Abstract
Paratarg-7 (P-7) is a frequent paraprotein target in monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), and Waldenström macroglobulinemia. Patients with P-7-specific paraproteins carry a hyperphosphorylated paratarg-7 (pP-7). Because pP-7 carrier state is dominantly inherited, we determined the paraprotein targets in 4 families with familial MGUS/MM. No antigenic target was identified for the paraproteins from 2 members of one family. Paraproteins from affected members of 2 other families targeted P-7, and paraproteins from 4 affected members of a fourth family targeted P-8, which is encoded by the ATG13 gene. P-8 was hyperphosphorylated in the affected family members (pP-8) and pP-8 carrier state is inherited in a dominant fashion. Six additional autoantigenic nonfamilial paraprotein targets were also hyperphosphorylated in the respective patients compared with normal controls. We conclude that paraproteins of affected members with familial MGUS/MM share family-typical hyperphosphorylated antigens and hyperphosphorylation of paraprotein targets might be a general mechanism underlying the pathogenesis of MGUS/MM.
Introduction
Using a human fetal brain-derived protein macroarray in a modified SEREX1 approach, we identified paratarg-7 (P-7) as the target of 15% of IgA or IgG paraproteins2 and 11% of IgM paraproteins.3 All patients with P-7 specific paraproteins are carriers of a hyperphosphorylated version of the protein (pP-7), and this hyperphosphorylation is inherited in a dominant fashion.2,4 pP-7 carrier state is associated with an increased risk of developing IgA/IgG monoclonal gammopathy of undetermined significance (MGUS)/multiple myeloma (MM) (odds ratio = 7.9)2 and IgM-MGUS (odds ratio = 6.5).3 Because pP-7 is the first molecularly defined risk factor for any hematologic neoplasm known to date,2,3 we set out to define the antigenic targets of paraproteins from affected members of families with familial MGUS/MM.
Methods
Patients and controls
This study was approved by the local ethical review board (Ethikkommission der Ärztekammer des Saarlandes) and conducted according to the Declaration of Helsinki. Peripheral blood and serum samples from 31 healthy and 10 affected members of 4 families with familial MGUS/MM were collected after obtaining written informed consent.
Serum screening on high-density protein arrays, immunoblot analyses, absorption studies, IEF, and phosphatase treatment of paraprotein targets
These were performed as described before.2,5 Absorption studies using His6-tagged P-8 protein or erythrocyte lysates were performed as described before for P-7.2,5 Similarly, Western blot analyses, isoelectric focusing (IEF), and phosphatase treatment of all paraprotein targets were performed as described before for P-7.2,5
P-7 and P-8 ELISA
Results and discussion
Four families with ≥ 2 cases of MGUS/MM were included in this study. No antigenic target was identified for the paraproteins from a brother and a sister of the first family, both affected by MM. Two sisters diagnosed with MGUS in the second family whose pedigree had been described before2 and 2 siblings with MM in the third family (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) all had an anti–P-7 specific paraprotein and were carriers of pP-7.
Identification and characterization of P-8
The paraproteins from 2 MGUS and 2 MM patients of the fourth family (Figure 1) did not react with P-7. Screening of the protein macroarray identified a single protein as the antigenic target of all 4 paraproteins from these family members affected by MGUS/MM. Sequence analysis showed that this protein was coded by the ATG13 gene, a member of the autophagy regulatory complex family of genes.7 The specificity and paraprotein-mediated nature of the observed anti–P-8 reaction were shown by the high titer of the reaction (1:108) and by absorption with recombinant P-8 (supplemental Figure 2).
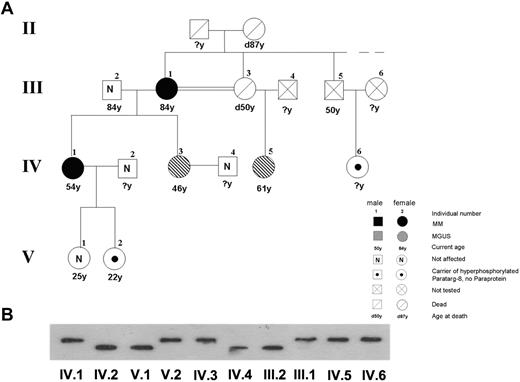
Pedigree of members of family 4, including 2 patients with MM and 2 with MGUS with a P-8 specific paraprotein carrying the hyperphosphorylated P-8. (A) The pedigree shows the family (family 4) of 2 patients with MM (III.1 and IV.1) and 2 patients with MGUS (IV.3 and IV.5), all having P-8 reactive paraproteins and carrying the hyperphosphorylated state of this protein. The pedigree is a part of a pedigree previously published.6 (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (III.2, IV.2, IV.4, and V.1) and hyperphosphorylated P-8 (III.1, IV.1, IV.6, and V.2) after IEF. The numbers indicate family members in different generations.
Pedigree of members of family 4, including 2 patients with MM and 2 with MGUS with a P-8 specific paraprotein carrying the hyperphosphorylated P-8. (A) The pedigree shows the family (family 4) of 2 patients with MM (III.1 and IV.1) and 2 patients with MGUS (IV.3 and IV.5), all having P-8 reactive paraproteins and carrying the hyperphosphorylated state of this protein. The pedigree is a part of a pedigree previously published.6 (B) Immunostaining of lysate bands derived from whole peripheral blood lysates from family members carrying wild-type (III.2, IV.2, IV.4, and V.1) and hyperphosphorylated P-8 (III.1, IV.1, IV.6, and V.2) after IEF. The numbers indicate family members in different generations.
To investigate the prevalence of paraproteins with P-8 specificity, 300 paraprotein-containing sera were tested for reactivity with P-8 by ELISA. Of these, only 1 reacted with P-8 (titer 1:107). Sequence analysis of P-8 derived from patients with a paraprotein-8 specific paraprotein and healthy controls excluded mutations or polymorphisms as a cause of the autoimmunogenicity of P-8. All lysates from patients with a paraprotein with anti–P-8 or non–P-8 specificity and healthy controls showed identical bands in the Western blots (data not shown). However, erythrocyte lysates from healthy controls and from patients with a P-8 specific paraprotein migrated differently in the IEF (supplemental Figure 3). After phosphatase treatment, all lysates showed identical bands in the IEF, regardless of whether they were derived from healthy controls, from patients having a paraprotein with specificity for P-8, or from patients with a paraprotein that did not bind to P-8. Differential banding in the IEF before and after treatment with alkaline phosphatase demonstrated that P-8 is phosphorylated in healthy persons, although to a lesser degree than in MGUS/MM patients with an anti–P-8 reactive paraprotein. All 4 MGUS/MM patients with an anti–P-8 paraprotein expressed hyperphosphorylated P-8, but hyperphosphorylation of P-8 was not observed in MM/MGUS patients whose paraprotein did not bind to P-8.
Only 1 of 200 healthy whites was shown to be carrier of pP-8 as shown by IEF, demonstrating that pP-8 has a much lower prevalence than pP-7 carrier state.
Phosphorylation state of other autoantigenic targets of paraproteins
All other molecularly defined autoantigenic targets of paraproteins from sporadic, nonfamilial cases of MGUS/MM identified by our group to date, where cells from the respective patients were available, were tested by IEF before and after phosphatase treatment (Table 1). In all these cases, the patients' paraprotein target was hyperphosphorylated compared with normal controls (supplemental Figure 4).
Hyperphosphorylation of autoantigenic paraprotein targets
| Paratarg no. . | Antigen . | Type of antigen . | No. of binding . | Subtype* . | Phosphorylation state . |
|---|---|---|---|---|---|
| 1 | Cylicin 2 | Allaontigen† | 1/115 nonfamilial cases | 1 × IgA-λ | No data available |
| 2 | TPP2 | Autoantigen‡ | 1/115 nonfamilial cases | 1 × IgG-κ | Hyperphosphorylated |
| 3 | IGFBP-2 | Autoantigen‡ | 1/115 nonfamilial cases | 1 × IgG-κ | No data available |
| 4 | Porcine kinesin | Heteroantigen | 1/115 nonfamilial cases | 1 × IgG-κ | No data available |
| 5 | Microtubule-associated protein | Autoantigen‡ | 2/103 nonfamilial cases | 1 × IgM-κ, 1 × IgG-λ | Hyperphosphorylated |
| 6 | LAPTM5 | Autoantigen‡ | 3/103 nonfamilial cases | 2 × IgG-λ, 1 × IgG-κ | Hyperphosphorylated |
| 7 | SLP-2 | Autoantigen‡ | 4 cases from 2 families; 76/650 nonfamilial cases | See Grass et al2 | Hyperphosphorylated |
| 8 | ATG13 | Autoantigen‡ | 4 cases from 1 family; 1/300 nonfamilial cases | 3 × IgG-λ, 1 × IgG-κ | Hyperphosphorylated |
| 9 | RSP16 | Autoantigen‡ | 1/300 nonfamilial cases | 1 × IgG-κ | Hyperphosphorylated |
| 10 | SPAG7 | Autoantigen‡ | 2/300 nonfamilial cases | 2 × IgG-λ | Hyperphosphorylated |
| 11 | SIVA | Autoantigen‡ | 2/300 nonfamilial cases | 1 × IgG-κ, 1 × IgA-λ | Hyperphosphorylated |
| Paratarg no. . | Antigen . | Type of antigen . | No. of binding . | Subtype* . | Phosphorylation state . |
|---|---|---|---|---|---|
| 1 | Cylicin 2 | Allaontigen† | 1/115 nonfamilial cases | 1 × IgA-λ | No data available |
| 2 | TPP2 | Autoantigen‡ | 1/115 nonfamilial cases | 1 × IgG-κ | Hyperphosphorylated |
| 3 | IGFBP-2 | Autoantigen‡ | 1/115 nonfamilial cases | 1 × IgG-κ | No data available |
| 4 | Porcine kinesin | Heteroantigen | 1/115 nonfamilial cases | 1 × IgG-κ | No data available |
| 5 | Microtubule-associated protein | Autoantigen‡ | 2/103 nonfamilial cases | 1 × IgM-κ, 1 × IgG-λ | Hyperphosphorylated |
| 6 | LAPTM5 | Autoantigen‡ | 3/103 nonfamilial cases | 2 × IgG-λ, 1 × IgG-κ | Hyperphosphorylated |
| 7 | SLP-2 | Autoantigen‡ | 4 cases from 2 families; 76/650 nonfamilial cases | See Grass et al2 | Hyperphosphorylated |
| 8 | ATG13 | Autoantigen‡ | 4 cases from 1 family; 1/300 nonfamilial cases | 3 × IgG-λ, 1 × IgG-κ | Hyperphosphorylated |
| 9 | RSP16 | Autoantigen‡ | 1/300 nonfamilial cases | 1 × IgG-κ | Hyperphosphorylated |
| 10 | SPAG7 | Autoantigen‡ | 2/300 nonfamilial cases | 2 × IgG-λ | Hyperphosphorylated |
| 11 | SIVA | Autoantigen‡ | 2/300 nonfamilial cases | 1 × IgG-κ, 1 × IgA-λ | Hyperphosphorylated |
The number of paraproteins with the respective specificity among all paraproteins tested for this specificity.
Cylicin 2 is sperm-specific; hence, it functioned as an alloantigen in the respective female patient.
Autoantigen, because it is expressed in the autologous patient's tissues.
Paraproteins from patients of families with multiple cases of MGUS/MM are directed against the same autoantigen: whereas no target could be identified for the paraproteins from 2 siblings of family 1, the paraproteins from each of 2 affected family members of family 2 and family 3 (supplemental Figure 1), respectively, recognized P-7, and the paraproteins from family 4 (Figure 1) reacted with P-8. The second intriguing finding of our study is that the autoantigens detected by the autologous paraproteins were hyperphosphorylated in the respective patients compared with normal controls in all (8 of 8) cases where this analysis was possible. The fact that all autoantigenic targets (and possible stimuli) of paraproteins molecularly defined to date are hyperphosphorylated suggests that hyperphosphorylation might be a general mechanism underlying the pathogenesis of MGUS/MM that is operative in many more cases than those with the 8 autoantigenic paraprotein targets described in this study. The elucidation of how this mechanism induces the clonal evolution of a B-cell clone with specificity for the respective autoantigen as well as the question why some carriers of hyperphosphorylated antigens develop MGUS/MM whereas others do not can now be addressed using specific molecular tools.
There have been a number of studies of familial MM, implicating both environmental and inherited factors,6,8-11 but results have been inconsistent.12 pP-7 and pP-8 are the first molecularly characterized structures that provide a plausible explanation for the familial clustering of cases of MGUS/MM, at least in cases with a P-7 (2 families) or P-8 (1 family to date) specific paraprotein. Although a molecular mimicry between these autoantigens and infectious agents cannot be definitely excluded, this mechanism is highly unlikely for 2 reasons: (1) a databank search comparing the autoantigenic targets with sequences from bacteria or viruses did not reveal significant homologies (data not shown); and (2) it would not explain why only the hyperphosphorylated, but not the wild-type, protein acts as an autoantigen. The hyperphosphorylation of the autoantigens appears to be the most obvious likely reason for their autoimmunogenicity. Indeed, “phosphoepitopes” were reported to induce stronger CD8+13 and CD4+14 T-cell responses than their nonphosphorylated counterparts. Whether the hyperphosphorylated autoantigenic targets of paraproteins induce the development of MGUS/MM by chronic antigenic stimulation or whether they are only a marker or an epiphenomenon of another susceptibility to develop MGUS/MM can now be investigated in the respective patients and their (not yet) affected relatives. Logical next steps are the identification of the kinases and phosphatases responsible for maintenance of the hyperphosphorylated state and the identification of the mutation or polymorphism(s) underlying the posttranslational modification of the autoantigenic targets of paraproteins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all American and German patients for participating in the study; Prof J. Geisel and M. Sand-Hill of the Saarland University Medical School Central Clinical Chemistry Laboratory; and Ms A. Bonaventura and Mr N. Zuschlag of Saarland University Central Immunology Laboratory for performing serum electrophoreses and immunofixations, respectively.
This work was supported by Förderverein Krebsforschung Saar-Pfalz-Mosel, HOMFOR (the research program of the Saarland University Faculty of Medicine) and Wilhelm Sander-Stiftung.
Authorship
Contribution: S.G. designed the experiments and wrote the manuscript; K.-D.P. designed the experiments; N.F. and E.R. performed and analyzed the experiments; F.Z. and L.T. recruited and helped analyze the family investigation for the study; S.T., D.D.W., V.W., and J.L. recruited and analyzed the American family for the study; and H.L. and M.P. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: K.-D.P. and M.P. have applied for a relevant patent. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, José-Carreras-Center for Immuno- and Gene Therapy, Department Internal Medicine I, Saarland University Medical School, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.
References
Author notes
H.L. and M.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal