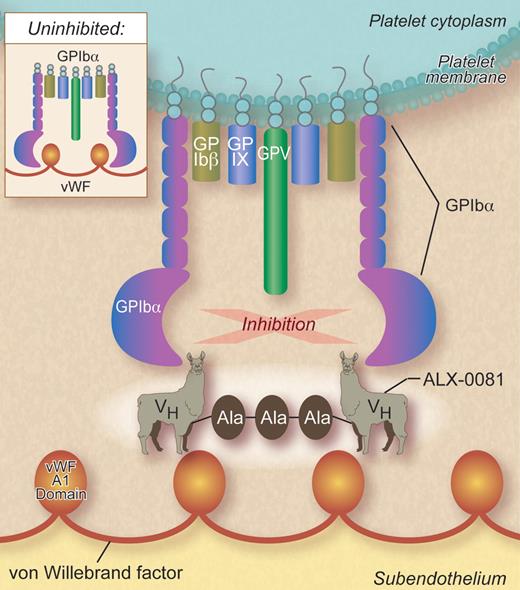
In this issue of Blood, Ulrichts and coworkers report preclinical studies of a novel bivalent heavy chain-only nanobody, ALX-0081, directed at the A1 domain of VWF that inhibits VWF binding to platelet glycoprotein Ibα1 (see figure).
The drugs currently available to treat patients with arterial vascular disease do so by impairing platelet aggregation, either indirectly by inhibiting platelet signal transduction pathways (eg, aspirin inhibits platelet thromboxane A2 synthesis by cyclooxygenase 1 and clopidogrel inhibits ADP-stimulated aggregation by binding to the P2Y12 ADP receptor) or directly by inhibiting ligand binding to the platelet fibrinogen receptor αIIbβ3 (eg, abciximab, eptifibatide, and tirofiban).2 Aspirin and clopidogrel are effective medications, but their efficacy is limited in that they only decrease the risk of secondary cardiovascular events by approximately 25%.3 Similarly, while parenterally administered αIIbβ3 inhibitors improve outcomes in high risk patients undergoing percutaneous transluminal coronary angioplasty (PTCA), their benefit in patients with acute coronary syndromes undergoing medical treatment without PTCA is uncertain and chronic administration of oral αIIbβ3 inhibitors to patients with coronary artery disease was inexplicably associated with increased mortality.4 Consequently, there are still opportunities to produce more effective and/or safer antiplatelet drugs.
In vivo, the platelet aggregation responsible for the formation of hemostatic platelet plugs is preceded by the adhesion of platelets to newly exposed subendothelium. When platelet adhesion occurs in blood vessels where shear rates are high (eg, in arterioles and microvascular vessels), it requires the presence of VWF bound to collagen. VWF is an elongated multimeric glycoprotein whose A1 domains contain a binding site for the platelet glycoprotein Ibα (GPIbα). GPIbα binding to immobilized VWF, via the A1 domain, enables unactivated platelets to translocate (roll) along the exposed subendothelial surface until platelet stimulation causes αIIbβ3- and α2β1-mediated firm platelet arrest. High shear facilitates the interaction of GPIbα with VWF, likely by perturbing the conformation of GPIbα, the A1 domain, or both. Thus, it is logical to presume that inhibiting platelet adhesion to subendothelial VWF would preempt the formation of the intravascular platelet aggregates that punctuate the course of atherosclerotic disease. Further, Ulrichts et al postulated that because the VWF A1 domain is only able to interact with GPIbα under high shear conditions, such as those present in stenosed arteries, a drug targeting the A1 domain could have an improved safety profile with respect to bleeding.1
The idea of targeting GPIbα binding to VWF to prevent arterial thrombosis is not new and a number of agents have been previously proposed for this purpose.5 These include the triphenylmethy dye aurintricarboxylic acid,6 monoclonal antibodies and their Fab fragments directed against GPIbα, snake venom C-type lectins, cysteine-constrained GPIbα-binding peptides,7 and the DNA/RNA aptamer ARC1779.8 However, the innovative approach to this idea taken by Ulrichts and coworkers is based on reports that members of the species Camelidae naturally produce heavy chain-only immunoglobulins.9 Thus, they immunized a llama with a recombinant form of the VWF A1 domain and subsequently produced the humanized nanobody PMP12A1hi from the heavy chain variable domain of the heavy chain-only llama immunoglobulins. PMP12A1hi was then dimerized using a 3 alanine linker to form the bivalent antithrombotic candidate drug ALX-0081.
The bivalent nanobody ALX-0081 inhibits von Willebrand factor (VWF) binding to platelet GPIbα by binding to the VWF A1 domain. VH indicates heavy chain variable domain; and Ala-Ala-Ala, alanine linker. Professional illustration by Debra T. Dartez.
The bivalent nanobody ALX-0081 inhibits von Willebrand factor (VWF) binding to platelet GPIbα by binding to the VWF A1 domain. VH indicates heavy chain variable domain; and Ala-Ala-Ala, alanine linker. Professional illustration by Debra T. Dartez.
Ulrichts et al report here that bivalent ALX-0081, but not univalent PMP12A1hi, binds with high affinity binding to VWF and inhibits VWF binding to formalin-fixed platelets.1 In addition, ALX-0081 inhibits platelet adhesion to collagen-bound VWF in vivo at arterial shear rates; potentiates the inhibitory effects of aspirin, clopidogrel, and unfractionated heparin on platelet adhesion ex vivo using blood from patients undergoing PTCA for acute coronary syndromes; and has activity comparable to abciximab, and somewhat better than clopidogrel, in preventing femoral artery thrombosis in a modified Folts thrombosis model in baboons.10 A noteworthy finding is that following a standardized wound in these animals, blood loss was less in animals given ALX-0081 than those given clopidogrel or abciximab. Based on these preclinical results, Ulrichts et al suggest that ALX-0081 has high efficacy, as well as an improved therapeutic index, compared with currently marketed anti-thrombotic agents.
ALX-0081 fills a hitherto empty niche in the spectrum of available antithrombotic agents. Nonetheless, it is important to remember that ALX-0081 acts by mimicking the pathophysiology of 2 human hemorrhagic disorders, VWD and the Bernard-Soulier syndrome.11 Thus, it is premature to draw conclusions on its therapeutic index in humans based on the data presented here. Moreover, while Ulrichts et al speculate that ALX-0081 will only have activity in the presence of high shear, like that present in a stenotic artery, patients with VWD and the Bernard-Soulier syndrome bleed at sites where high shear is unlikely to be a factor. Hopefully, ALX-0081 will fulfill the promise its designers expect in clinical trails.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal