The antimicrobial potential of neutrophil granulocytes is tightly linked to their adhesiveness and migratory behavior. In this issue of Blood, separate papers by Gambardella et al and by Costa et al present genetic evidence for important contributions of Rho family GAPs (GTPase activating proteins) in restraining neutrophil functions.
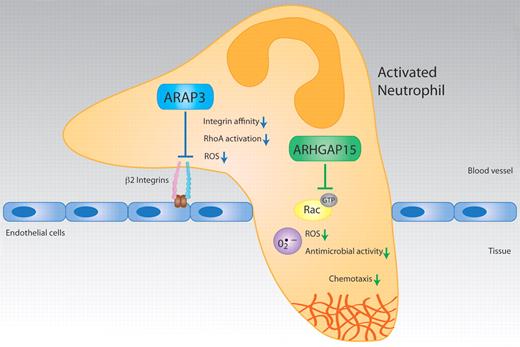
New papers by Gambardella et al and Costa et al, respectively, reveal that ARAP3 and ArhGAP15 are negative regulators of important neutrophil functions in vitro and in vivo, including integrin-mediated cell adhesion, ROS production, and chemotaxis. Illustration by Christine De Nardo and Waldemar Kolanus.
New papers by Gambardella et al and Costa et al, respectively, reveal that ARAP3 and ArhGAP15 are negative regulators of important neutrophil functions in vitro and in vivo, including integrin-mediated cell adhesion, ROS production, and chemotaxis. Illustration by Christine De Nardo and Waldemar Kolanus.
Neutrophil granulocytes are key contributors to fast and vigorous innate immune defenses against invading microorganisms. To this end, these normally blood-borne phagocytes are recruited into tissues via a process called diapedesis, in which these cells first activate their β-2 integrin adhesion receptors to stick firmly to the vessel walls of inflamed tissues, and egress from the bloodstream through the endothelial cell layer to reach the site of infection, where they can kill microorganisms, for example, by the production of reactive oxygen species (ROS).1 Integrin activation can occur very fast (in < a second following stimulation) and is controlled by intracellular signaling processes, which enable these receptors to alter their conformation or to form clusters (inside-out signaling).2 The neutrophil compartment is renewed every day, because systemic damage of these cells, which harbor a powerful but rather nonselective antimicrobial arsenal, would lead to disastrous consequences. At the site of infection, exhausted neutrophils enter programmed cell death (apoptosis) and are cleared by macrophages. Interestingly, the pro-inflammatory cytokine TNF can cause neutrophil apoptosis locally, but not systemically. To achieve this, TNF signaling in neutrophils is enabled by ligand-bound integrins, to ensure that nonadherent neutrophils are not inadvertently killed in the blood during an infection.3 Therefore, integrins do not function here as mere molecular glue, but they restrict and control other important signaling pathways in this cell type. Lack of β-2 integrins in neutrophils leads to a severe immune deficiency termed LAD I (leukocyte adhesion deficiency type I), which is characterized by relentless, recurrent bacterial infections and a high incidence of death.4 Neutrophils are among the most important cell types to control bacterial sepsis and septic shock, which remains a highly lethal problem.
Small GTPases of the Rho family are the most important intracellular switches to govern cellular shape and the ability of cells to move. They also are important regulators of neutrophil functions.5 The main action of these highly conserved proteins is the subcellular control of actin polymerization, but they are also involved in a variety of cellular activation pathways.6 RhoA and Rac are 2 members of this family, which regulate cell contractions and the formation of pseudopods, respectively.6 These proteins are active in the GTP-bound state, to which they are converted by guanine nucleotide exchange factors (GEFs). The latter remove the normally bound GDP so that GTP can enter, which is present at an excess concentration in the cytoplasm. GTPase activating proteins (GAPs), which enhance the intrinsic ability of the GTPase to hydrolyze GTP, complete the cycle. For reasons that are still not well understood, a very large variety of proteins exists that contain Rho GEF and GAP domains, and they are often embedded in modular proteins that bear numerous other activities and adapter functions.
One such protein is ARAP3,7 which includes GAP activities for RhoA and ARF6, another small GTPase involved in receptor recycling. ARAP3 also associates with Rap1, which, like RhoA, is a key regulator of integrin activation in immune cells.8 Gambardella et al9 have now generated a conditional ARAP3−/− mouse model in which they analyzed leukocyte functions. ARAP3 emerged as an important negative regulator of neutrophil activation. Globally, neutrophils from conditional ARAP3−/− mice showed unaltered ROS production, compared with wild-type cells (see figure). However, by employing a multivalent integrin ligand (polyRGD), which can activate ROS production in the absence of other inflammatory stimuli, Gambardella et al found that integrin-dependent ROS formation was significantly enhanced in ARAP3−/− neutrophils. This suggested an altered integrin function in these cells. Indeed, Gambardella et al found that the β-2 integrins from ARAP3−/− granulocytes showed significantly enhanced binding to their ligand ICAM-1 in solution, as well as stronger clustering of the adhesion receptors. Apparently, the control of β-2 integrin affinity and possibly also their avidity for relevant ligands was affected by the loss of ARAP3. Consequently, a number of other adhesion-dependent functions was found up-regulated in ARAP3−/− neutrophils, including adhesion and spreading in vivo and in vitro to several substrates, chemotaxis, or intracellular signaling involving protein kinase B or MAP kinase p38. On the mechanistic side, the authors analyzed the potential contribution of ARAP3 to GTPase activation pathways in neutrophils. Interestingly, a selective modulation of RhoA activity by ARAP3 was observed, while Rap1 activation was not affected in these experiments. Because ARAP3 can bind to Rap1, these findings apparently place ARAP3 as a negative regulator of RhoA downstream of Rap1, but this hypothesis needs further genetic and biochemical evaluation.
In the other study, Costa et al10 have produced a complete null allele for ArhGAP15,11 which had been identified earlier by the same group as a specific GAP for the GTPase Rac1.12 Rac1 is an important regulator of neutrophil migration, but it is also involved in the control of ROS production in this cell type by direct binding to p67phox, a NADPH oxidase. Costa et al analyzed leukocyte functions of the otherwise normal ArhGAP15−/− mice. First, they saw that the numbers of circulating neutrophils and macrophages were significantly decreased. Furthermore, macrophages from ArhGAP15−/− mice showed an altered morphology but normal migration. Finally, they discovered that neutrophil motility was strongly altered, indicating an important cell-selective role of ArhGAP15 (see figure). Neutrophils from these knockout animals showed increased motility with primarily the directional migration and cell polarization affected, as was shown by time-lapse video. The investigators used agarose chemotxis assays, where the cells have to squeeze underneath a solid agarose block to follow the chemotactic gradient, which causes strong flattening of the cells and thus more informative images. The enhancement of ArhGAP15−/− neutrophil migratory functions was accompanied by increases in phagocytosis, bacterial killing, and ROS generation. The enhanced ROS production seen in the knockout cells also depended on the stimulus employed: while ROS triggered by Fc receptors (ie, receptors of antibody-coated immune complexes) was normal in the knockout cells, the generation of oxygen species via fMLP or C5a (ie, G-protein coupled receptors) was strongly enhanced. Most importantly, the ArhGAP15−/− animals showed a clinically important phenotype because they were protected from severe polymicrobial abdominal sepsis. To this end, Costa et al had exposed wild-type and ArhGAP15−/− mice to a model of cecal ligation and perforation inducing sepsis. This resulted in a significantly increased neutrophil load in the cecum and in the peritoneum of the ArhGAP15−/− animals, while noninfected organs did not show any signs of altered neutrophil recruitment. Septic injury and mortality are strongly associated with exorbitant systemic inflammation and multiple cytokine production. Importantly, ArhGAP15−/− mice showed a dramatically reduced production of multiple cytokines during the experimental sepsis and the animals survived for extended periods of time while all control mice died within 48 hours.
Taken together these 2 papers provide novel and remarkable evidence for important negative regulatory roles of the GAP proteins ARAP3 and ArhGAP15 in neutrophil cell biology and pathophysiology. As has been shown for ArhGAP15, these findings might be exploited in the future in the clinical treatment of sepsis, for example, by the generation of specific, small molecular GAP inhibitors. Of course, there must be a reason for the evolution of such proteins, likely the maintenance of a high threshold for the onset of systemic inflammation. Thus, any future clinical application based on these factors would have to get around potentially significant side effects. Mechanistically, work needs to be done to fully understand the cell-specific actions of these GAPs. ArhGAPs bind to other GTPases such as the ARFs,8 and it would be interesting to see whether these factors are involved here as well. ARAP3 can function as an ARF-GAP in vitro, although its Rho-GAP activity appears to be more required for restraining neutrophil functions, as shown by Gambardella et al. Intriguingly, the ARF-GEF cytohesin-1 has been previously shown to be required for integrin-dependent leukocyte chemotaxis and, significantly, for RhoA activation.13 Future studies will tell whether the important discoveries presented in these 2 papers are linked more tightly at the molecular level than what is obvious now.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal