen in the clinic; other choices are possible.
A mathematical framework accurately predicts the dynamics of TKI treatment responses. (A-D) The panels display the abundance of healthy (black) and leukemic (blue) stem cells (SCs), progenitor cells (PCs), differentiated cells (DCs), and terminally differentiated cells (TCs) over time (years) since the initiation of imatinib mesylate therapy, as predicted by the mathematical framework.10,12 (E-F) The panels display the median (orange circles) and quartiles of the short-term (E) and long-term (F) imatinib mesylate response data together with the results of the mathematical framework. Based on the model presented in the supplemental Information, the mathematical model prediction is given by αy3/(2x3 + y3). Here x3 and y3 denote the abundance of normal and leukemic terminally differentiated cells, and α specifies the average number of copies of BCR-ABL1 per cell times a factor representing batch effects between cohorts. Parameter values are d0 = 0.0008, d1 = 0.0057, d2 = 0.0519, d3 = 1, rx = 0.008, ry = 0.01, px = 9 × 10−6, py = 1.15 × 10−6, ax = 0.57, bx = 5.19, cx = 100, ay = 2ax, by = 2bx, cy = cx, a'y = ay/200, b'y = by/300, c'y = cy, r'y = ry/15, and α = 3 (E) and α = 4 (F) (see supplemental Information). Apart from the dimension-less parameters, all values are given in units per day. Note that these parameter choices represent only one example that can recapitulate the dynamics of the treatment response seen in the clinic; other choices are possible.
In most patients of the long-term imatinib mesylate cohort, the third slope is negative, suggesting that imatinib mesylate may be capable of decreasing the abundance of leukemic stem cells in some patients. In a small subset of patients, however, there is a slow increase in the total leukemic burden during the third phase. In some cases, the third slope cannot be observed, probably due to insufficient follow-up or due to missing data. Our mathematical framework, together with data of the longest currently available follow up of TKI-treated patients, thus predicts that in this subset of highly selected patients, those who tolerated continuous imatinib mesylate or nilotinib therapy for several years without the evolution of resistance, progression of disease, or adverse effects, TKIs may be able to decrease the abundance of leukemic stem cells in some patients.
Discussion
It has been suggested that it is impossible to cure CML with the use of targeted therapy because leukemic stem cells cannot be eradicated.19 For months or years after achieving a complete cytogenetic response, most patients with CML treated with imatinib mesylate have measurable disease by RQ-PCR and would relapse if imatinib mesylate therapy were discontinued.20,21 However, in most patients who respond to imatinib mesylate, there is a progressive decline in the BCR-ABL1 counts over time, such that after several years of treatment an increasing number of patients achieve a complete molecular response within the limitations of the assay. Whether all CML cells have been eradicated in any of these patients is a question of significant clinical and scientific importance. Furthermore, the dynamics of responses to second-generation inhibitors have not yet been investigated.
In this study, we performed statistical analyses of both short- and long-term imatinib mesylate as well as nilotinib treatment responses, encompassing patients who were treated with 600 mg of imatinib mesylate and followed for 12 months, patients who were treated with 400 mg of imatinib mesylate with a follow-up of ≤ 10 years, and patients who were treated with first-line nilotinib (400 mg twice daily) with a follow-up of ≤ 66 months. On the basis of these data and our mathematical framework,11,13 we concluded that the leukemic treatment response displays 3 phases: during the first phase, the abundance of leukemic cells decreases with a slope of −0.052 ± 0.018 (imatinib mesylate) or −0.042 ± 0.015 (nilotinib) per day, representing a depletion of differentiated leukemic cells by treatment. During the second phase, the leukemic burden declines with a slope of −0.0057 ± 0.0038 (imatinib mesylate) or −0.0019 ± 0.0013 (nilotinib) per day, signifying a decrease of the abundance of leukemic progenitors. Both the first and second slopes are significantly different between the imatinib mesylate and nilotinib patient cohorts, signifying variability because of differences in follow-up, missing data, pharmacokinetic effects, or potentially different effects of imatinib mesylate versus nilotinib on the death rates of leukemic cells during therapy. During the third phase, the leukemic cell number decreases in a subset of patients treated with imatinib mesylate (mean, −0.0008 ± 0.0003) and 3 patients treated with nilotinib (−0.00028, −0.00002, and −0.00697) while increasing in 1 patient treated with imatinib mesylate (0.0014). In a few patients, this third slope could not be observed, probably due to insufficient follow-up or missing data. The number of patients with a third slope was smaller in the nilotinib than in the imatinib mesylate cohort, because the follow-up of patients treated with first-line nilotinib was much shorter than the follow-up of patients treated with imatinib mesylate. Importantly, this third slope has never before been described in TKI response data and offers novel insights into the biology of the disease.
The existence of a third slope suggests that it may be possible to infer the kinetics of a population of immature leukemic cells, possibly stem cells, from long-term TKI response data. Our findings support the hypothesis that targeted therapy is capable of depleting leukemic stem cells at a very slow rate in a subset of patients. In a few patients, however, the third slope was positive or could not be identified with statistical significance. The variability in response to long-term targeted therapy may be due to inconsistent patient compliance to drug; indeed, poor compliance was hypothesized to be the predominant reason for the inability of some patients with CML to obtain or maintain adequate molecular responses to imatinib mesylate therapy.22 Alternatively, this variability in patient response may be caused by the presence of low-level resistance in some patients, by heterogeneity in the disease characteristics, or by both. In many situations, there is marked heterogeneity in phenotype even if genetically, cells are identical.23-26 Similarly, different patients may present with leukemic cell phenotypes with disparate growth and differentiation kinetics. This hypothesis is supported by experimental evidence suggesting that both the amount of BCR-ABL1 mRNA and second-site mutations alter the fitness of leukemic cells.27,28 Furthermore, even leukemic stem cells within one patient may be highly heterogeneous, harboring clones with different growth kinetics.29,30 Inclusion of such intrapatient variability into our framework would not alter the results, because the mathematical framework describes the behavior of the dominant clone in each differentiation stage (supplemental Information). Finally, this variability in the third slope observed among patients may be because of the limitations in the sensitivity of the RQ-PCR assay to detect BCR-ABL1 transcripts at such low levels (supplemental Information).
Note that the long-term TKI treatment follow-up data used for our analyses represent a strongly selected population of patients who were not only able to tolerate TKI therapy for a decade but also achieved at least a complete cytogenetic response. Therefore, the conclusions that are based on this patient group may not apply in general to all patients with CML. Furthermore, the shape of the imatinib mesylate treatment response curve was identified on the basis of converted BCR-ABL1 transcript values (supplemental Information); as long as the shape of the curve using converted values accurately represents the shape of the curve of true values, this data can be used to identify the behavior of immature CML cells during imatinib mesylate therapy. However, the majority of patients (> 93%) had ≥ 1 positive (nonconverted) value after their specific turning points between the second and third slopes, and > 79% of those patients had multiple positive measurements thereafter. Therefore, the estimation of the third slope is not purely based on converted values. Furthermore, a subset of nilotinib-treated patients, who did not have any converted data points, also displayed a triphasic decline of the leukemic cell burden.
Our results stand in contrast to extensive in vitro studies of CML stem cells suggesting that these cells are intrinsically resistant to TKIs.31 Such studies identified multiple pathways that contribute to stem cell resistance, including decreased intracellular uptake and retention of cytotoxic drugs and TKIs 31 and resistance to apoptosis.32,33 A lower expression of human leukocyte antigen costimulatory molecules and targets of adaptive immunity may also protect stem cells from immune surveillance.34,35 Furthermore, treatment with TKIs in vitro results in an increase in quiescent immature CML cells that retain proliferative capacity after treatment is withdrawn.36,37 Finally, human CML stem cell survival was recently found to be independent of BCR-ABL1 activity,38 and, although short-term in vitro imatinib mesylate treatment reduced the expansion of CML stem/progenitors, cytokine support permitted growth and survival in the absence of BCR-ABL1 activity that was comparable to that of normal stem/progenitor counterparts. These data suggest that stem cells may not be inhibited by imatinib mesylate therapy. These observations can be reconciled with our findings by the fact that in vitro studies may not predict the in vivo behavior of CML stem cells, that in vitro settings arguably use a more differentiated cell population than the most primitive leukemic stem cells present in patients, and that our study population represents a very selected group of patients who may not display the same disease characteristics and long-term response to TKIs as those patients from whom samples for in vitro studies were obtained. Furthermore, the possibility remains that the third slope represents the behavior not of leukemic stem cells but of an immature subpopulation that is more differentiated than stem cells; in that case we would predict the existence of another slope that signifies the behavior of leukemic stem cells.
Our findings provide a fresh perspective for the discussion of whether TKIs are capable of curing CML. The identification of a biphasic depletion of leukemic cells in response to imatinib mesylate therapy11 led to the design of several computational models; although our framework predicted that there would be 3 phases of BCR-ABL1 depletion in patients with CML,12,13 representing the behavior of differentiated cells, progenitors, and potentially leukemic stem cells, other models suggested that there are only 2 slopes, caused by a decline of cycling and quiescent leukemic stem cells,12 or by a decline of leukemic progenitors and stem cells.39 The validity of any of these interpretations of the biphasic depletion of leukemic cells11,12,39 and thus the inferred effect of TKIs on leukemic stem cells remains unresolved. However, these computational models led to distinct predictions of the long-term imatinib mesylate response. Our analyses thus contribute to the discussion of the applicability of these alternative interpretations to long-term treatment response data.
Our findings are consistent with the results of imatinib mesylate discontinuation trials,14-16 which found that most patients relapsed a few months after discontinuation of imatinib mesylate, whereas ∼ 40% of patients remained BCR-ABL1-negative for the duration of their follow up. However, several instances of late relapse and fluctuations of BCR-ABL1 levels after imatinib mesylate discontinuation also occurred, thus questioning the ability of imatinib mesylate to lead to a lasting cure of the disease in these patients. One treatment option that has been suggested to succeed in curing CML is allogeneic stem cell transplantation. Complete molecular response (CMR) is commonly achieved after allogeneic transplantation and is associated with long-term disease-free survival; furthermore, CMR induced by allografts was found to be more stable than CMR induced by imatinib mesylate therapy.40 However, late molecular relapses were reported even after this treatment option.9 In the allograft setting it is possible that ongoing immune surveillance is essential to suppress a pool of residual CML cells. Similarly, for the small number of patients who achieve CMR after administration of IFN-α therapy, ongoing immune surveillance may be important. This agent may represent an attractive therapeutic option because several recent clinical studies,15,41-43 as well as in vitro data,44 have suggested that IFN-α selectively impairs proliferation of primitive CML progenitors. Although the ability of allografts and IFN-α to cure CML remains incompletely understood, we believe that, based on our analyses, continuous TKI therapy has the potential to diminish the leukemic stem cell population at least in a subset of patients. The rate of depletion might vary between patients and may also depend on other factors such as the immune system and previous treatment with IFN-α, cytosine arabinoside, or other agents. In addition, it remains a possibility that those patients who tolerate TKI therapy for ≤ 10 years without adverse effects or progression of disease represent a distinct subset of patients whose leukemia is exquisitely sensitive to treatment. Future clinical studies will show the general applicability of these findings and will allow for the identification of predictors of the long-term response to TKI therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ross L. Levine, Ruth M. Pfeiffer, and Eric V. Slud for helpful discussions and comments.
National Institutes of Health
Authorship
Contribution: M.T., M.G., and F.M. designed the study; M.T. performed the analyses; M.G. and F.M. contributed analysis tools; S.B., T.P.H., A.Q.-C., J.C., H. K., and C.F. contributed data; and M.T., M.G., S.B., and F.M. interpreted the results and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Franziska Michor, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: michor@jimmy.harvard.edu.