Abstract
Unlike conventional T cells, which are exported from the thymus as naive cells and acquire effector functions upon antigen encounter in the periphery, a subset of γδ T cells differentiates into effectors that produce IL-17 within the fetal thymus. We demonstrate here that intrathymic development of the naturally occurring IL-17–producing γδ T cells is independent of STAT3 and partly dependent on RORγt. Comparative gene-expression analysis identified Hes1, one of the basic helix-loop-helix proteins involved in Notch signaling, as a factor specifically expressed in IL-17–producing γδ T cells. Hes1 is critically involved in the development of IL-17–producing γδ T cells, as evidenced by their severe decrease in the thymi of Hes1-deficient fetal mice. Delta-like 4 (Dll4)–expressing stromal cells support the development of IL-17–producing γδ T cells in vitro. In addition, conditional Hes1 ablation in peripheral γδ T cells decreases their IL-17 production but not their IFN-γ production. These results reveal a unique differentiation pathway of IL-17–producing γδ T cells.
Introduction
Conventional TCR αβ cells are exported from the thymus as naive T cells. After activation by exposure to their cognate antigens in the periphery, naive CD4+ αβ T cells differentiate into different helper T-cell lineages such as Th1, Th2, and Th17 cells, depending on the cytokine milieu, which induce different combinations of transcription factors. STAT3, RORγt, and RORα, which are induced by combined signals from TGFβ and IL-6 receptors, play important roles in the differentiation of Th17 cells by binding to the promoter or the enhancer region of the IL17 gene.1-3 STAT3 also inhibits the expression of Foxp3, which suppresses the functions of RORγt.4
In addition to Th17 cells, several subsets of T cells produce IL-17. These include T cells lacking CD4 and CD8, CD8+ T cells, invariant natural killer T cells (NKT cells), and TCR γδ T cells.5-8 There is accumulating evidence that TCR γδ T cells could be the major source of IL-17 in various murine models of infection such as Mycobacterium tuberculosis, Escherichia coli, and Listeria monocytogenes.9 IL-17–producing γδ T cells are also involved in the pathogenesis of autoimmune diseases such as experimental allergic encephalomyelitis, collagen-induced arthritis, chronic granulomatous disease, and ischemic brain injury.10 Interestingly, even freshly isolated γδ T cells from the thymus produce IL-17 in response to phorbol myristate acetate (PMA) and ionomycin stimulation, indicating the functional differentiation within the thymus.11 Such naturally occurring IL-17–producing γδ T cells were already detected at the fetal stage as early as on embryonic day 15 (E15), when γδ T cells began to develop.11 Therefore, in γδ T cells, the development and functional differentiation to IL-17 producers coincidentally occur within the fetal thymus. Intrathymic functional differentiation has been well documented for NKT cells.12 During intrathymic development, NKT cell precursors acquire either an IL-4– or an IFN-γ–producing function at different stages.13 Furthermore, there is a population of NKT cells that differentiates into IL-17–producing cells in the thymus independently of IL-6 and STAT3.6,14 Nevertheless, like Th17 cells, IL-17–producing NKT cells require RORγt for their development.15 At present, the molecular mechanisms for the development of IL-17–producing γδ T cells have not been defined, although it has been shown that IL-17–producing γδ T cells developed normally in IL-6–deficient mice but were decreased in the absence of TGF-β1.16,17
In the present study, we found that Hes1, one of the basic helix-loop-helix (bHLH) proteins induced by Notch signaling, was specifically expressed in IL-17–producing γδ T cells. Furthermore, Hes1, rather than STAT3 and RORγt, was critically involved in intrathymic development of IL-17–producing γδ T cells. Expression of Hes1 is also important for IL-17 production by γδ T cells in the periphery. Therefore, although Notch signaling is well known for its role in thymocyte development, it also regulates innate functions of γδ T cells in the thymus and in the periphery.
Methods
Mice
STAT3flox/flox mice,18 Tie-2-Cre transgenic (Tg) mice,19 and RORc−/− mice20 were used. Cβ−/− mice were purchased from The Jackson Laboratory. Hes1−/− mice21 and Hes1flox/flox mice22 were kindly provided by R. Kageyama (Institute for Virus Research, Kyoto, Japan). IL-17–green fluorescent protein (GFP) reporter mice were kindly provided by Y. Iwakura (Institute of Medical Science, University of Tokyo, Japan). For analyzing disruption of floxed STAT3, genomic DNA from purified γδ T cells was analyzed by PCR.23 For conditional ablation by inducing Cre recombinase driven by the IFN-inducible MX-1 promoter, 5 × 300 μg of poly(I)-poly(C) (P-1530; Sigma-Aldrich) was injected intraperitoneally into 4-week-old mice at 2-day intervals. Fetal mice were obtained from timed matings in which the day of finding a vaginal plug was designated as day 0 of embryonic development. Mice were maintained in specific pathogen-free conditions at our institute. This study was approved by the Committee of Ethics on Animal Experiments in the Faculty of Medicine, Kyushu University. Experiments were carried out under the control of the Guidelines for Animal Experiments.
Cell preparations from various tissues
Single-cell suspensions were prepared from fetal thymi, adult thymi, adult spleens, lamina propria lymphocytes (LPLs), and intraepithelial lymphocytes (IELs), as described previously.11
Antibodies and flow cytometric analysis
FITC-conjugated anti-TCRγδ (GL3) and anti-CD4 (L3T4) mAb; PE-conjugated anti–mIL-17 (eBio17B7), anti-TCRγδ (GL3), anti-CD25 (PC61.5), anti-NK1.1 (PK136), and anti-cKit (2B8) mAb; allophycocyanin-conjugated anti-TCRγδ (GL3) and anti-B220 (RA3-6B2) mAb; and PerCP-eFluor710–conjugated anti-TCRγδ (GL3) mAb were purchased from eBioscience. FITC-conjugated anti-Vγ4 (UC3-10A6) and anti-Vγ5(536) mAbs, Alexa Fluor 647–conjugated anti–mIL-17 (TC11-18H10) mAb, and allophycocyanin-conjugated mIFN-γ (XMG1.2) mAb were purchased from BD Biosciences. PE-conjugated anti-CD27 (LG.3A10) mAb was purchased from BioLegend. PE-conjugated anti-Vγ5(536) mAb was purchased from Santa Cruz Biotechnology. Stained cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences). Propidium iodide (1 μg/mL) was added to the cell suspension just before running on a flow cytometer to detect and exclude dead cells for the analysis of surface staining. The data were analyzed using CellQuest software Version 3.3 (BD Biosciences).
Intracellular cytokine staining
Cells were stimulated with 25 ng/mL of PMA (P-8139, Sigma-Aldrich) and 1 μg/mL of ionomycin (I-0634; Sigma-Aldrich) for 4 hours at 37°C; 10 μg/mL of brefeldin A (B-7651; Sigma-Aldrich) was added for the last 3 hours of incubation. After cells were stained with various mAbs for 20 minutes at 4°C, intracellular staining was performed according to the manufacturer's instructions (BD Biosciences).
qRT-PCR
Total RNA from hybridomas or cells sorted by FACSAria (BD Biosciences) was purified using the RNeasy Mini or Micro Kit (QIAGEN). The efficacy of cell sorting was consistently > 98%. The first-strand cDNA synthesis was done using Superscript I (Invitrogen) according to the manufacturer's instructions. Gene-specific primers were used as follows: Hes1, 5′-ACACCGGACAAACCAAAGAC-3′, 5′-ATGCCGGGAGCTATCTTTCT-3′; Hes5, 5′-CAAGGAGAAAAACCGACTGC-3′, 5′-GGCTTTGCTGTGTTTCAGGT-3′; RORγt, 5′-AGCTTTGTGCAGATCTAAGG-3′, 5′-TGTCCTCCTCAGTAGGGTAG-3′; Notch1, 5′-ACAACAACGAGTGTGAGTCC-3′, 5′-ACACGTGGCTCCTGTATATG-3′; β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. Quantitative RT-PCR (qRT-PCR) was performed on an ABI PRISM thermal cycler (Applied Biosystems) using SYBR Premix Ex Taq (RP041A; Takara). The 2−ΔΔCt equation was used to calculate the relative expression of target genes against that of β-actin.
Coculture with stromal cells
To induce T-cell differentiation in vitro, TSt-4 thymic stromal cells (TSt4/no) expressing murine Dll-4 gene (TSt-4/Dll4) were used as described previously.24 Three thousand fetal thymocytes (E15) were cocultured on a layer of TSt-4/no or TSt-4/Dll4 cells in 24-well plates for the indicated days. Culture was performed without additional cytokines, and half of the medium was changed every 3 days.
Statistics
Statistical significance was calculated using the Student t test using Prism software Version 4.0a (GraphPad). P < .05 was considered to be statistically significant.
Results
STAT3 is dispensable for the development of IL-17–producing γδ T cells
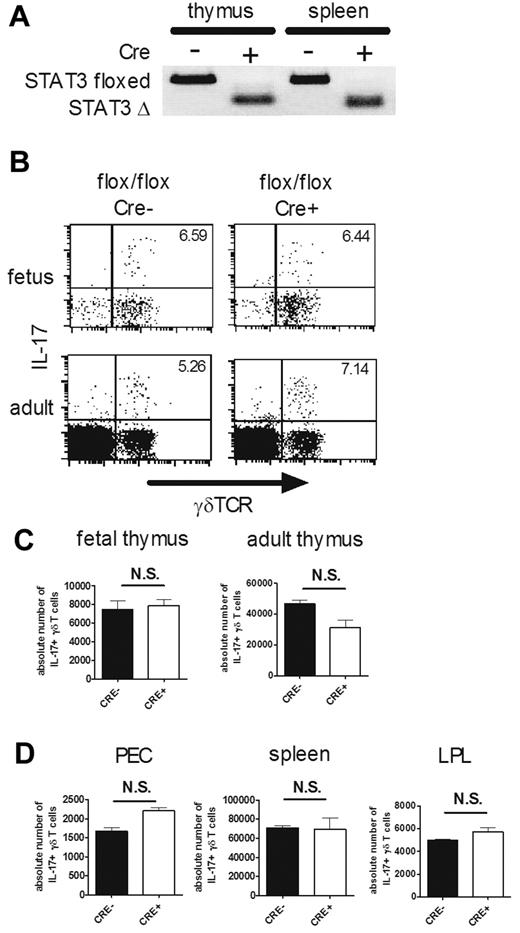
STAT3 is an important transcription factor for the differentiation of Th17 cells, which are IL-17–producing CD4+ αβ T cells.25 To explore the roles of STAT3 in the development of IL-17–producing γδ T cells, we crossed STAT3flox/flox mice with Tie2-Cre Tg mice, because STAT3−/− mice are embryonically lethal.26 Tie2 is a tyrosine kinase specifically expressed by hematopoietic progenitors and endothelial cells from E9.5.27 We confirmed that the floxed STAT3 allele was successfully disrupted in γδ T cells purified from the thymi and spleens of the conditional STAT3-deficient mice (Figure 1A). IL-17 production by γδ T cells in fetal and adult thymi was examined after brief stimulation with PMA and ionomycin, but there was no significant difference in the absolute number of IL-17–producing γδ T cells between STAT3-deficient and control mice (Figure 1B-C). Similarly, IL-17–producing γδ T cells were found equally in the periphery of the conditional STAT3-deficient and control mice (Figure 1D).
STAT3 is dispensable for the development of IL-17–producing γδ T cells. (A) γδ T-cell–specific disruption of the STAT3 gene in STAT3flox/floxTie2-Cre mice. Genomic DNA was prepared from purified γδ T cells from the thymi and spleens of STAT3flox/flox (Cre−) and STAT3flox/floxTie2-Cre (Cre+) mice and used for allele-specific PCR analysis. Floxed allele (STAT3 floxed, 350 bp) and disrupted allele (STAT3 Δ, 150 bp) are shown. (B-D) IL-17–producing γδ T cells in STAT3flox/flox (Cre−) and STAT3flox/floxTie2-Cre (Cre+) mice. Single-cell suspensions of fetal (E16) and adult thymocytes (4 weeks old; B-C) or PEC, spleens, and LPLs (4 weeks old; D) from STAT3flox/flox (flox/flox Cre−) and STAT3flox/floxTie2-Cre (flox/flox Cre+) mice were stimulated with PMA and ionomycin and analyzed for intracellular staining for IL-17. (B) Representative dot plots of intracellular staining for IL-17 in fetal (top panels) and adult (bottom panels) thymi are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδTCR+ cells. (C-D) Absolute numbers of IL-17+ γδ T cells in each organ are shown. Data shown are the means ± SD of 5 mice. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
STAT3 is dispensable for the development of IL-17–producing γδ T cells. (A) γδ T-cell–specific disruption of the STAT3 gene in STAT3flox/floxTie2-Cre mice. Genomic DNA was prepared from purified γδ T cells from the thymi and spleens of STAT3flox/flox (Cre−) and STAT3flox/floxTie2-Cre (Cre+) mice and used for allele-specific PCR analysis. Floxed allele (STAT3 floxed, 350 bp) and disrupted allele (STAT3 Δ, 150 bp) are shown. (B-D) IL-17–producing γδ T cells in STAT3flox/flox (Cre−) and STAT3flox/floxTie2-Cre (Cre+) mice. Single-cell suspensions of fetal (E16) and adult thymocytes (4 weeks old; B-C) or PEC, spleens, and LPLs (4 weeks old; D) from STAT3flox/flox (flox/flox Cre−) and STAT3flox/floxTie2-Cre (flox/flox Cre+) mice were stimulated with PMA and ionomycin and analyzed for intracellular staining for IL-17. (B) Representative dot plots of intracellular staining for IL-17 in fetal (top panels) and adult (bottom panels) thymi are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδTCR+ cells. (C-D) Absolute numbers of IL-17+ γδ T cells in each organ are shown. Data shown are the means ± SD of 5 mice. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
RORγt is partly required for the intrathymic development of IL-17–producing γδ T cells
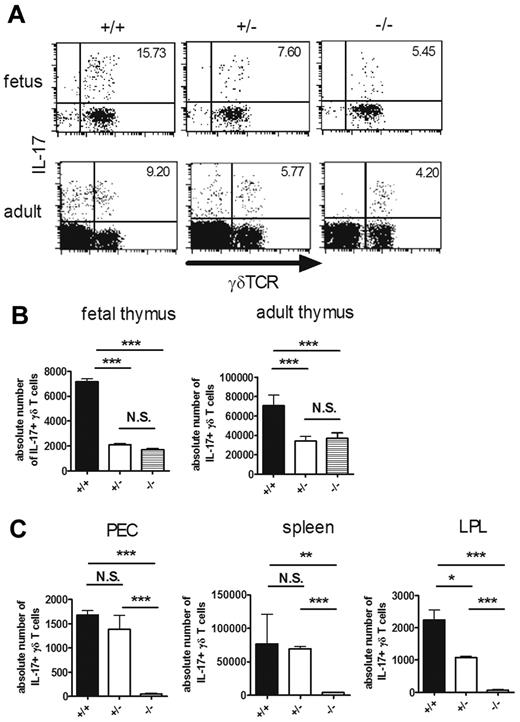
RORγt is indispensable for the differentiation of IL-17–producing NKT cells and Th17 cells.15,28 We next examined the role of RORγt in the development of IL-17–producing γδ T cells using RORγt-deficient (RORc−/−) mice. As described previously,20 the percentage of DP thymocytes was decreased in RORc−/− mice, although there was no significant decrease in the percentage or the absolute number of γδ T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IL-17–producing γδ T cells in fetal and adult thymi were significantly decreased in RORc−/− and RORc+/− mice compared with RORc+/+ mice (Figure 2A-B). However, there was no difference in the number of IL-17–producing γδ T cells between RORc+/− and RORc−/− mice. IL-17–positive cells in γδTCR-negative cells, which were detected in the adult thymi of RORc+/+ or RORc+/− mice, were strikingly decreased in RORc−/− mice (Figure 2A). We found that most of these non-γδ T cells producing IL-17 expressed αβ TCR and CD4 (supplemental Figure 2). In contrast to the thymus, IL-17–producing γδ T cells were virtually absent in the periphery of RORc−/− mice (Figure 2C). These results suggest that RORγt is only partly required for intrathymic development of IL-17–producing γδ T cells, but plays indispensable roles in their maintenance in the periphery.
RORγt is partly required for the intrathymic development of IL-17–producing γδ T cells. (A-C) IL-17–producing γδ T cells in RORc+/+ (+/+), RORc+/− (+/−), and RORc−/− (−/−) mice. Single-cell suspensions of fetal (E16) and adult thymocytes (6 weeks old; A-B) or PEC, spleen, and LPL (4 weeks old; C) from RORc+/+ (+/+), RORc+/− (+/−), and RORc−/− (−/−) mice were stimulated with PMA and ionomycin and analyzed for intracellular staining for IL-17. (A) Representative dot plots of intracellular staining for IL-17 in fetal (top panels) and adult (bottom panels) thymi are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδTCR+ cells. (B-C) Absolute numbers of IL-17+ γδ T cells in each organ are shown. Data shown are the means ± SD of 4-5 mice. *P < .05; **P < .01; ***P < .001. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
RORγt is partly required for the intrathymic development of IL-17–producing γδ T cells. (A-C) IL-17–producing γδ T cells in RORc+/+ (+/+), RORc+/− (+/−), and RORc−/− (−/−) mice. Single-cell suspensions of fetal (E16) and adult thymocytes (6 weeks old; A-B) or PEC, spleen, and LPL (4 weeks old; C) from RORc+/+ (+/+), RORc+/− (+/−), and RORc−/− (−/−) mice were stimulated with PMA and ionomycin and analyzed for intracellular staining for IL-17. (A) Representative dot plots of intracellular staining for IL-17 in fetal (top panels) and adult (bottom panels) thymi are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδTCR+ cells. (B-C) Absolute numbers of IL-17+ γδ T cells in each organ are shown. Data shown are the means ± SD of 4-5 mice. *P < .05; **P < .01; ***P < .001. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
Hes1 expression is correlated with IL-17 production of γδ T cells
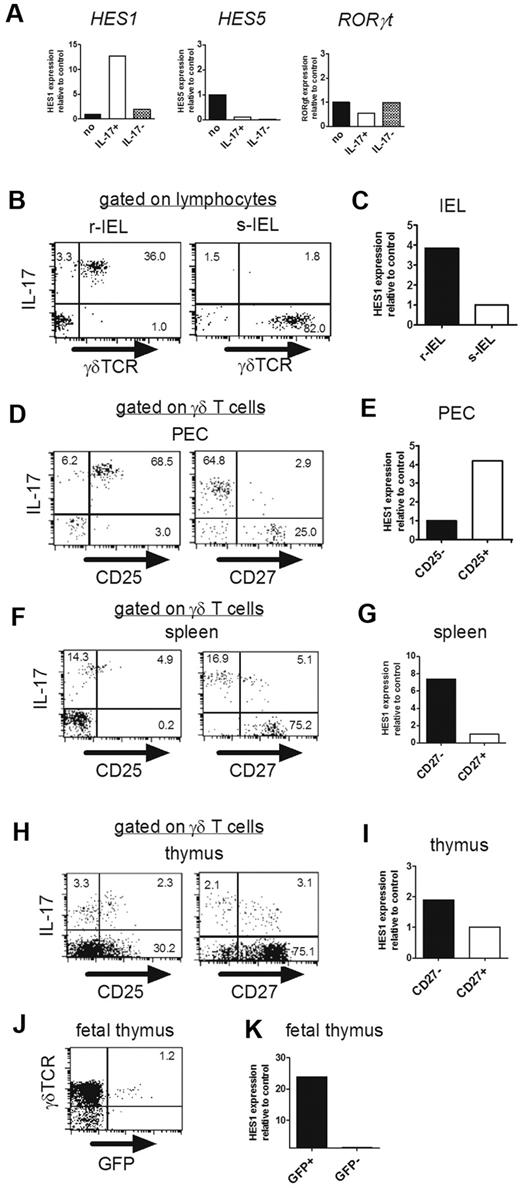
To search for the molecule(s) critically involved in the development of IL-17–producing γδ T cells, we established IL-17–producing γδ T-cell hybridoma clones by fusing peritoneal γδ T cells with the AKR thymoma BW5147 (supplemental Figure 3). As the negative control, γδ T-cell hybridoma clones were generated from Vγ5+ γδ T cells in skin IELs (s-IELs), none of which produced IL-17.11 Comparative gene-expression analysis identified 19 genes that were highly expressed in IL-17+ clones (supplemental Table 1). Among these candidates, we focused on Hes1, a member of the family of mammalian bHLH transcriptional repressors, because Hes1 is highly expressed in fetal thymocytes around E15,29 when IL-17–producing γδ T cells begin to be detected.11 We confirmed expression of Hes1 mRNA selectively in IL-17+ clones by qRT-PCR (Figure 3A). Because Hes5 and Hes1 have redundant functions in neural stem cells,30 we also analyzed Hes5 expression in these clones, and it was not detected in either (Figure 3A). The expression level of RORγt was not up-regulated in IL-17+ clones (Figure 3A).
Hes1 is specifically expressed in IL-17–producing γδ T cells. (A) Hes1 is specifically expressed in IL-17–producing hybridoma clones. (A) mRNA expressions of Hes1, Hes5, and RORγt in parental hybridomas (no), IL-17–producing (IL-17+), and nonproducing (IL-17−) γδ TCR+ hybridoma clones were analyzed by qRT-PCR. (B-K) Hes1 is specifically expressed in IL-17–producing γδ T cells. (B,D,F,H) Purified γδ T cells from IELs (B), PECs (D), spleens (F), and thymi (8 weeks old; H) were stimulated with PMA and ionomycin and analyzed for intracellular IL-17. Representative dot plots are shown after gating on lymphocytes identified by FSC/SSC profile (B) or γδ TCR+ cells (D,F,H). The numbers in the respective quadrants indicate the percentages of positive cells. (C,E,G,I) Purified γδ T cells from IELs (6 weeks old; C) or sorted CD25high, CD25low, CD27high, and CD27low γδ T cells from PECs (E), spleens (G), and thymi (I) of CβKO mice (6 weeks old) were analyzed for the relative expression of Hes1. Hes1 expression of s-IELs (C), CD25− γδ T cells (E), or CD27+ γδ T cells (G,I) was set to 1. (J) Fetal thymocytes (E18) of IL-17–GFP reporter mice were analyzed after stimulation with PMA and ionomycin for 4 hours. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδ TCR+ cells. (K) After sorting of GFP+ and GFP− cells, Hes1 mRNA expression was analyzed by qRT-PCR. Hes1 expression of GFP- γδ T cells was set to 1. Data are representative of 3 independent experiments.
Hes1 is specifically expressed in IL-17–producing γδ T cells. (A) Hes1 is specifically expressed in IL-17–producing hybridoma clones. (A) mRNA expressions of Hes1, Hes5, and RORγt in parental hybridomas (no), IL-17–producing (IL-17+), and nonproducing (IL-17−) γδ TCR+ hybridoma clones were analyzed by qRT-PCR. (B-K) Hes1 is specifically expressed in IL-17–producing γδ T cells. (B,D,F,H) Purified γδ T cells from IELs (B), PECs (D), spleens (F), and thymi (8 weeks old; H) were stimulated with PMA and ionomycin and analyzed for intracellular IL-17. Representative dot plots are shown after gating on lymphocytes identified by FSC/SSC profile (B) or γδ TCR+ cells (D,F,H). The numbers in the respective quadrants indicate the percentages of positive cells. (C,E,G,I) Purified γδ T cells from IELs (6 weeks old; C) or sorted CD25high, CD25low, CD27high, and CD27low γδ T cells from PECs (E), spleens (G), and thymi (I) of CβKO mice (6 weeks old) were analyzed for the relative expression of Hes1. Hes1 expression of s-IELs (C), CD25− γδ T cells (E), or CD27+ γδ T cells (G,I) was set to 1. (J) Fetal thymocytes (E18) of IL-17–GFP reporter mice were analyzed after stimulation with PMA and ionomycin for 4 hours. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδ TCR+ cells. (K) After sorting of GFP+ and GFP− cells, Hes1 mRNA expression was analyzed by qRT-PCR. Hes1 expression of GFP- γδ T cells was set to 1. Data are representative of 3 independent experiments.
We next examined expression of Hes1 in different subsets of γδ T cells freshly isolated from various organs of normal mice. As shown in Figure 3B, virtually all γδ T cells in the epithelium of female reproductive organs (reproductive IELs [r-IELs]) produced IL-17. Consistent with this, Hes1 was highly expressed in γδ T cells purified from r-IELs compared with those from s-IELs (Figure 3C). We and others have reported previously that CD25 and CD27 can be used as markers discriminating IL-17- and IFN-γ–producing γδ T cells.11,31 In the present study, we demonstrated that nearly all CD25+ γδ T cells but not all CD27− cells in the peritoneal cavity produced IL-17 (Figure 3D). Conversely, the expression level of CD25 on IL-17–producing γδ T cells in the spleen was lower than that in the PEC, whereas nearly all CD27− cells in the spleen produced IL-17 (Figure 3F). Based on these findings, we compared the expression of Hes1 in different subpopulations of γδ T cells in the PEC and spleen, and found that Hes1 was specifically expressed in CD25+ γδ T cells and CD27− γδ T cells, respectively (Figure 3E,G). We also examined expression of Hes1 in thymic γδ T cells. IL-17–producing γδ T cells in adult thymi contains CD27+ cells in addition to CD27− cells (Figure 3H). Similarly, there were both CD25+ and CD25− γδ T cells positive for IL-17 in the thymus. Consistent with the expression level of IL-17, Hes1 expression in CD27− γδ T cells was slightly higher than that in CD27+ γδ T cells (Figure 3H-I). To more directly examine the relationship between Hes1 expression and IL-17 production by γδ T cells, we analyzed the fetal thymi of IL-17-GFP reporter mice. After brief stimulation with PMA and ionomycin, some of the γδ T cells became positive for GFP (Figure 3J), which exclusively expressed Hes1 (Figure 3K). The expression of Hes1 correlated well with the IL-17–producing function of γδ T cells.
Hes1 is critical for the development of IL-17–producing γδ T cells in the thymus
To determine whether Hes1 is required for the development of IL-17–producing γδ T cells, we analyzed Hes1-deficient mice. Because Hes1-deficient mice die during gestation or just after birth with severe defects of neural tubes and eye morphogenesis,21 we analyzed fetal thymi on E16 and E18. The absolute number of γδ T cells in the thymi of Hes1-deficient mice was lower than that of control mice (Figure 4A), which is consistent with a previous report in which Hes1 was shown to play an important role in the expansion of early T-cell precursors.29 All γδ T-cell repertoires developed normally in the absence of Hes1 (Figure 4B); however, IL-17–producing γδ T cells were strikingly decreased in Hes1-deficient mice (Figure 4C-D), indicating that Hes1 plays a critical role in the development of IL-17–producing γδ T cells in fetal thymi.
Hes1 is critical for the development of IL-17–producing γδ T cells. (A-B) Hes1 is dispensable for the development of γδ T cells. (A) Absolute numbers of γδ T cells in fetal thymi (E16, left; E18, right) of Hes1+/− (+/−) and Hes1−/− (−/−) mice were calculated after analyzing by flow cytometry. Data shown are the means ± SD of 4 fetuses for each group. (B) Representative dot plots for the expression of Vγ4 and Vγ5 in fetal thymi (E16) are shown after gating on γδ TCR+ cells. The numbers under each panel indicate the percentages of positive cells in the respective quadrants. (C-D) Hes1 is required for the development of IL-17–producing γδ T cells. IL-17 production by γδ T cells from E16 (top panels) and E18 (bottom panels) of Hes1+/− (+/−) and Hes1−/− (−/−) mice were analyzed after stimulation with PMA and ionomycin. (C) Representative dot plots are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδ TCR+ cells. (D) Absolute numbers of IL-17+ γδ T cells are shown. Data shown are the means ± SD of 4 mice. *P < .05. Data are representative of 3 independent experiments.
Hes1 is critical for the development of IL-17–producing γδ T cells. (A-B) Hes1 is dispensable for the development of γδ T cells. (A) Absolute numbers of γδ T cells in fetal thymi (E16, left; E18, right) of Hes1+/− (+/−) and Hes1−/− (−/−) mice were calculated after analyzing by flow cytometry. Data shown are the means ± SD of 4 fetuses for each group. (B) Representative dot plots for the expression of Vγ4 and Vγ5 in fetal thymi (E16) are shown after gating on γδ TCR+ cells. The numbers under each panel indicate the percentages of positive cells in the respective quadrants. (C-D) Hes1 is required for the development of IL-17–producing γδ T cells. IL-17 production by γδ T cells from E16 (top panels) and E18 (bottom panels) of Hes1+/− (+/−) and Hes1−/− (−/−) mice were analyzed after stimulation with PMA and ionomycin. (C) Representative dot plots are shown after gating on CD3+ cells. The number in the top right quadrant indicates the percentage of IL-17+ cells in γδ TCR+ cells. (D) Absolute numbers of IL-17+ γδ T cells are shown. Data shown are the means ± SD of 4 mice. *P < .05. Data are representative of 3 independent experiments.
Notch-Hes1 pathway is involved in the development of IL-17–producing γδ T cells
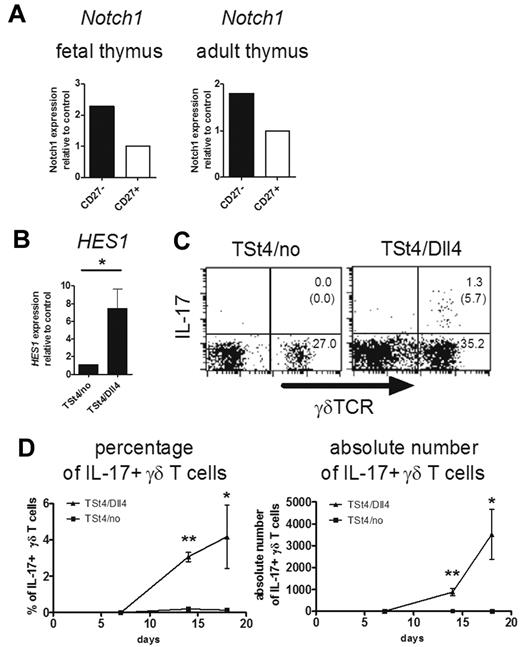
Hes1 is known as a downstream signaling molecule of Notch receptors, such as Notch1, Notch2, Notch3, and Notch4.32 It was shown previously that Notch1 expression was detected on fetal thymic γδ T cells on E16.33 Consistent with Hes1 expression (Figure 3I and data not shown), CD27− γδ T cells expressed a relatively higher level of Notch1 than CD27+ γδ T cells in the fetal and adult thymus (Figure 5A). It is of interest to examine the role of Notch and its ligands, including Dll1, Dll3, Dll4, and Jagged 1 and 2, in the development of IL-17–producing γδ T cells in the thymus. However, Notch signaling, especially the Notch1-Dll4 interaction, is indispensable for early T-cell development in the thymus.34,35 In addition, mice deficient for Notch1 or Dll4 are embryonically lethal.36,37 Therefore, we analyzed the involvement of Dll4-mediated Notch signaling in γδ T-cell differentiation in vitro using TSt4 stromal cells. In addition to CD4+CD8+ double-positive cells, γδ T cells, some of which produced IL-17, developed from hematopoietic progenitor cells in the fetal liver after culture with TSt4/Dll4 cells (data not shown). However, consistent with the previous report,24 only NK and B cells, but not T cells, developed in the absence of Notch signaling. Therefore, it was impossible to separately analyze the development and function of γδ T cells in this system. To circumvent this problem, we cultured fetal thymocytes (E15), in which T-cell–committed progenitors had already developed, with the stromal cells. In this system, γδ T cells developed even in the absence of Notch signaling (Figure 5C). However, the expression level of Hes1 in γδ T cells was greatly diminished in the absence of Notch signaling, indicating the importance of Notch signaling in Hes1 expression (Figure 5B). Furthermore, the development of IL-17–producing γδ T cells was not detected in the culture without Notch signaling (Figure 5C-D).
Critical role of Notch-Hes1 pathway for the development of IL-17–producing γδ T cells. (A) Notch1 expression of γδ T cells in the thymus. Sorted CD27high and CD27low γδ T cells from E18 fetal (left) or adult (right) thymi of CβKO mice (6 weeks old) were analyzed for the relative expression of Notch1. Notch1 expression of CD27+ γδ T cells was set to 1. (B-D) Dll4-mediated Notch signaling induces the development of IL-17–producing γδ T cells. Three thousand fetal thymocytes (E15) were cocultured with TSt-4/no or TSt-4/Dll4 stromal cells. (B) Hes1 expression was analyzed by qRT-PCR 14 days after the culture. Data shown are the means ± SD of 4 individual wells. *P < .05. (C-D) After coculture with TSt-4/no or TSt-4/Dll4 cells, IL-17 production by γδ T cells was analyzed after stimulation with PMA and ionomycin (C). Representative dot plots are shown after gating on CD45.2+ cells. The number in top right or bottom right quadrant indicates γδTCR+IL-17+ or γδTCR+IL-17− cells in CD45.2+ cells, respectively. The number in the parentheses shows the percentage of IL-17+ cells in γδ TCR+ cells. (D) Kinetics of percentages (left) or absolute numbers (right) of IL-17–producing γδ T cells are shown at the indicated days. Data shown are the means ± SD of 4 individual wells at each time point. *P < .05; **P < .01. Data are representative of 3 independent experiments.
Critical role of Notch-Hes1 pathway for the development of IL-17–producing γδ T cells. (A) Notch1 expression of γδ T cells in the thymus. Sorted CD27high and CD27low γδ T cells from E18 fetal (left) or adult (right) thymi of CβKO mice (6 weeks old) were analyzed for the relative expression of Notch1. Notch1 expression of CD27+ γδ T cells was set to 1. (B-D) Dll4-mediated Notch signaling induces the development of IL-17–producing γδ T cells. Three thousand fetal thymocytes (E15) were cocultured with TSt-4/no or TSt-4/Dll4 stromal cells. (B) Hes1 expression was analyzed by qRT-PCR 14 days after the culture. Data shown are the means ± SD of 4 individual wells. *P < .05. (C-D) After coculture with TSt-4/no or TSt-4/Dll4 cells, IL-17 production by γδ T cells was analyzed after stimulation with PMA and ionomycin (C). Representative dot plots are shown after gating on CD45.2+ cells. The number in top right or bottom right quadrant indicates γδTCR+IL-17+ or γδTCR+IL-17− cells in CD45.2+ cells, respectively. The number in the parentheses shows the percentage of IL-17+ cells in γδ TCR+ cells. (D) Kinetics of percentages (left) or absolute numbers (right) of IL-17–producing γδ T cells are shown at the indicated days. Data shown are the means ± SD of 4 individual wells at each time point. *P < .05; **P < .01. Data are representative of 3 independent experiments.
To further confirm the importance of Notch-Hes1 pathway in the development of IL-17–producing γδ T cells in vivo, fetal liver cells were introduced with intracellular Notch1 (ICN1), which activates Notch signaling by bypassing Notch-Notch ligand interaction. The ICN1-transduced cells were transferred into irradiated recipient mice, which were analyzed after 4 weeks. Constitutively active Notch signaling augmented Hes1 expression in thymocytes (supplemental Figure 4A). Consistent with a previous report,34 an increased percentage of CD4+CD8+ double-positive cells in the thymus was observed (supplemental Figure 4B). It was also found that introducing ICN1 greatly increased the percentage of IL-17–producing γδ T cells (supplemental Figure 4C-D). Therefore, the Notch-Hes1 pathway is critical for the development of naturally occurring IL-17–producing γδ T cells in the thymus.
Importance of Hes1 in mature IL-17–producing γδ T cells in the periphery
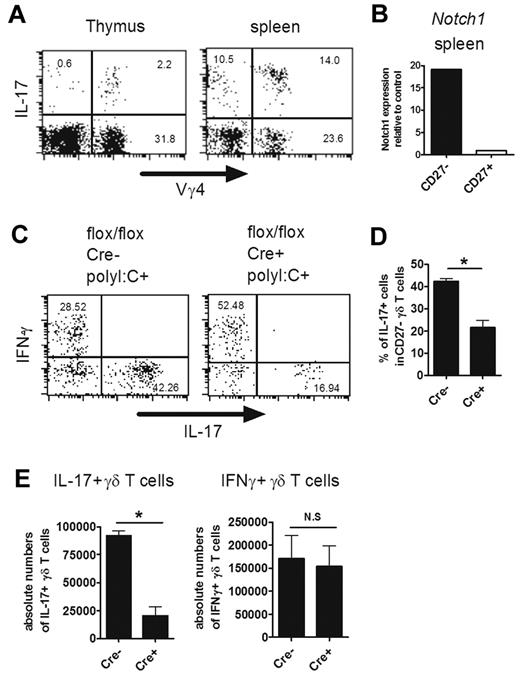
Notch1 as well as Hes1 was expressed in IL-17–producing (CD27−) γδ T cells in the periphery, suggesting the involvement of the Notch-Hes1 pathway at this stage (Figures 3G and 6B). Therefore, we generated conditional Hes1-deficient mice by crossing Hes1flox/flox mice with MX1-Cre Tg mice, in which the Hes1 gene was deleted by poly I:C injection. Vγ4+ γδ T cells are known to be part of the major repertoire of IL-17–producing γδ T cells in the periphery.38 In fact, IL-17–producing Vγ4+ γδ T cells were abundantly found in the thymus as well as in the periphery of adult mice (Figure 6A). Because Hes1 expression was efficiently eliminated in γδ T cells in the spleen but not in the thymus after poly I:C injection (supplemental Figure 5), splenic IL-17–producing γδ T cells were analyzed. We found that IL-17 production by γδ T cells was significantly reduced in the absence of Hes1 (Figure 6C-E). The significant decrease of the percentage of IL-17–producing cells was detected within CD27− γδ T cells (Figure 6D). The number of IFN-γ–producing γδ T cells was not reduced by Hes1 ablation (Figure 6C,E). The number of IL-17–producing γδ TCR− splenocytes, most of which were CD4+ αβ T cells, was also unaffected by the absence of Hes1 (supplemental Figure 6, data not shown). These results suggest that Hes1 is selectively involved in the IL-17–producing function of mature γδ T cells in the periphery.
Important role of Hes1 in IL-17–producing γδ T cells in the periphery. (A) Vγ4+ IL-17–producing γδ T cells in the thymi and spleens of adult mice. Single-cell suspensions of thymocytes and splenocytes from adult mice (6 weeks old) were stimulated with PMA and ionomycin and analyzed for IL-17–producing cells. Representative dot plots are shown after gating on γδ TCR+ cells. (B) Sorted Vγ4+ CD27high and CD27low γδ T cells from spleens were analyzed for the relative expression of Notch1. Notch1 expression of CD27+ γδ T cells was set to 1. (C-E) Hes1 is selectively involved in IL-17 production by γδ T cells in the periphery. Splenocytes from HES1flox/flox (flox/flox Cre−) and HES1flox/floxMX1-Cre (flox/flox Cre+) mice (4 weeks old) were prepared 4 days after the last poly I:C injection. (C-D) IFN-γ and IL-17 production by Vγ4+ γδ T cells was analyzed after stimulation with PMA and ionomycin. (C) Representative dot plots are shown after gating on Vγ4+ γδ T cells. The numbers in the respective quadrants indicate the percentage of cells. (D) Percentage of IL-17+ cells within CD27− Vγ4+ γδ T cells are shown. Data shown are the means ± SD of 3 mice. (E) Absolute numbers of IFN-γ- or IL-17–producing Vγ4+ γδ T cells are shown. Data shown are the means ± SD of 5 mice. *P < .05. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
Important role of Hes1 in IL-17–producing γδ T cells in the periphery. (A) Vγ4+ IL-17–producing γδ T cells in the thymi and spleens of adult mice. Single-cell suspensions of thymocytes and splenocytes from adult mice (6 weeks old) were stimulated with PMA and ionomycin and analyzed for IL-17–producing cells. Representative dot plots are shown after gating on γδ TCR+ cells. (B) Sorted Vγ4+ CD27high and CD27low γδ T cells from spleens were analyzed for the relative expression of Notch1. Notch1 expression of CD27+ γδ T cells was set to 1. (C-E) Hes1 is selectively involved in IL-17 production by γδ T cells in the periphery. Splenocytes from HES1flox/flox (flox/flox Cre−) and HES1flox/floxMX1-Cre (flox/flox Cre+) mice (4 weeks old) were prepared 4 days after the last poly I:C injection. (C-D) IFN-γ and IL-17 production by Vγ4+ γδ T cells was analyzed after stimulation with PMA and ionomycin. (C) Representative dot plots are shown after gating on Vγ4+ γδ T cells. The numbers in the respective quadrants indicate the percentage of cells. (D) Percentage of IL-17+ cells within CD27− Vγ4+ γδ T cells are shown. Data shown are the means ± SD of 3 mice. (E) Absolute numbers of IFN-γ- or IL-17–producing Vγ4+ γδ T cells are shown. Data shown are the means ± SD of 5 mice. *P < .05. N.S. indicates statistically not significant between groups. Data are representative of 3 independent experiments.
Discussion
The acquisition of effector T-cell functions is regulated by various cell-intrinsic and cell-extrinsic factors. STAT3 and RORγt are required for the differentiation of Th17- and IL-17–producing NKT cells. In the present study, we found that Hes1, a bHLH protein induced by Notch signaling, is critically involved in the development of naturally occurring IL-17–producing γδ T cells, which develop profoundly in the perinatal thymus.
In Th17 cells, STAT3 plays an important role in transducing IL-6 receptor signaling. Consistent with our finding that IL-17–producing γδ T cells developed in STAT3-deficient mice, it was reported that IL-6 was required for the differentiation of IL-17–producing αβ T cells, but not for γδ T cells.16 Interestingly, IL-17–producing NKT cells develop normally in IL-6–deficient mice,14 suggesting that the development of IL-17–producing γδ T cells and NKT cells is regulated by a similar mechanism. However, whereas intrathymic development of IL-17–producing γδ T cells is partly dependent on RORγt, that of IL-17–producing NKT cells requires RORγt expression.15 RORγt-independent development of IL-17–producing γδ T cells is supported by our previous finding that RORγt expression was detected in the CD25−CD122+ population of γδ T cells, which only produce IFN-γ.11 In addition, a study using RORγt reporter mice demonstrated expression of RORγt in Vγ5+ γδ T cells in the skin, which do not produce IL-17.16 Therefore, the expression level of RORγt is not necessarily related to IL-17 production in γδ T cells. Interestingly, IL-17–producing γδ T cells were absent in the periphery of RORγt-deficient mice, suggesting the importance of RORγt at this stage. In agreement with this, Ribot et al observed increased expression of RORγt in IL-17–producing CD27− γδ T cells in the periphery.31 RORγt was initially identified as a molecule that is highly expressed in double-positive thymocytes and promotes their survival by up-regulating the expression of Bcl-xL.20 It has yet to be determined whether RORγt plays a similar antiapoptotic role in IL-17–producing γδ T cells in the periphery. A small number of Th17 cells naturally develop in STAT3-deficient and RORγt-deficient mice,25,28 suggesting that some αβ T cells share a differentiation mechanism with γδ T cells.
Hes1 is known to regulate the fate of various cell lineages in developing organs, including T cells.39 It was shown previously that Hes1-deficient fetal liver cells had a severe defect in reconstituting T cells in Rag1-deficient mice.29 Fetal thymic organ culture revealed that the cellularity was significantly reduced in Hes1-deficient thymic lobes.40 Hes1 overexpression in T cells potentially induces T-cell lymphoma.41,42 In addition, Hes1 promotes the commitment of αβ T cells toward the CD8 lineage by repressing expression of the CD4 receptor.43 In the present study, we identified a novel role of Hes1 in developing T cells. Hes1 is involved in the development of IL-17–producing γδ T cells in the thymus. At present, the molecular mechanisms through which Hes1 induces the development of IL-17–producing γδ T cells is unclear. Hes1 is known to promote or inhibit cell-cycle progression by regulating the expression of cell-cycle regulators such as p27Kip1 or E2F-1, respectively, depending on the level of Hes1 expression.44,45 Indeed, the number of γδ T cells in the fetal thymi of Hes1-deficient mice was slightly decreased (Figure 4A). Although there was no significant bias in the TCR repertoire, Hes1 might promote the expansion of the IL-17–producing γδ T-cell population. Because Hes1 is known as a transcriptional repressor, it seems unlikely that Hes1 directly induces IL-17 production by binding to regulatory regions of the IL-17 gene. Nevertheless, recent studies have demonstrated an involvement of Notch signaling in the regulation of IL-17 production by Th17 cells in vitro and in vivo.46,47 Direct interaction of RBPjκ (also known as CSL), a DNA-binding protein downstream of Notch signaling, with the IL17 promoter was also shown.47
We found here that inactivation of the Hes1 gene in peripheral γδ T cells selectively reduced IL-17 production, which is consistent with the expression of Hes1 in IL-17–producing γδ T cells. Furthermore, Dll4 expression was detected in peripheral tissues such as the intestine and lung, where IL-17–producing γδ T cells are abundantly found.48 These findings imply that Notch receptors continuously transmit signals to maintain Hes1 expression in IL-17–producing γδ T cells in the periphery. It is possible that Hes1 promotes the survival of IL-17–producing γδ T cells in the periphery. Alternatively, Hes1 may be directly involved in IL-17 production of γδ T cells, as discussed in the preceding paragraph; further studies are required to clarify the molecular mechanisms. In contrast to γδ T cells, the number of peripheral IL-17–producing αβ T cells, most of which were CD4+, was not reduced by conditional deletion of Hes1, indicating lineage-specific roles of Hes1. Although we detected a few IL-17+ CD4+ αβ T cells in adult but not in fetal thymi, it is unclear whether their development is also independent of Hes1, because Hes1-deficient mice do not live longer than the perinatal period and the Hes1 gene in adult thymocytes was not efficiently deleted in the conditional knockout mice (supplemental Figure 5).
Notch signaling is well known for its role in the development of αβ T cells, but it is also required for the development of γδ T cells.34,35 Notch signaling is also known to regulate the differentiation of helper CD4+ αβ T cells, although the requirement for Notch signaling is not always absolute in this case.32 The results of the present study revealed that Notch signaling is involved in and even indispensable for the development of IL-17–producing γδ T cells. IFNγ-producing γδ T cells, which are also known to be generated in the thymus, were infrequently detected in our system (data not shown). It has been suggested that additional signals, including TCR- and/or CD27-mediated signals, are required for the functional differentiation of IFNγ-producing γδ T cells.31,49 Although our results clearly reveal the importance of Hes1 in developing γδ T cells, the induction of Hes1 does not account for all of the effects of Notch signaling in γδ T cells. The reduced but significant number of γδ T cells in the thymi of Hes1-deficient mice revealed the Hes1-independent mechanism of Notch signaling in the development of γδ T cells. Because accumulating evidence indicates the importance of IL-17–producing γδ T cells in vivo,9,10 our results shed light on novel roles of the Notch-Notch ligand system in the regulation of immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kiyomi Akasaki and Akiko Yano for secretarial assistance, Takanori Nakamura and Amago Michi for microarray analysis, and Toshio Kitamura for providing packaging cell lines and vectors for retroviral transduction.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science, by The Mochida Memorial Foundation for Medical and Pharmaceutical Research, and by the program of Founding Research Centers for Emerging and Reemerging Infectious Diseases launched as a project commissioned by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Authorship
Contribution: K.S. and H.Y. designed the research; K.S., T.D., M.N., T.S., and H.T. performed experiments and analyzed data; R.K., Y.I., H.H., S.Y., T.I., and H.K. provided essential materials and protocols; Y.Y. supervised the experimental work; and K.S. and H.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kensuke Shibata or Hisakata Yamada, Division of Host Defense, Medical Institute of Bioregulation, Kyushu University, 3-1-1, Maidashi, Higashi-ku, Fukuoka, Japan; e-mail: k_shibata@bioreg.kyushu-u.ac.jp or hisakata@bioreg.kyushu-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal