Abstract
Induction of indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in tryptophan degradation along the kynurenine pathway, acts as a potent immunoregulatory loop. To address its role in human allogeneic stem cell transplantation, we measured major tryptophan metabolites, such as quinolinic acid and kynurenine, in serial urine specimens from 51 patients by liquid chromatography-tandem mass spectrometry. Samples were collected between admission and day 90 after transplantation, and metabolite levels were correlated with early clinical events and outcome. In selected patients, IDO gene expression was assessed by quantitative RT-PCR in intestinal biopsies. Surviving patients had significantly lower metabolite levels on days 28, 42, and 90, respectively, compared with patients dying of GVHD and associated complications (n = 10). Kynurenine levels were directly correlated with severity and clinical course of GVHD: Mean urinary quinolinic acid levels were 4.5 ± 0.3 μmol/mmol creatinine in the absence of acute GVHD, 8.0 ± 1.1 μmol/mmol creatinine for GVHD grade 1 or 2, and 13.5 ± 2.7 μmol/mmol creatinine for GVHD grade 3 or 4 (P < .001), respectively. GVHD-dependent induction of IDO was further suggested by increased expression of IDO mRNA in intestinal biopsies from patients with severe GVHD. Our data indicate reactive release of kynurenines in GVHD-associated inflammation.
Introduction
Graft-versus-host disease (GVHD) is a major cause of treatment-related mortality (TRM) and morbidity after allogeneic stem cell transplantation (SCT) in patients with hematologic malignancies. Activation of donor T lymphocytes by recipient antigen-presenting cells is the central pathophysiologic event, but specific alloreaction is augmented by nonspecific inflammation and activation of innate immunity, both in the activation and effector phases of GVHD.1 Activation of both innate and specific immunity induces immunoregulatory mechanisms to control and balance the proinflammatory immune responses.
An important immunoregulatory mechanism broadly involved in the induction of peripheral tolerance is controlled by the enzyme indoleamine 2,3-dioxygenase (IDO), which is the initial rate-limiting enzyme of tryptophan catabolism along the kynurenine (KYN) pathway.2 The tryptophan catabolites KYN, 3-hydroxykynurenine, and 3-hydroxyanthranilic acid are potent inhibitors of T-cell activation and induce T-cell apoptosis.3 IDO is induced by IFN-γ in epithelial cells as well as in antigen-presenting cells and endothelial cells.3,4 Importantly, increased expression of IDO in antigen-presenting cell links innate to specific immunity as cytotoxic and Th17-like responses are reduced in favor of Treg-type responses.5,6
To date, tryptophan catabolism along the KYN pathway has been analyzed mainly in experimental models of allogeneic SCT: Jasperson et al reported both accelerated GVHD in IDO−/− mice after allogeneic bone marrow transplantation and aggravation of GVHD in IFN-γR−/− recipients that were unable to produce IDO as an important counter-regulatory mechanism.7,8 In patients, increased IDO activity has been reported in monocytes of pregnant women and stem cell transplant recipients without GVHD,9 and recently in intestinal epithelial cells as well as intestinal CD4+ cells from patients with GVHD.10 Systematic analyses, however, are still missing. We therefore addressed the role of the KYN pathway in early complications after clinical SCT by means of liquid chromatography-tandem mass spectrometry (LC-MS/MS) of tryptophan and intermediates of the KYN pathway in urine samples and by quantitative real-time RT-PCR of IDO mRNA expression in selected intestinal biopsies. Our data suggest a strong association of IDO activity with the severity and outcome of clinical GVHD.
Methods
Patients
Fifty-four consecutive patients were included in the study irrespective of the type of donor or conditioning. Three patients were excluded from analysis because of confounding early EBV-posttransplantation lymphoproliferative disease or early relapse. In the 51 study patients, indications for allogeneic SCT were acute myeloid leukemia (n = 28), acute lymphoblastic leukemia (n = 9), myelodysplastic syndrome (n = 4), myeloma (n = 4), and other hematologic malignancies. Eighteen patients were in early, 20 in intermediate, and 13 in advanced phases of the underlying disease. Median age of patients at SCT was 47 years (range, 16-70 years), 14 received unmanipulated grafts from HLA identical siblings (n = 14) or unrelated donors (n = 37) after standard (n = 18) or reduced intensity (n = 33) conditioning. Immunosuppressive prophylaxis consisted of calcineurin inhibitors and methotrexate. In case of unrelated donors, anti–thymocyte globulin (ATG) serotherapy was given in the days before SCT. Acute GVHD, treatment, outcome of treatment, and time course of GVHD, major complications, and causes of deaths were recorded. The study had been approved by the medical ethics committee at the University of Regensburg (approval no. 02/220). All patients gave written informed consent to sampling of urine as well as to endoscopic examination, intestinal biopsies, and collection of clinical data, in accordance with the Declaration of Helsinki.
Urine levels of tryptophan and KYNs were correlated with clinical events. Comparisons between urinary catabolite levels in different clinical groups were made by t tests. t tests, the area under the receiver operator curve, and log-rank tests for cumulative nonrelapse mortality were calculated by means of the PASW Version 18 statistical software. Although all relevant metabolites were analyzed for all time points, we focus here for clarity on urinary KYN and quinolinic acid (QA) levels. In selected patients, lower gastrointestinal endoscopy was performed and biopsies were obtained for routine histopathology as well as IDO gene expression analysis.
Analysis of tryptophan and KYNs
Urine specimens were collected before conditioning and at defined time points (days 0, 7, 14, 21, 28, 42, and 90) until at least day 90 after SCT or death and in defined relation to onset of clinically relevant GVHD (days 0, 4, 7, and 28). Specimens were stored at −20°C until liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) of tryptophan, KYN, kynurenic acid, 3-hydroxykynurenine, QA, xanthurenic acid, anthranilic acid, and 3-hydroxyanthranilic acid was performed according to published protocols.11 The urinary concentrations of tryptophan and KYNs are given as molar ratios relative to urinary creatinine, which was measured over a linear dynamic range of 0.01 to 100μM by LC-ESI-MS/MS under isocratic conditions (0.1% formic acid in 50% aqueous acetonitrile) at a flow rate of 400 μL/min with 0.5μM deuterated [2H3]-creatinine from C/D/N Isotopes Inc serving as internal standard.
RT-PCR of IDO in intestinal biopsies
Intestinal biopsies were collected in RNA later (QIAGEN) and immediately stored at 4°C. Total RNA was isolated using the RNeasy Mini Kit (QIAGEN), and reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase (Promega) following the manufacturer's instructions. Reverse-transcribed products were analyzed on a Mastercyler Ep Realplex (Eppendorf) using the QuantiFast SYBR Green PCR Kit (QIAGEN) according to the manufacturer's instructions. As gene-specific primers, the following oligo-DNAs were used (5′-3′): IDO, GGTCATGGAGATGTCCGTAAGGT; and GAPDH, CCACATCGCTCAGACACCAT. Melting curves were analyzed to control for specificity of the PCR reactions. Expression of IDO was normalized to GAPDH. The relative units were calculated from a standard curve plotting 3 different concentrations of log dilutions against the PCR cycle number.
Results and discussion
Tryptophan metabolites associate with GVHD and TRM
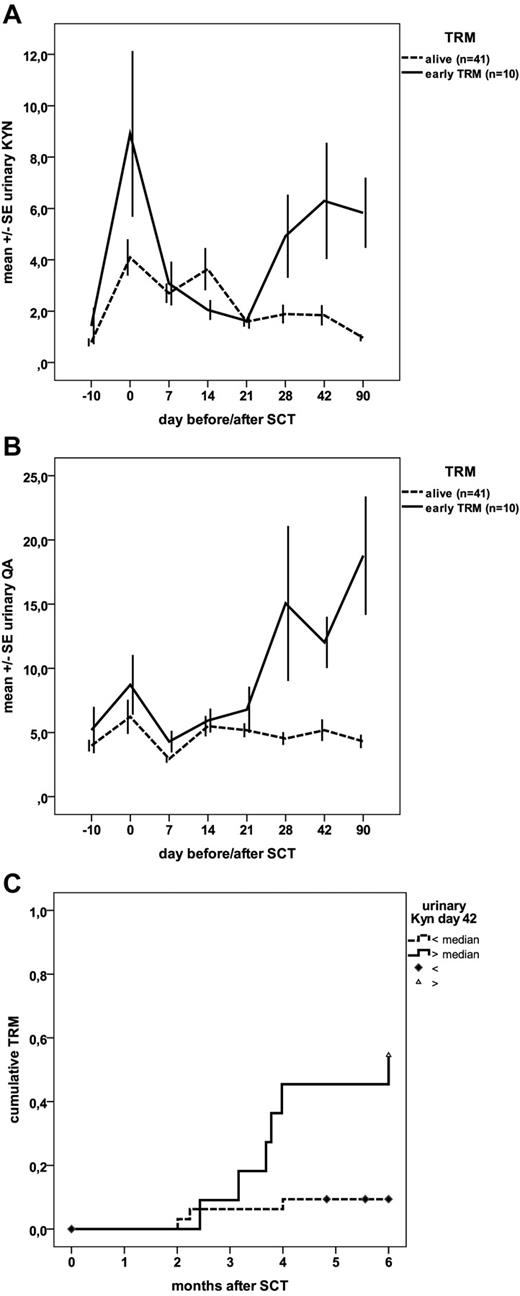
Forty-one patients survived at least until day 90 after SCT, whereas 10 patients died either because of severe GVHD alone (n = 7) or GVHD associated with infection (n = 2) or late veno-occlusive disease (n = 1). Urinary QA and KYN levels showed a first peak on the day of SCT, followed by a drop during the aplastic phase and continuously low levels in surviving patients, whereas patients dying of GVHD and related complications had significantly increased levels on days 28, 42, and 90 after SCT (Figure 1A-B). Vice versa, increased urinary KYN and QA levels were associated with TRM: The area under the curve of the receiver operator curve was 0.79 and 0.73 for QA and KYN, respectively, on day 28, and 0.83 and 0.77 on day 42. Patients with KYN levels above median had a significantly increased 6-month TRM of 50% and 55%, respectively, on days 28 and 42 (Figure 1C), whereas nonrelapse mortality was as low as 10% and 14% in patients with KYN levels below median.
Prognostic significance of kynurenine catabolites. (A) Time courses of urinary levels of KYN as a function of TRM. Data are the mean ± SE of the molar ratio of KYN to creatinine (μmol/mmol) for both surviving patients (n = 41) and those who died of GVHD and associated complications (n = 10) as a function of time; differences were significant: P < .05 for day 28 and P < .01 for days 42 and 90. (B) Time courses of urinary levels of QA as a function of TRM. Date are the mean ± SE of the molar ratio of urinary QA to creatinine (μmol/mmol) for both surviving patients (n = 41) and those who died of GVHD and associated complications (n = 10) as a function of time; differences were significant: P < .01 for day 28 and P < .001 for days 42 and 90. (C) Cumulative nonrelapse mortality as a function of urinary KYN levels on day 42. Patients with KYN levels above median had significantly (log-rank, P = .005) increased TRM compared with patients with levels below median.
Prognostic significance of kynurenine catabolites. (A) Time courses of urinary levels of KYN as a function of TRM. Data are the mean ± SE of the molar ratio of KYN to creatinine (μmol/mmol) for both surviving patients (n = 41) and those who died of GVHD and associated complications (n = 10) as a function of time; differences were significant: P < .05 for day 28 and P < .01 for days 42 and 90. (B) Time courses of urinary levels of QA as a function of TRM. Date are the mean ± SE of the molar ratio of urinary QA to creatinine (μmol/mmol) for both surviving patients (n = 41) and those who died of GVHD and associated complications (n = 10) as a function of time; differences were significant: P < .01 for day 28 and P < .001 for days 42 and 90. (C) Cumulative nonrelapse mortality as a function of urinary KYN levels on day 42. Patients with KYN levels above median had significantly (log-rank, P = .005) increased TRM compared with patients with levels below median.
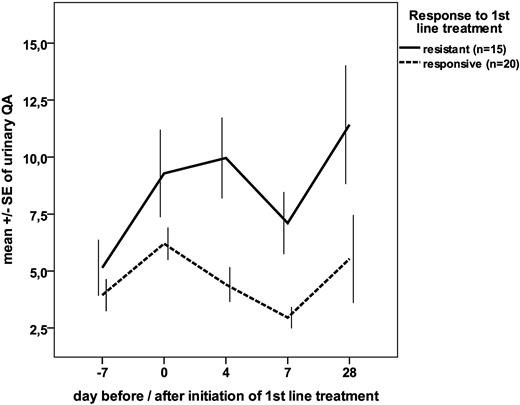
Overall, there was a strong correlation of urinary QA and KYN with grade of GVHD at the time of sampling at all time points after SCT: QA levels were 4.1 ± 0.2 μmol/mmol creatinine in the absence of GVHD, 8.0 ± 1.1 μmol/mmol for GVHD grade 1 or 2, and 13.5 ± 2.7 μmol/mmol for GVHD grade 3 or 4 (P < .001 for grade 0 vs grade 1 or 2; P = .04 for grade 1 or 2 vs grade 3 or 4). KYN levels rose from 2.0 ± 0.2 μmol/mmol creatinine in the absence of GVHD to 3.3 ± 0.6 μmol/mmol creatinine for GVHD grade 1 or 2 and 5.7 ± 2.0 μmol/mmol creatinine for GVHD grade 3 or 4 (P < .001 for grade 0 vs grade 1 or 2; not significant for grade 1 or 2 vs grade 3 or 4). In addition, urinary QA and KYN levels on days 4, 7, and 28 after onset of GVHD treatment differed significantly between patients requiring second-line treatment of GVHD within 28 days and those responding to first-line treatment of GVHD with corticosteroids (Figure 2). The area under the curve of the receiver operator curve to detect GVHD requiring second-line treatment within 28 days after initiation of corticosteroids was 0.81 for urinary QA (P < .05) and 0.63 for urinary KYN (not significant) on day 4, and after exclusion of 2 patients who had received second-line treatment between day 4 and day 7 highly significant with 0.84 for urinary QA and 0.93 for urinary KYN on day 7 (P < .001).
Time courses of urinary QA levels as a function of outcome of first-line treatment of acute GVHD. Data are the mean ± SE of the molar ratio of QA to creatinine (μmol/mmol) for both patients responding to first-line corticosteroids (n = 20) and those requiring second-line treatment within 28 days (“resistant”) (n = 15); differences were significant (P < .05) for days 4 and 7.
Time courses of urinary QA levels as a function of outcome of first-line treatment of acute GVHD. Data are the mean ± SE of the molar ratio of QA to creatinine (μmol/mmol) for both patients responding to first-line corticosteroids (n = 20) and those requiring second-line treatment within 28 days (“resistant”) (n = 15); differences were significant (P < .05) for days 4 and 7.
As shown in Figure 1, a first peak of urinary metabolites was observed on the day of SCT. This peak was exclusively seen in patients receiving pretransplant serotherapy with ATG, but not in patients receiving sibling transplants without ATG treatment. Mean urinary QA and KYN levels were 2.5 ± 0.4 μmol/mmol creatinine and 2.1 ± 0.5 μmol/mmol creatinine in 15 patients not receiving ATG but 8.0 ± 1.4 μmol/mmol creatinine and 6.5 ± 1.2 μmol/mmol creatinine in 37 treated with ATG (P < .002).
IDO expression is increased in patients with severe intestinal GVHD
In 41 of 51 patients, endoscopic examination of the lower gastrointestinal tract was performed either on a routine basis or for confirmation of clinically suspected gastrointestinal GVHD. As shown in Figure 3, gastrointestinal IDO expression increased with the severity of GVHD. In 3 patients, serial biopsies before onset or after resolution of GVHD and at onset of GVHD were available: Again, mean (± SE) expression of IDO was 0.00006 (± 0.00003) in the absence of GVHD, but 0.0076 (± 0.0068) at the onset of GVHD (P = .02).
Relative expression of IDO in biopsies from lower gastrointestinal tract as a function of GVHD grade at the time of biopsy. Relative expression of IDO (± SE) as assessed by quantitative RT-PCR is shown. Differences between patients without GVHD and GVHD grade 1 or 2 as well as severe GVHD were significant (P = .03).
Relative expression of IDO in biopsies from lower gastrointestinal tract as a function of GVHD grade at the time of biopsy. Relative expression of IDO (± SE) as assessed by quantitative RT-PCR is shown. Differences between patients without GVHD and GVHD grade 1 or 2 as well as severe GVHD were significant (P = .03).
Discussion
Our study indicates activation of the KYN pathway in GVHD after human allogeneic SCT, as it has been reported in experimental models of GVHD.7,8 Underlying induction of IDO is confirmed by GVHD-dependent IDO expression in lower gastrointestinal biopsies. As IFN-γ is a central mediator in acute GVHD1 and has been shown to be a potent inducer of IDO, our findings seem to confirm the presence of an immunoregulatory loop involving IFN-γ and IDO not only in animals but also in patients.
Although induction of IDO was protective in experimental models of GVHD,7,8 the association of increased KYN levels and IDO activity with severity of GVHD and worse outcome observed in our clinical study seems contradictory. However, this may reflect a reactive release of immunosuppressive mediators in GVHD-related inflammation, which is initiated to balance immune reactions. Similar reactive release has been observed for the anti–inflammatory cytokine IL-10, which is secreted in response to TNF-α and also indicates more severe GVHD. Obviously, this reactive induction of IDO is not sufficient to control GVHD in severe and established GVHD. In concordance, increased KYN/tryptophan ratios indicating activation of IDO have also been associated with kidney and lung allograft rejection.12,13
An open question is the cellular source of IDO activity. A recent study from Ratacjzak et al suggests production of IDO not only by epithelial cells and dendritic cells, but also by a newly described population of CD4+ IDO-producing cells.10 Interestingly, expression of IDO in CD4+ cells was down-regulated in more severe GVHD, whereas epithelial expression increased with severity. Our data reflect net activation of IDO and, thus, do not allow identification and differentiation of the underlying cellular source. Future careful studies are needed to dissect the contribution of different cellular sources, which may undergo differential regulation at different stages of GVHD.
An important aspect of our study is that KYNs do not only correlate with GVHD severity but also differ significantly between steroid-sensitive and steroid-resistant GVHD as early as 7 days after treatment initiation. As none of the patients had been treated with ATG as second-line treatment, explanation of this prognostic difference by a drug-related induction of metabolites as seen on day 0 (see next paragraph) can be excluded. If verified in larger patient cohorts by multivariate analysis, LC-ESI-MS/MS of KYNs, which can be performed in a routine clinical laboratory, may permit the early prediction of therapeutic responses and the assessment of GVHD prognosis and, thus, allow the early risk-adaption of GVHD treatment.
Reactive and inflammation-related release of IDO also explains the first sharp increase in urinary KYN and QA on day 0, which was clearly related to the use of ATG. All ATGs are known to induce cytokine release syndromes with fever, chills, and other inflammatory reactions.14,15 Thus, release of IFN-γ may induce reactive activation of IDO. Whether ATG-induced activation of IDO contributes to immunosuppressive mechanism of action of ATGs needs further experimental analysis.
In conclusion, we demonstrate a strong activation of the KYN pathway in clinical allogeneic SCT. KYN levels in urine and serum appear to be indicators of an anti–inflammatory response, and activation of IDO may be used for monitoring of SCT complications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Conny Winter and Heike Bremm who collected, cryopreserved, and organized the specimens.
This work was supported in part by the intramural ReForM program of the Medical Faculty of the University of Regensburg, BayGene, BayImmuNet, the Jose Carreras Leukemia Foundation, and the Wilhelm Sander Foundation.
Authorship
Contribution: K.L. designed and performed clinical research, collected and analyzed data, and helped write the manuscript; W.Z. performed research in the laboratory, analyzed and interpreted data, and performed statistical analysis; M.C.W. performed research in the laboratory and analyzed data; U.S., K.P., and M.K. performed quantitative RT-PCR, analyzed and interpreted data, and helped write the manuscript; J.A. and B.H. collected clinical data; D.W. and M.E. collected and interpreted data and helped write the manuscript; R.A. designed research and helped write the manuscript; P.J.O. designed and overviewed research in the laboratory, analyzed and interpreted data, and wrote the manuscript; and E.H. designed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript.
Conflict-of-interest disclosure: E.H. and D.W. consulted and received grants for scientific conferences from Fresenius Hemocare, Germany. The remaining authors declare no competing financial interests.
Correspondence: Ernst Holler, Department of Haematology/Oncology, University Medical Center, Franz-Josef-Strauss Allee 11, 93042 Regensburg, Germany; e-mail: ernst.holler@klinik.uni-regensburg.de.
References
Author notes
K.L. and W.Z. contributed equally to this study.