Abstract
Adoptive T-cell therapy for malignancies using redirected T cells genetically engineered by tumor antigen-specific T-cell receptor (TCR) gene transfer is associated with mispairing between introduced and endogenous TCR chains with unknown specificity. Therefore, deterioration of antitumor reactivity and serious autoimmune reactivity are major concerns. To address this problem, we have recently established a novel retroviral vector system encoding siRNAs for endogenous TCR genes (siTCR vector). In this study, to test the clinical application of siTCR gene therapy for human leukemia, we examined in detail the efficacy and safety of WT1-siTCR–transduced T cells. Compared with conventional WT1-TCR (WT1-coTCR) gene-transduced T cells, these cells showed significant enhancement of antileukemia reactivity resulting from stronger expression of the introduced WT1-specific TCR with inhibition of endogenous TCRs. Notably, WT1-siTCR gene-transduced T cells were remarkably expandable after repetitive stimulation with WT1 peptide in vitro, without any deterioration of antigen specificity. WT1-siTCR gene–transduced T cells from leukemia patients successfully lysed autologous leukemia cells, but not normal hematopoietic progenitor cells. In a mouse xenograft model, adoptively transferred WT1-siTCR gene-transduced T cells exerted distinct antileukemia efficacy but did not inhibit human hematopoiesis. Our results suggest that gene-immunotherapy for leukemia using this WT1-siTCR system holds considerable promise.
Introduction
Recent identification of various tumor-associated antigens has encouraged the clinical development of cell-mediated immunotherapy for leukemia targeting leukemia-associated antigens.1,2 Among various kinds of immunotherapy, adoptive tumor-specific T-cell therapy using ex vivo expansion of autologous tumor-responsive T cells seems to be an attractive option. Indeed, patients with advanced metastatic melanoma have been treated successfully with melanoma-specific T cells obtained from tumor-infiltrating T cells.3-5 Although adoptive transfer of tumor-specific tumor-infiltrating T cells is a promising strategy, its general application for therapy would appear to be unlikely because of the complex procedures and difficulties involved in the timely preparation of sufficient numbers of tumor-specific cytotoxic T lymphocytes (CTLs) with adequate therapeutic quality.6,7 To address these problems, an innovative approach involving substituting redirected T cells using predefined tumor antigen-specific T-cell receptor (TCR) gene transfer has been developed. In recent clinical trials, melanoma antigen-specific TCR gene-transferred T cells have been used for treatment of patients with advanced melanoma.8,9 However, the clinical efficacy of TCR gene-engineered T cells is still not satisfactory, and serious autoimmune responses have been observed in some melanoma patients. In addition, adoptive immunotherapy using tumor antigen-specific TCR gene-transferred T cells targeting malignancies other than melanoma still remains in its infancy. Therefore, the development of TCR gene-immunotherapy targeting universal tumor-associated antigens is essential to popularize this strategy for cancer treatment.
Wilms tumor gene product 1 (WT1) is one of the zinc-finger transcriptional regulators that is abundantly expressed in the vast majority of acute leukemias, but not in normal cells.10,11 In addition, the expression level of WT1 in tumor cells is clinically correlated with disease aggressiveness and prognosis.12,13 Furthermore, a study using a model involving immunodeficient mice engrafted with human acute myelogenous leukemia has recently obtained important evidence that WT1 is expressed abundantly in chemotherapy-resistant acute myelogenous leukemia stem cells.14 These data have prompted us and other groups to develop adoptive T-cell immunotherapy targeting leukemia stem cells using WT1-specific TCR gene transfer.15-17
To facilitate the clinical application of adoptive immunotherapy using genetically engineered WT1-specific CTLs, some important issues need to be addressed. First, there is the problem of mispairing between endogenous and introduced TCR chains that would reduce the expression of introduced TCR on the surface of gene-modified T cells, resulting in lower functionality.18 Mispairing of TCR could also carry a risk of evoking severe autoimmunity.19,20 Therefore, it is essential to clarify both the on- and off-target adverse effects mediated by WT1-TCR gene-engineered T cells using in vivo as well as in vitro systems. The other issue of concern is bone marrow suppression mediated by WT1-specific T cells because it has been reported that hematopoietic progenitor cells express WT1 mRNA.21,22 In previous trials of WT1 peptide vaccine, suppression of normal hematopoiesis has not been reported in most cases, even though WT1-specific CTLs were generated following vaccination.23,24 However, long-term adverse effects on hematopoietic progenitors mediated by adoptively transferred WT1-TCR gene-engineered T cells should be considered because, in this therapy, a larger number of WT1-specific T cells are infused at one time into patients.
To overcome the aforementioned problems, we have recently developed a novel retroviral vector system for TCR gene transfer that can selectively express target antigen-specific TCR, whereas expression of intrinsic TCRs is suppressed by built-in siRNAs (siTCR vector).25 MAGE-A4–specific TCR gene-engineered T cells prepared by this vector system successfully showed both up-regulated expression of the introduced TCR and enhanced anti-MAGE-A4 reactivity. We also constructed a novel WT1-siTCR retroviral vector encoding human leukocyte antigen (HLA)–A*24:02-restricted and WT1-specific TCR genes cloned from a CTL clone, TAK-1.26 This WT1-siTCR vector similarly appeared capable of increasing the expression of the introduced WT1-specific TCR; however, the usefulness of WT1-siTCR for clinical application remains to be clarified before clinical trials can begin. In the present study, with the aim of clinically applying this WT1-siTCR gene transfer system for treatment of leukemia, we assessed in detail the efficacy and safety of adoptive immunotherapy using WT1-specific TCR gene-modified CTLs using both in vivo as well as in vitro experimental systems. On the basis of the data we have obtained, we discuss the feasibility of this novel gene immunotherapy for leukemia using a WT1-siTCR retroviral vector.
Methods
Cloning of WT1-specific TCR gene and construction of WT1-TCR retroviral vectors
The HLA-A*24:02-restricted and WT1235–243-specific TCR-α and TCR-β genes were cloned from our originally established CTL clone, TAK-1, using the 5′ RACE method (Clontech).27 The TCR-α and TCR-β genes of TAK-1 appeared to be Vα20/J33/Cα and Vβ5.1/J2.1/Cβ2, respectively. Retrovirus vectors expressing TAK-1-derived TCR (WT1-TCR) genes were constructed as reported previously.25 Briefly, the WT1-TCR-α and TCR-β genes were bicistronically integrated into a conventional MS-bPa retroviral vector (WT1-coTCR vector).28 Partially codon-optimized TCR-α and TCR-β genes were similarly integrated into a novel MS-bPa–based retroviral vector encoding shRNAs that complementarily bind to the constant regions of the endogenous TCR-α and TCR-β genes (WT1-siTCR vector) (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Ecotropic retroviral vectors were obtained by transient cotransfection of WT1-TCR-expression retroviral vector and other components (Takara Bio) to HEK293 cell line; subsequently, GaLV-pseudotyped retroviral vectors were obtained by sequential infection of ecotropic retroviral vectors to PG13 cell line. The Jurkat/MA cell line, lacking endogenous TCR and engineered with the hCD8α and NFAT-luciferase construct,29 was transduced with the WT1-TCR vector to confirm that our retroviral vector was actually able to express functional WT1-specific and HLA-A*24:02-restricted TCR molecules on the cell surface (supplemental Figure 2).
Cell lines, freshly isolated leukemia cells, and normal cells
Approval for this study was obtained from the Institutional Review Board of Ehime University Hospital. Written informed consent was given by all patients, healthy volunteers, and the parents of the cord blood donors in accordance with the Declaration of Helsinki. All cell lines and freshly isolated cells were cultured as described previously.30 B-lymphoblastoid cell lines (B-LCLs) were established by transformation of peripheral blood B lymphocytes with Epstein-Barr virus. An HBZ26–34 peptide-specific and HLA-A*02:01-restricted CTL clone, designated HBZ-1, was established as reported previously.31 The HLA-A*24:02 gene-transduced C1R cell line (C1R-A*24:02) was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 0.5 mg/mL hygromycin B (Invitrogen) and the HLA-A*24:02 gene-transduced K562 cell line (K562-A*24:02) was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 1.0 μg/mL puromycin (Sigma-Aldrich). Peripheral blood mononuclear cells and bone marrow mononuclear cells from leukemia patients and healthy volunteers, and cord blood mononuclear cells from healthy donors were isolated and stored in liquid nitrogen until use. All leukemia samples contained more than 95% leukemia cells. In some experiments, CD34+ cells from cord blood mononuclear cells were isolated using CD34+ cell-isolating immunomagnetic beads (MACS beads; Miltenyi Biotec).
Establishment of WT1-TCR gene-transduced CTL lines
CD8+ T cells were isolated from peripheral blood mononuclear cells of healthy volunteers and leukemia patients in complete remission, and cord blood mononuclear cells using CD8+ cell-isolating MACS beads and stimulated with 1 μg/mL anti-CD3 monoclonal antibody (MoAb, OKT-3; BioLegend). CD8+ T cells were cultured in GT-T503 medium (Takara Bio) supplemented with 5% human serum, 0.2% human albumin, 50 U/mL recombinant human IL-2 (R&D Systems), 5 ng/mL IL-7 (R&D Systems), 10 ng/mL IL-15 (PeproTech), and 10 ng/mL IL-21 (Shenandoah Biotechnology). Then, CD8+ T cells were transfected with the WT1-TCR retrovirus vector using RetroNectin (Takara Bio)-coated plates as described previously.25 In some experiments, Vβ5.1-positive cells among WT1-TCR gene-transduced CD8+ T cells were further isolated using fluorescein isothiocyanate (FITC)-conjugated Vβ5.1 MoAb (Beckman Coulter) and an anti-FITC-conjugated MACS beads system. To measure the expression levels of introduced WT1-specific TCR in gene-engineered CD8+ T cells, the cells were labeled with anti-CD8, anti-CD4, anti-CD3 (BD Biosciences), and anti-Vβ5.1 MoAbs and phycoerythrin-conjugated HLA-A*24:02/WT1235–243-tetramer or HLA-A*24:02/HIV-1 Env584–592-tetramer (supplemental Figure 3).32 The labeled cells were analyzed using a Gallios flow cytometer (Beckman Coulter) and FlowJo Version 7.2.2 software (TreeStar). To compare the expandability of WT1-coTCR–transduced and WT1-siTCR-transduced CD8+ T cells by stimulation with WT1 peptide, WT1-TCR gene-transduced CD8+ T cells were weekly stimulated with mitomycin-C (Kyowa Hakko)-treated and heteroclitic WT1235–243 peptide (CYTWNQMNL)-pulsed HLA-A*24:02-positive LCLs.
51Cr-release assays
To determine the cytotoxic activity of WT1-TCR gene-transduced CD8+ T cells, standard 51Cr-release assays were performed as described previously.30 Briefly, 5 × 10351Cr (Na251CrO4; New England Nuclear)–labeled target cells and various numbers of effector cells in 200 μL of RPMI 1640 medium supplemented with 10% fetal calf serum were seeded into 96-well round-bottomed plates. The target cells were incubated with or without WT1 peptide for 2 hours before adding the effector cells. To assess the HLA class I-restricted cytotoxicity, target cells were incubated with an anti-HLA class I framework MoAb (w6/32; ATCC) or an anti-HLA-DR MoAb (L243; ATCC) at an optimal concentration (10 μg/mL) for 1 hour before adding the effector cells. After incubation with the effector cells for 5 hours, 100 μL of supernatant was collected from each well. The percentage of specific lysis was calculated as: (experimental release cpm − spontaneous release cpm)/(maximal release cpm − spontaneous release cpm) × 100 (%).
Detection of CD107a and intracellular IFN-γ expression in WT1-TCR gene-transduced CD8+ T cells
CD107a expression in WT1-TCR gene-transduced CD8+ T cells in response to stimulation with WT1 peptide was assessed as described previously.33 Briefly, 1 × 105 C1R-A*24:02 cells were seeded into a 96-well round-bottom plate and incubated with or without WT1 peptide for 2 hours. Then, 2 × 105 WT1-TCR gene-transduced CD8+ T cells were seeded into each well along with FITC-conjugated CD107a MoAb (BioLegend) for 3 hours. Similarly, CD107a expression in the HBZ-1 cell line and WT1-TCR gene-transduced HBZ-1 cells in response to stimulation with HBZ26–34 peptide-loaded HLA-A*02:01-positive T2 cells was analyzed. To investigate intracellular interferon-γ (IFN-γ) production, effector cells were incubated with target cells and 10 μg/mL brefeldin A for 4 hours. They were then collected, fixed, and permeabilized with fluorescence-activated cell sorter lysing solution and fluorescence-activated cell sorter permeabilizing solution (BD Biosciences).34 After permeabilization, the washed cells were stained with FITC-conjugated IFN-γ MoAb (BD Biosciences). Finally, these effector cells were stained with anti-CD3, anti-CD8 MoAbs (BD Biosciences) and phycoerythrin-conjugated HLA-A*24:02/WT1-tetramer, and then analyzed using a Gallios flow cytometer and FlowJo Version 7.2.2 software.
Quantitative analysis of WT1 mRNA expression
Total RNA was extracted from each sample with an RNeasy Mini Kit (QIAGEN) in accordance with the manufacturer's instructions. Quantitative real-time polymerase chain reaction of WT1 mRNA was performed using the QuantiTect SYBR Green PCR Kit (QIAGEN) and primers as follows: forward; 5′-AGCACAGGGTACGAGAGCGATAAC-3′, reverse; 5′-TATTGCAGCCTGGGTAAGCACA-3′ (Takara Bio). GAPDH mRNA as an internal control was prepared as described previously.30 These samples were analyzed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems). The expression level of WT1 mRNA was corrected by reference to that of GAPDH mRNA, and the amount of WT1 mRNA in each sample relative to that in the K562 leukemia cell line, which strongly expresses WT1 mRNA (shown as 1.0), was calculated by the comparative ΔCt method.
In vivo antileukemia effect of WT1-siTCR gene-transduced CTLs
Six-week-old NOD/scid/γcnull (NOG) female mice35 were purchased from the Central Institute for Experimental Animals and maintained in the institutional animal facility at Ehime University. All in vivo experiments were approved by the Ehime University animal care committee. For xenografting of human leukemia cells, NOG mice were inoculated subcutaneously in the left flank with 5 × 106 K562-A*24:02 cells, which had been preincubated with 2.5 × 107 effector cells for 5 hours. Then, 1 × 107 effector cells were additionally administered intravenously via the tail vein every week for a total of 5 times. The mice were monitored for tumor growth and survival after inoculation; the tumors were measured at 5-day intervals, and the tumor area was determined.
In vivo differentiation of human hematopoietic stem cells in humanized mice
CD34+ cells were isolated from cord blood mononuclear cells (hCB-CD34+ cells), and then 5 × 104 of the cells were coincubated with 2.5 × 105 autologous WT1-siTCR–transduced or non–gene-modified CTLs generated from cord blood CD8+ T cells for 5 hours. hCB-CD34+ cells were then reisolated from the cell mixture and injected intravenously into 7-week-old NOG mice that had been irradiated with 1.5 Gy. Three months later, these mice were killed to study the engraftment and differentiation of human hematopoietic cells in both the bone marrow and spleen. Human leukocytes were discriminated from murine cells using anti-hCD45 MoAb (BD Biosciences). Human cells were further stained with MoAbs against cell lineage-related surface molecules, including CD3, CD8, CD4, CD19, CD33, CD34, CD38, CD41a, and GPA (BD Biosciences). The expression of HLA-A*24:02 in the engrafted human cells was also measured using FITC-conjugated anti-HLA-A24 MoAb (One Lambda), and the immunostained cells were analyzed using a Gallios flow cytometer and FlowJo Version 7.2.2 software.
Results
Comparison of WT1-TCR expression and WT1-specific cytotoxic reactivity of WT1-siTCR- and WT1-coTCR–transduced CD8+ T cells
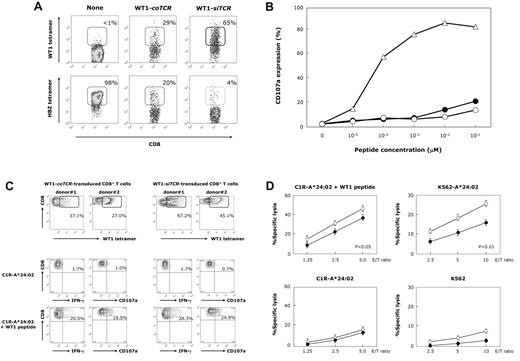
First, we confirmed the augmented and inhibitory efficacies of the WT1-siTCR vector for expression of the respectively introduced and endogenous TCRs. To do so, we transduced WT1-siTCR into HBZ-1, which is an HLA-A*02:01-restricted and HBZ26–34-specific CD8+ T-cell clone. As shown in Figure 1A, positivity for HLA-A*24:02/WT1-tetramer staining in nontreated, WT1-coTCR- and WT1-siTCR–transduced HBZ-1 was < 1%, 29%, and 65%, respectively, whereas the corresponding values for HLA-A*02:01/HBZ-tetramer staining were 98%, 20%, and 4%, respectively. For functional assessment of the efficacy of the siTCR vector for suppression of endogenous HBZ-TCR, CD107a assays were performed. We evaluated the extent of the decreased responsiveness to the cognate HBZ peptide mediated by WT1-coTCR– and WT1-siTCR–transduced HBZ-1 compared with that mediated by the parent HBZ-1. As shown in Figure 1B, WT1-siTCR–transduced HBZ-1 exhibited an apparent loss of responsiveness to the HBZ peptide-loaded T2 cells, indicating that sufficient functional suppression of endogenous TCR is achievable using the WT1-siTCR vector. The reactivity of WT1-coTCR–transduced HBZ-1 to stimulation with HBZ peptide also appeared to decrease compared with that of the parental HBZ-1 clone; however, the inhibitory effect of the siTCR vector appeared to be higher than that of the coTCR vector at high concentrations of the HBZ peptide. On the basis of these data, we were able to confirm the efficacy of the WT1-siTCR vector for augmentation of introduced TCR expression and inhibition of endogenous TCR expression.
Enhanced expression of introduced WT1-specific TCR and augmented functionality in WT1-siTCR–transduced CD8+ T cells. (A) An HLA-A*02:01-restricted HBZ26–34-specific CTL clone (HBZ-1) was transduced with the WT1-siTCR or WT1-coTCR vector. Expression of the introduced WT1-specific and intrinsic HBZ-specific TCRs in TCR gene-modified HBZ-1 cells was examined using either HLA-A*24:02/WT1 tetramer or HLA-A*02:01/HBZ tetramer. A non–gene-modified HBZ-1 clone was used as a negative control. (B) HBZ-1 cells (▵), WT1-coTCR–transduced HBZ-1 cells (●), and WT1-siTCR–transduced HBZ-1 cells (○) were cocultured with HLA-A*02:01-positive T2 cells loaded with various concentrations of HBZ peptide for 3 hours. Thereafter, surface CD107a expression was analyzed as detailed in “Detection of CD107a and intracellular IFN-γ expression in WT1-TCR gene–transduced CD8+ T cells.” (C) IFN-γ production and degranulation of WT1-siTCR–transduced and WT1-coTCR–transduced CD8+ T cells in response to stimulation with WT1 peptide. Populations of WT1 tetramer-positive cells in WT1-coTCR– and WT1-siTCR–transduced CD8+ T cells before stimulation are shown in the upper column. The CD8+/WT1-tetramer+ cells shown with a broken line in each sample were analyzed for intracellular IFN-γ production and surface CD107a expression. One set of data obtained from experiments performed using CD8+ T cells from 2 different donors are representatively shown. (D) Cytotoxic activities of WT1-siTCR–transduced CD8+ T cells (○) and WT1-coTCR–transduced CD8+ T cells (●) against C1R-A*24:02 cells loaded with or without WT1 peptide and K562 cells transduced with or without HLA-A*24:02 gene were examined by standard 5-hour 51Cr-release assays at various effector/target (E/T) ratios.
Enhanced expression of introduced WT1-specific TCR and augmented functionality in WT1-siTCR–transduced CD8+ T cells. (A) An HLA-A*02:01-restricted HBZ26–34-specific CTL clone (HBZ-1) was transduced with the WT1-siTCR or WT1-coTCR vector. Expression of the introduced WT1-specific and intrinsic HBZ-specific TCRs in TCR gene-modified HBZ-1 cells was examined using either HLA-A*24:02/WT1 tetramer or HLA-A*02:01/HBZ tetramer. A non–gene-modified HBZ-1 clone was used as a negative control. (B) HBZ-1 cells (▵), WT1-coTCR–transduced HBZ-1 cells (●), and WT1-siTCR–transduced HBZ-1 cells (○) were cocultured with HLA-A*02:01-positive T2 cells loaded with various concentrations of HBZ peptide for 3 hours. Thereafter, surface CD107a expression was analyzed as detailed in “Detection of CD107a and intracellular IFN-γ expression in WT1-TCR gene–transduced CD8+ T cells.” (C) IFN-γ production and degranulation of WT1-siTCR–transduced and WT1-coTCR–transduced CD8+ T cells in response to stimulation with WT1 peptide. Populations of WT1 tetramer-positive cells in WT1-coTCR– and WT1-siTCR–transduced CD8+ T cells before stimulation are shown in the upper column. The CD8+/WT1-tetramer+ cells shown with a broken line in each sample were analyzed for intracellular IFN-γ production and surface CD107a expression. One set of data obtained from experiments performed using CD8+ T cells from 2 different donors are representatively shown. (D) Cytotoxic activities of WT1-siTCR–transduced CD8+ T cells (○) and WT1-coTCR–transduced CD8+ T cells (●) against C1R-A*24:02 cells loaded with or without WT1 peptide and K562 cells transduced with or without HLA-A*24:02 gene were examined by standard 5-hour 51Cr-release assays at various effector/target (E/T) ratios.
Next, we further investigated whether enhanced expression of the introduced TCR by the WT1-siTCR vector actually up-regulated the function of engineered CD8+ T cells. To do this, we compared the amounts of intracellular IFN-γ production and degrees of granular exocytosis by WT1-coTCR– and WT1-siTCR– transduced CD8+ T cells in response to stimulation with WT1 peptide. As shown in the upper column of Figure 1C, the proportion of tetramer-positive cells in WT1-siTCR–transduced CD8+ T cells was higher than that in WT1-coTCR–transduced CD8+ T cells. The data for intracellular IFN-γ production and CD107a expression in WT1 tetramer-positive cells are shown in the lower columns. The degrees of both IFN-γ production and CD107a expression in WT1-siTCR–transduced CD8+ T cells in response to stimulation with WT1 peptide appeared to be higher than those in WT1-coTCR-transduced CD8+ T cells.
Similarly, as shown in Figure 1D, up-regulation of cytotoxicity mediated by WT1-siTCR–transduced CD8+ T cells against both WT1 peptide-loaded target cells and HLA-A*24:02-positive leukemia cells was detected compared with that mediated by WT1-coTCR–transduced CD8+ T cells.
Enhanced expandability without deterioration of antigen-specific responsiveness of WT1-siTCR–transduced CD8+ T cells
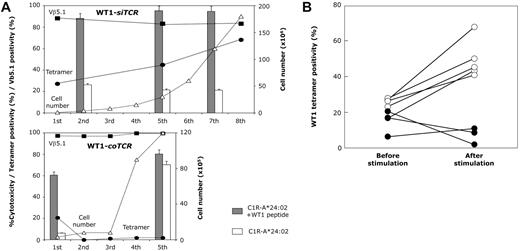
Efficient expansion of transferred T cells in vivo as well as in vitro is an important issue affecting the efficacy of adoptive T-cell therapy. Therefore, we compared the expandability of WT1-siTCR–transduced and WT1-coTCR–transduced CD8+ T cells after repeated stimulation with WT1 peptide. Representative data are shown in Figure 2A. WT1-siTCR–transduced CD8+ T cells showed good expansion after stimulation with WT1 peptide and maintained their antigen specificity. In contrast, WT1-coTCR–transduced CD8+ T cells showed rapid growth, but their WT1 specificity declined rapidly. A summary of this experiment using WT1-siTCR–transduced CD8+ T cells generated from 5 donors and WT1-coTCR–transduced CD8+ T cells generated from 3 donors is shown in Figure 2B.
Enhanced expandability of WT1-siTCR–transduced CD8+ T cells by repetitive stimulation with WT1 peptide in vitro. (A) WT1-siTCR–transduced CD8+ T cells (top) and WT1-coTCR–transduced CD8+ T cells (bottom) were repetitively stimulated with HLA-A*24:02-positive LCLs loaded with WT1 peptide in vitro. Total cell number (▵), percentage of Vβ5.1-positive cells (■), and percentage of HLA-A*24:02/WT1 tetramer-positive cells (●) were monitored after stimulation with WT1 peptide. Cytotoxic activities of WT1-siTCR–transduced CD8+ T cells and WT1-coTCR–transduced CD8+ T cells against WT1 peptide-loaded (gray bars) or -unloaded C1R-A*24:02 cells (white bars) are also shown. (B) Percentages of HLA-A*24:02/WT1 tetramer-positive cells among WT1-siTCR–transduced CD8+ T cells from 5 donors (○) and those in WT1-coTCR–transduced CD8+ T cells from 3 donors (●) before and after stimulation with WT1 peptide.
Enhanced expandability of WT1-siTCR–transduced CD8+ T cells by repetitive stimulation with WT1 peptide in vitro. (A) WT1-siTCR–transduced CD8+ T cells (top) and WT1-coTCR–transduced CD8+ T cells (bottom) were repetitively stimulated with HLA-A*24:02-positive LCLs loaded with WT1 peptide in vitro. Total cell number (▵), percentage of Vβ5.1-positive cells (■), and percentage of HLA-A*24:02/WT1 tetramer-positive cells (●) were monitored after stimulation with WT1 peptide. Cytotoxic activities of WT1-siTCR–transduced CD8+ T cells and WT1-coTCR–transduced CD8+ T cells against WT1 peptide-loaded (gray bars) or -unloaded C1R-A*24:02 cells (white bars) are also shown. (B) Percentages of HLA-A*24:02/WT1 tetramer-positive cells among WT1-siTCR–transduced CD8+ T cells from 5 donors (○) and those in WT1-coTCR–transduced CD8+ T cells from 3 donors (●) before and after stimulation with WT1 peptide.
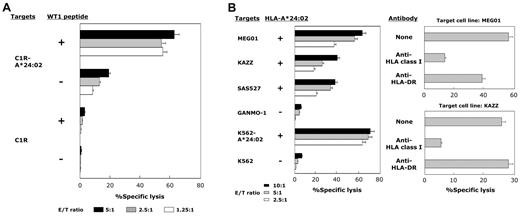
We further confirmed the WT1 specificity and HLA-A*24:02 restriction of cytotoxicity mediated by WT1-siTCR–transduced CD8+ T cells that had been cultured and expanded for more than 2 months with repeated WT1 peptide stimulation. Representative data for 5 experiments are shown in Figure 3A. WT1-siTCR–transduced CD8+ T cells after > 2 months of culture appeared to be totally positive for TCR-Vβ5.1 expression, and > 70% of the cells were positive for HLA-A*24:02/WT1-tetramer staining. These WT1-siTCR–transduced CTLs exerted strong cytotoxicity against WT1 peptide-loaded but not peptide-unloaded C1R-A*24:02 cells. Similarly, they exerted strong cytotoxicity against HLA-A*24:02-positive but not HLA-A*24:02-negative leukemia cell lines, as shown in Figure 3B. Their cytotoxicity against HLA-A*24:02-positive leukemia cell lines was significantly abrogated by anti-HLA class I MoAb. These data indicate that stimulation of WT1-siTCR–transduced CD8+ T cells with WT1 peptide might be effective for their expansion while maintaining their antigen specificity.
HLA-A*24:02-restricted and WT1 peptide-specific cytotoxicity mediated by WT1-siTCR–transduced CTLs after repeated stimulation with WT1 peptide. (A) Cytotoxicity of WT1-siTCR-transduced CD8+ T cells that had been cultured continuously for > 2 months against WT1 peptide-loaded or -unloaded C1R-A*24:02 cells and C1R cells was examined by 5-hour 51Cr-release assays. (B) Cytotoxicity of WT1-siTCR–transduced CD8+ T cells that had been cultured continuously for > 2 months against HLA-A*24:02-positive and HLA-A*24:02-negative leukemia cell lines was examined by 5-hour 51Cr-release assays. HLA class I-restriction of cytotoxicity mediated by WT1-siTCR–transduced CTLs against MEG01 and KAZZ cell lines was examined by 5-hour 51Cr-release assays at an E/T ratio of 5:1 in the presence or absence of anti-HLA class I MoAb or anti-HLA-DR MoAb.
HLA-A*24:02-restricted and WT1 peptide-specific cytotoxicity mediated by WT1-siTCR–transduced CTLs after repeated stimulation with WT1 peptide. (A) Cytotoxicity of WT1-siTCR-transduced CD8+ T cells that had been cultured continuously for > 2 months against WT1 peptide-loaded or -unloaded C1R-A*24:02 cells and C1R cells was examined by 5-hour 51Cr-release assays. (B) Cytotoxicity of WT1-siTCR–transduced CD8+ T cells that had been cultured continuously for > 2 months against HLA-A*24:02-positive and HLA-A*24:02-negative leukemia cell lines was examined by 5-hour 51Cr-release assays. HLA class I-restriction of cytotoxicity mediated by WT1-siTCR–transduced CTLs against MEG01 and KAZZ cell lines was examined by 5-hour 51Cr-release assays at an E/T ratio of 5:1 in the presence or absence of anti-HLA class I MoAb or anti-HLA-DR MoAb.
Lysis of autologous leukemia cells and lack of damage to autologous hematopoietic progenitor cells by WT1-siTCR–transduced CTLs
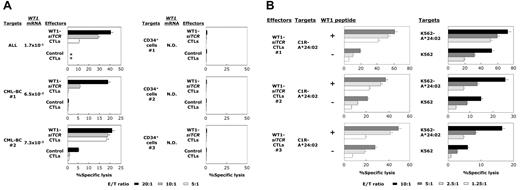
For clinical application of adoptive T-cell therapy using WT1-TCR gene-engineered CTLs, it is essential to obtain evidence that autologous leukemia cells are indeed lysed and that autologous hematopoietic progenitor cells are not damaged by WT1-siTCR–transduced CTLs. We therefore performed cytotoxicity assays using WT1-siTCR–transduced CTLs as effector cells, and autologous leukemia cells from leukemia patients and hematopoietic progenitors from cord blood as target cells. As shown in Figure 4A, WT1-siTCR–transduced CTLs generated from peripheral blood CD8+ T cells of patients with leukemia exerted cytotoxicity against autologous leukemia cells. On the other hand, those generated from cord blood CD8+ T cells exerted no cytotoxicity against autologous hematopoietic progenitor cells. As shown in Figure 4B, all WT1-siTCR–transduced CTL lines made from cord blood CD8+ T cells used in this experiment appeared to efficiently lyse WT1 peptide-loaded HLA-A*24:02-positive LCLs and HLA-A*24:02-positive leukemia cell lines without the addition of exogenous WT1 peptide.
Cytotoxicity mediated by WT1-siTCR–transduced CTLs against autologous leukemia cells and hematopoietic progenitor cells. (A) Cytotoxic activities of WT1-siTCR–transduced CTLs against autologous leukemia cells and autologous normal hematopoietic progenitor cells were examined by 5-hour 51Cr-release assays. WT1-siTCR–transduced CTLs were generated from peripheral blood CD8+ T cells from a patient with acute lymphoblastic leukemia (ALL) in complete remission, 2 patients with blastic crisis of chronic myelogenous leukemia (CML-BC) in chronic phase after chemotherapy, and cord blood CD8+ T cells from 3 donors. Their cytotoxicity against autologous freshly isolated leukemia cells or autologous hCB-CD34+ cells was examined by standard 51Cr-release assays at various E/T ratios. The relative expression levels of WT1 mRNA in target cells are shown. N.D. indicates not detectable. (B) Cytotoxicity mediated by WT1-siTCR–transduced CTLs generated from cord blood CD8+ T cells against C1R-A*24:02 cells with or without loaded WT1 peptide, K562-A*24:02 cells, and K562 cells was examined by 5-hour 51Cr-release assays at various E/T ratios. Each number of effector cells (#1, #2, and #3) corresponds to that of the hCB-CD34+ cell sample shown in Figure 4A, respectively.
Cytotoxicity mediated by WT1-siTCR–transduced CTLs against autologous leukemia cells and hematopoietic progenitor cells. (A) Cytotoxic activities of WT1-siTCR–transduced CTLs against autologous leukemia cells and autologous normal hematopoietic progenitor cells were examined by 5-hour 51Cr-release assays. WT1-siTCR–transduced CTLs were generated from peripheral blood CD8+ T cells from a patient with acute lymphoblastic leukemia (ALL) in complete remission, 2 patients with blastic crisis of chronic myelogenous leukemia (CML-BC) in chronic phase after chemotherapy, and cord blood CD8+ T cells from 3 donors. Their cytotoxicity against autologous freshly isolated leukemia cells or autologous hCB-CD34+ cells was examined by standard 51Cr-release assays at various E/T ratios. The relative expression levels of WT1 mRNA in target cells are shown. N.D. indicates not detectable. (B) Cytotoxicity mediated by WT1-siTCR–transduced CTLs generated from cord blood CD8+ T cells against C1R-A*24:02 cells with or without loaded WT1 peptide, K562-A*24:02 cells, and K562 cells was examined by 5-hour 51Cr-release assays at various E/T ratios. Each number of effector cells (#1, #2, and #3) corresponds to that of the hCB-CD34+ cell sample shown in Figure 4A, respectively.
In vivo antileukemia efficacy of adoptively transferred WT1-siTCR–transduced CTLs in a xenograft mouse model
We further examined the in vivo antileukemia efficacy of adoptive transfer with WT1-siTCR–transduced CTLs using 3 cohorts of a therapeutic xenograft mouse model. In the first group, NOG mice were inoculated with K562-A*24:02 cells that had been preincubated with non–gene-modified CD8+ T cells (control CTLs), and additionally treated by intravenous infusion of control CTLs weekly for a total of 5 times. Control CTLs were prepared by stimulation of peripheral blood CD8+ T cells with anti-CD3 MoAb and cultured in IL-2–containing medium. In the second group, NOG mice were inoculated with K562-A*24:02 cells that had been preincubated with WT1-siTCR–transduced CTLs, without additional cell transfer. In the third group, NOG mice were inoculated with K562-A*24:02 cells that had been preincubated with WT1-siTCR–transduced CTLs and received additional cell transfer with WT1-siTCR–transduced CTLs weekly 5 times. The growth curves of the inoculated leukemia cells are shown in Figure 5. K562-A*24:02 cells grew rapidly in all mice treated with control CTLs and died within 40 days. Compared with mice treated with control CTLs, the survival of mice inoculated with K562-A*24:02 cells that had been preincubated with WT1-siTCR–transduced CTLs was significantly prolonged. Furthermore, additional transfer of WT1-siTCR–transduced CTLs further significantly prolonged the survival period of K562-A*24:02-inoculated mice. Notably, no tumor formation was detected in mice during adoptive transfer of WT1-siTCR–transduced CTLs. These results clearly show the efficacy of adoptive T-cell therapy using WT1-siTCR–transduced CTLs for treatment of human leukemia.
Antileukemia effect of adoptively transferred WT1-siTCR–transduced CTLs in a xenograft mouse model. WT1-siTCR–transduced CTLs and non–gene-modified human CD8+ T cells (control CTLs) were prepared from peripheral blood CD8+ T cells. NOG mice were inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs or control CTLs with or without additional cell therapy. (○) represents the growth of leukemia cells in 2 control mice inoculated with K562-A*24:02 cells preincubated with control CTLs and into which control CTLs were transferred weekly; gray circles, leukemia cell growth in 2 mice inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs without additional transfer of CTLs; and (●), the growth of leukemia cells in 2 mice inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs, and into which WT1-siTCR–transduced CTLs were additionally transferred weekly. The time points (in days) after transplantation of K562-A*24:02 cells when the mice died are indicated.
Antileukemia effect of adoptively transferred WT1-siTCR–transduced CTLs in a xenograft mouse model. WT1-siTCR–transduced CTLs and non–gene-modified human CD8+ T cells (control CTLs) were prepared from peripheral blood CD8+ T cells. NOG mice were inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs or control CTLs with or without additional cell therapy. (○) represents the growth of leukemia cells in 2 control mice inoculated with K562-A*24:02 cells preincubated with control CTLs and into which control CTLs were transferred weekly; gray circles, leukemia cell growth in 2 mice inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs without additional transfer of CTLs; and (●), the growth of leukemia cells in 2 mice inoculated with K562-A*24:02 cells preincubated with WT1-siTCR–transduced CTLs, and into which WT1-siTCR–transduced CTLs were additionally transferred weekly. The time points (in days) after transplantation of K562-A*24:02 cells when the mice died are indicated.
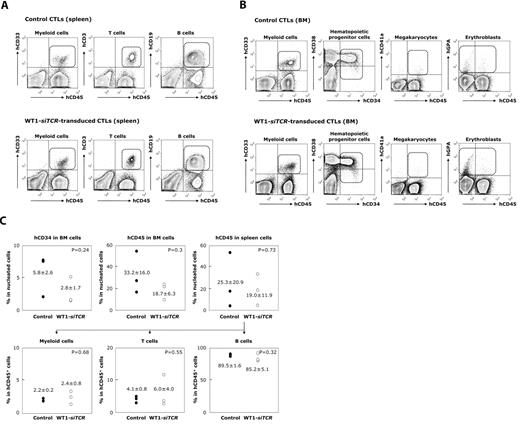
No deteriorative effect of WT1-siTCR–transduced CTLs on engraftment and differentiation of autologous hematopoietic progenitor cells in humanized mice
Finally, we addressed the issue of whether WT1-siTCR–transduced CTLs exert an inhibitory effect on the proliferation and differentiation of normal hematopoietic progenitor cells, as it has been reported that WT1 expression is detectable in normal hematopoietic progenitor cells.21,22,36 The hCB-CD34+ cells that had been preincubated with WT1-siTCR–transduced CTLs or control CTLs were transplanted into NOG mice. Three months later, these mice were killed and analyzed for engraftment and differentiation of human hematopoietic cells. HLA-A*24:02 appeared to be efficiently expressed in human blood cells that had proliferated in humanized mice (data not shown). Representative data for 3 experiments are shown in Figure 6A. It is clearly evident that human CD34+ cells preincubated with WT1-siTCR–transduced CTLs were successfully engrafted and differentiated into human peripheral blood cell components, including hCD45+/CD33+ myeloid cells, hCD45+/CD19+ B cells, and hCD45+/CD3+ T cells in the spleen, as was the case for NOG mice transplanted with human CD34+ cells that had been preincubated with control CTLs. In bone marrow, not only hCD45+/CD33+ myeloid cells, but also hCD45+/CD34+ hematopoietic progenitor cells, hCD45+/hGPA+ erythroid immature cells, and hCD45+/CD41a+ megakaryocytic immature cells were efficiently engrafted and differentiated from human CD34+ cells preincubated with WT1-siTCR–transduced CTLs as well as control CTLs. Although interindividual differences in engraftment efficacy were detected among NOG mice transplanted with human CD34+ cells, it was concluded thatWT1-siTCR–transduced CTLs never damage human CD34+ hematopoietic progenitor cells (Figure 6B).
Lack of an inhibitory effect of WT1-siTCR–transduced CTLs on human hematopoiesis in a humanized mouse model. HLA-A*24:02-positive hCB-CD34+ cells preincubated with autologous WT1-siTCR–transduced CTLs or non–gene-modified autologous CTLs (as a negative control) were transplanted into NOG mice. Three months later, the mice were killed and examined for engraftment and differentiation of human hematopoietic progenitor cells in the spleen and bone marrow. (A) Representative data for 3 experiments. (B) Summary of long-term hematopoiesis of engrafted human hematopoietic progenitor cells in the bone marrow and spleen of mice transplanted with hCB-CD34+ cells that had been preincubated with control CTLs or WT1-siTCR–transduced CTLs.
Lack of an inhibitory effect of WT1-siTCR–transduced CTLs on human hematopoiesis in a humanized mouse model. HLA-A*24:02-positive hCB-CD34+ cells preincubated with autologous WT1-siTCR–transduced CTLs or non–gene-modified autologous CTLs (as a negative control) were transplanted into NOG mice. Three months later, the mice were killed and examined for engraftment and differentiation of human hematopoietic progenitor cells in the spleen and bone marrow. (A) Representative data for 3 experiments. (B) Summary of long-term hematopoiesis of engrafted human hematopoietic progenitor cells in the bone marrow and spleen of mice transplanted with hCB-CD34+ cells that had been preincubated with control CTLs or WT1-siTCR–transduced CTLs.
Discussion
In our previous study, we developed a novel retroviral vector system that can express antigen-specific TCR more efficiently based on the concept of siRNA-targeting of the constant regions of the endogenous TCR-α and TCR-β genes and siRNA-resistant codon-optimization of exogenous TCR genes.25 To apply the basic concept of WT1-siTCR clinically for treatment of human leukemia, we investigated in detail the efficacy and safety of this strategy. Consequently, we demonstrated the marked advantages of WT1-siTCR gene transfer for adoptive immunotherapy in terms of both enhancement of the antileukemia effect and safety. First, we clearly demonstrated that up-regulated expression of introduced WT1-specific TCR and sufficient inhibition of endogenous TCR could be achieved using an experimental system in which the WT1-siTCR gene was transduced into a HBZ-specific T-cell clone. Enhanced expression of the introduced WT1-TCR on WT1-siTCR–transduced CTLs resulted in augmentation of WT1-specific cytotoxicity as compared with that mediated by WT1-coTCR–transduced CTLs. In addition, through repetitive stimulation with cognate peptide in vitro, WT1-siTCR–transduced CTLs showed marked expandability while maintaining their antigen specificity. Furthermore, the WT1-siTCR–transduced CTLs were able to successfully lyse autologous leukemia cells but not normal hematopoietic progenitor cells. Importantly, experiments using a xenograft mouse model revealed that adoptively transferred WT1-siTCR–transduced CTLs effectively inhibited leukemia cell growth in vivo. In contrast, the engraftment and differentiation abilities of normal hematopoietic progenitors showed no deterioration in the presence of WT1-siTCR–transduced CTLs, thus negating the possibility that WT1-specific CTLs might mediate severe bone marrow failure.
One of the major advantages of TCR gene-engineered T-cell immunotherapy revealed by our present series of experiments is the establishment of augmented antigen-specific cytotoxicity and safety through silencing of endogenous TCR gene expression. Recent clinical studies using TCR gene-transduced T cells have indicated that almost all of these cells disappeared in patients within 2 months after infusion.8,37 Moreover, there appeared to be a significant correlation between clinical response and the persistence of infused T cells in the peripheral blood of patients.9 Therefore, it would be important to maintain a sufficient number of TCR gene-engineered T cells with adequate antigen specificity in patients for a long period to achieve a good clinical response. In our present study, the antigen specificity of WT1-coTCR–transduced CTLs declined rapidly during culture, even though they were stimulated repeatedly with WT1 peptide. This might have been because of the formation of mispaired TCRs that had acquired nonspecific reactivity. In contrast, WT1-siTCR–transduced CTLs appeared to be markedly expanded while maintaining WT1 specificity and showing enhanced WT1-specific cytotoxicity for more than 2 months as a result of repetitive stimulation with WT1 peptide. Recently, it has been reported that adoptively transferred gp100-specific murine T cells were expandable up to 1000-fold after cognate peptide vaccination, resulting in an effective antitumor response in vivo.38 These data strongly support the practical value of WT1-siTCR for maintaining the WT1 specificity of TCR gene-modified CTLs for a long period in vivo and also suggest that WT1 peptide vaccination after adoptive transfer of WT1-siTCR–transduced CTLs would facilitate the expansion of WT1-specific CTLs in human patients.
One of the major concerns related to adoptive transfer of TCR gene-engineered T cells is the possibility of evoking severe autoimmunity mediated by mispaired TCR. Recently, an elegant study of TCR gene therapy using a mouse model has revealed that mispairing of introduced and endogenous TCR chains in TCR gene-modified T cells leads to the formation of self-reactive TCRs that are responsible for lethal graft-versus-host disease.19 Furthermore, it has been reported that adjustments in the design of gene therapy vectors for preventing the formation of mispaired TCRs could reduce the risk of TCR gene therapy-induced lethal autoimmunity.19 This evidence obtained from basic research strongly supports the clinical advantage of our WT1-siTCR vector.
It is also notable that autologous HLA-restricted and foreign antigen-derived peptide-specific TCRs can exert allogeneic HLA responsiveness. Recently, the frequent incidence of allogeneic HLA reactivity mediated by redirected T cells against predefined virus antigens has been reported.39 Using an LCL panel, we similarly observed that our HLA-A*24:02-restricted and WT1 peptide-specific TCR gene-transduced CTLs responded to the HLA-B*57:01 molecule in the absence of cognate WT1 peptide.40 Therefore, TCR gene-engineered T cells should be tested for their allogeneic HLA reactivity against recipient cells before administration.
Another notable finding in the present study was that WT1-siTCR-transduced CTLs never exerted a cytotoxic effect on normal hematopoietic progenitor cells. Although it has been proposed that WT1 is an ideal tumor-associated antigen,41 previous reports have indicated that WT1 expression is certainly detectable in normal hematologic progenitor cells,21,22,36 suggesting a risk of bone marrow failure mediated by WT1-specific CTLs. Furthermore, the occurrence of severe leukocytopenia after WT1 peptide vaccination in 2 patients with myelodysplastic syndrome has been reported.42 Therefore, we examined in detail the inhibitory effect of WT1-siTCR–transduced CTLs on normal human hematopoiesis in a humanized mouse model. We clearly demonstrated that WT1-siTCR–transduced CTLs never damaged normal human hematopoietic progenitor cells. There are 3 possible explanations for the mechanism underlying the resistance of normal hematopoietic progenitors to WT1-specific CTL-mediated cytotoxicity. The first is that the amount of WT1 expressed in normal hematopoietic progenitors is not enough to be recognized by CTLs. This possibility seems likely because quantitative analysis has revealed that the expression level of WT1 mRNA in normal hematopoietic progenitor cells is relatively low compared with that in leukemia cells.43,44 The second is that normal hematopoietic progenitors have the potential to resist CTL-mediated cytotoxicity. We previously reported that, although the levels of WT1 expression in myeloma and lymphoma cells were almost the same, only myeloma cells were lysed efficiently by WT1-specific CTLs. The extent of membrane damage induced by purified perforin appeared to be significantly higher in myeloma cells than in lymphoma cells.32 Therefore, the susceptibility of membranes to perforin is an important factor determining the sensitivity of target cells to CTL-mediated cytotoxicity, and normal hematopoietic cells are relatively resistant to CTL-mediated granule exocytosis. The third is that the introduced WT1-specific TCR in this study might have had an optimal range of avidity for recognition of leukemia cells but that spare normal hematopoietic progenitor cells physiologically expressed WT1, as avidity enhancement of the introduced TCR was able to evoke “on-target” adverse events against normal tissues.9
In conclusion, the present study has revealed that our WT1-siTCR retrovirus vector system shows considerable promise in terms of efficacy and safety for adoptive immunotherapy for leukemia using TCR gene-engineered T cells. On the basis of our data, we intend to begin clinical trials of adoptive WT1-siTCR–transduced T-cell therapy with WT1 peptide vaccination for chemotherapy-resistant leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kenji Kameda, Ehime University, Japan, for skilled technical assistance; Dr Yoshiki Akatsuka, Department of Hematology, Fujita Health University, Japan, for supplying the K562-A*24:02 cell line; Dr Midori Okumura and Dr Tomihiro Katayama, Department of Obstetrics and Gynecology, Ehime University Graduate School of Medicine, Japan, for supplying cord blood samples; Dr Hiroo Saji, HLA Laboratory, Japan, for HLA typing; and Dr Erik Hooijberg, Vrije Universiteit Medisch Centrum, The Netherlands, for supplying the Jurkat/MA cell line.
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (H.F. and M.Y.), the Ministry of Health, Labor and Welfare (Grant-in-Aid for Cancer Research; M.Y.), and the Third Term Comprehensive Control Research for Cancer (K.K.).
Authorship
Contribution: T.O. designed and performed the research and wrote the paper; H.F. designed and performed the research, wrote and edited the paper, and provided financial support; S.O., J.A., K.N., T.S., J.M., and H.S. discussed and interpreted the experimental results and provided materials; K.K. made and supplied the tetramer; and M.Y. discussed and interpreted the experimental results, wrote and edited the paper, and provided financial support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiroshi Fujiwara, Department of Bioregulatory Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; e-mail: yunarief@m.ehime-u.ac.jp; and Masaki Yasukawa, Department of Bioregulatory Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; e-mail: yasukawa@m.ehime-u.ac.jp.