To the editor:
In contrast to the familial predisposition observed in somatically acquired myeloproliferative neoplasms (low penetrance, clonal hematopoiesis), the hereditary thrombocythemias (HT) are characterized by Mendelian inheritance, high penetrance, and polyclonal hematopoiesis, and appear to only affect the megakaryocytic lineage.1 All the molecular alterations identified thus far in patients with HT have involved either THPO (thrombopoietin) or its receptor MPL (myeloproliferative leukemia virus oncogene) genes, with 4 and 3 distinct mutations reported, respectively. The HT- associated THPO mutations were either confirmed or expected to increase the translational efficiency of thrombopoietin without altering the sequence of the mature protein.1 Thrombopoietin, the primary regulator of megakaryopoiesis and platelet production, is produced in the liver, kidney, spleen, and bone marrow.2 Thrombopoietin binds to its receptor and activates the JAK-STAT signaling pathway.3 The presence of multiple upstream AUG codons (uAUG) within the 5′-untranslated region (5′-UTR) precludes efficient translation and prevents harmful overproduction of this potent cytokine.2
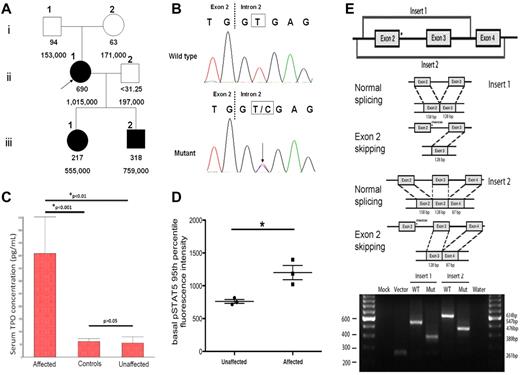
We identified a novel point mutation at the splice donor site of THPO intron 2 (position +2) in a Filipino family with HT. Approval was obtained from the Stanford University institutional review board for these studies and informed consent was provided according to the Declaration of Helsinki. The proband and her 2 children manifested with moderate to severe elevations of the serum thrombopoietin levels and platelet counts, while those were normal in her biologic parents and husband (Figure 1A). The absence of the mutation in either parent suggests the de novo nature of the mutation. Sequencing of the 5′-UTR of THPO in all the family members revealed a heterozygous T > C transition at the splice donor of intron 2 (Figure 1B) in subjects II-1, III-1 and III-2, but not in I-1, I-2 and II-2, indicating the co-segregation of the mutation with the thrombocytosis phenotype. The patients were negative for all known HT-associated MPL mutations. Serum thrombopoietin levels (measured with the human TPO Quantikine kit, R&D Systems) in the affected family members were significantly higher than in the non-affected family members or healthy controls, while there was no statistically significant difference between the latter 2 groups (Figure 1C). To examine the functional effects of increased thrombopoietin levels on downstream JAK-STAT signaling, phospho-specific flow cytometry was performed as previously described4 on peripheral blood samples from the family members. As shown in Figure 1D, basal phosphorylated STAT5 (pSTAT5) levels in myeloid progenitors were significantly higher in the affected group (P = .024). Because of the unavailability of the appropriate tissue specimens for direct patient THPO RNA analysis, the in vitro pSLP3b exon trapping system5 was used to prove the inactivation of the splice donor and exon 2 skipping as the main consequence of the mutation (Figure 1E). This novel T > C mutation at position +2 involves the same splice donor site as the previously reported G > C mutation at position +1 in a Dutch family.6 Thus, it appears that mutations in either position +1 or +2 of the THPO intron 2 splice donor site may result in exon 2 skipping and loss of inhibitory 5′-UTR sequence, leading to increased thrombopoietin expression and thrombocytosis.
THPO mutation inactivates the intron 2 splice donor and correlates with elevation of thrombopoietin level. (A) Pedigree of the 6 family members with serum thrombopoietin concentrations (pg/mL) in the 1st row and platelet counts (/mm3) in the 2nd row. The proband (II-1) and her 2 children (III-1, III-2) exhibit thrombocytosis and high serum thrombopoietin levels. The proband's parents (I-1, I-2) and husband (II-2) have normal platelet counts and thrombopoietin levels. (B) A novel heterozygous T > C point mutation at the splice donor site of THPO gene intron 2 was identified through Sanger sequencing in the proband and her children, but not in her parents or husband. (C) The serum thrombopoietin concentrations of family members with the THPO mutation were significantly higher than those family members without the mutation (P < .01), or healthy controls (P < .001). The thrombopoietin levels in the latter 2 groups showed no statistically significant difference. This analysis is performed with 1-way ANOVA followed by the Student-Newman-Keuls multiple comparisons test and the data are presented in the mean with SEM format. D) Basal phosphorylated STAT5 (pSTAT5) levels in CD3−/CD66−/CD14− myeloid progenitors were evaluated and the 95th percentile is presented in the mean with SEM format. An un-paired t test revealed that pSTAT5 levels are significantly higher in the affected group (*P = .024). (E) Top panel: schematic of the cloned inserts used for exon trapping. These inserts were amplified from patient genomic DNA, cloned into the pCR2.1-TOPO Vector, and then subcloned into the pSPL3b exon trapping vector. Middle panel: expected splicing products from the constructs of the cloned sequence within pSPL3b. In the presence of a mutation in intron 2, exon 2 is expected to be spliced out of the resulting product. Bottom panel: electrophoretic visualization of cDNA-PCR products amplified from the constructs after transfection into COS-7 cells. RNA was extracted and reverse transcribed to cDNA 48 hours after the transfection of the pSPL3b-Insert construct into COS-7 cells. PCR was performed using primers SD6 and SA2, and products were resolved on a 2% agarose gel. Splicing of the vector alone yields a 261bp fragment resulting from the flanking vector exons. Splicing of the wild-type Insert 1 and Insert 2 constructs results in 547bp and 634 bp fragments, respectively. The mutant splice products display fragments that are 158bp shorter, indicating complete splicing-out of exon 2.
THPO mutation inactivates the intron 2 splice donor and correlates with elevation of thrombopoietin level. (A) Pedigree of the 6 family members with serum thrombopoietin concentrations (pg/mL) in the 1st row and platelet counts (/mm3) in the 2nd row. The proband (II-1) and her 2 children (III-1, III-2) exhibit thrombocytosis and high serum thrombopoietin levels. The proband's parents (I-1, I-2) and husband (II-2) have normal platelet counts and thrombopoietin levels. (B) A novel heterozygous T > C point mutation at the splice donor site of THPO gene intron 2 was identified through Sanger sequencing in the proband and her children, but not in her parents or husband. (C) The serum thrombopoietin concentrations of family members with the THPO mutation were significantly higher than those family members without the mutation (P < .01), or healthy controls (P < .001). The thrombopoietin levels in the latter 2 groups showed no statistically significant difference. This analysis is performed with 1-way ANOVA followed by the Student-Newman-Keuls multiple comparisons test and the data are presented in the mean with SEM format. D) Basal phosphorylated STAT5 (pSTAT5) levels in CD3−/CD66−/CD14− myeloid progenitors were evaluated and the 95th percentile is presented in the mean with SEM format. An un-paired t test revealed that pSTAT5 levels are significantly higher in the affected group (*P = .024). (E) Top panel: schematic of the cloned inserts used for exon trapping. These inserts were amplified from patient genomic DNA, cloned into the pCR2.1-TOPO Vector, and then subcloned into the pSPL3b exon trapping vector. Middle panel: expected splicing products from the constructs of the cloned sequence within pSPL3b. In the presence of a mutation in intron 2, exon 2 is expected to be spliced out of the resulting product. Bottom panel: electrophoretic visualization of cDNA-PCR products amplified from the constructs after transfection into COS-7 cells. RNA was extracted and reverse transcribed to cDNA 48 hours after the transfection of the pSPL3b-Insert construct into COS-7 cells. PCR was performed using primers SD6 and SA2, and products were resolved on a 2% agarose gel. Splicing of the vector alone yields a 261bp fragment resulting from the flanking vector exons. Splicing of the wild-type Insert 1 and Insert 2 constructs results in 547bp and 634 bp fragments, respectively. The mutant splice products display fragments that are 158bp shorter, indicating complete splicing-out of exon 2.
Authorship
Acknowledgments: The authors thank Dr Reinhard Sedlmeier at Ingenium Pharmaceuticals GmbH for kindly providing them with the pSPL3b exon trapping vector and Dr Alex McMillan at Stanford University for statistical consultation. They also express gratitude to Parveen Abidi and Larry Okumoto of the Stanford Hematology Division/Cancer Institute Tissue Bank. G.P.N. was supported by National Institutes of Health grants 1R01CA130826, U54CA149145, and 5U54CA143907. S.T.O. was supported by National Institutes of Health training grant 5T32AI07290. This research is funded by the Charles and Ann Johnson Foundation.
Contribution: B.Z., D.N., C.J., S.T.O., G.P.N., S.S., J.L.Z., and J.G. designed and/or conducted experiments; W.W. and J.G. were involved in patient care; and B.Z., D.N., S.T.O., J.L.Z. and J.G. wrote and/or edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Jason Gotlib, Associate Professor of Medicine (Hematology), Stanford Cancer Institute and Stanford University School of Medicine, 875 Blake Wilbur Dr, Rm 2324, Stanford, CA 94305-5821; e-mail: jason.gotlib@stanford.edu.
References
National Institutes of Health
Author notes
J.L.Z. and J.G. contributed equally to this work

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal