Variability in presentation and prognosis of transient myeloproliferative disorder (TMD) in infants with Down syndrome (DS) has perplexed clinicians and scientists for decades and is explored in this issue of Blood by Gamis and colleagues from the Children's Oncology Group (COG).1
Molecular, biologic, and clinical data, such as the study by Gamis et al,1 indicate that transient myeloproliferative disorder (TMD) and Down syndrome–associated acute myeloid leukemia (ML-DS) are initiated before birth when fetal liver hematopoietic stem and/or progenitor cells trisomic for chromosome 21 acquire GATA1 mutations. TMD usually presents around birth with a spectrum of clinical features from life-threatening hepatic fibrosis to asymptomatic leukocytosis. Although most cases of TMD spontaneously and permanently remit by the age of 6 months, in 15% to 30% of cases additional genetic events lead to further expansion of the trisomic GATA1-containing clone(s) resulting in ML-DS before the age of 5 years.
Molecular, biologic, and clinical data, such as the study by Gamis et al,1 indicate that transient myeloproliferative disorder (TMD) and Down syndrome–associated acute myeloid leukemia (ML-DS) are initiated before birth when fetal liver hematopoietic stem and/or progenitor cells trisomic for chromosome 21 acquire GATA1 mutations. TMD usually presents around birth with a spectrum of clinical features from life-threatening hepatic fibrosis to asymptomatic leukocytosis. Although most cases of TMD spontaneously and permanently remit by the age of 6 months, in 15% to 30% of cases additional genetic events lead to further expansion of the trisomic GATA1-containing clone(s) resulting in ML-DS before the age of 5 years.
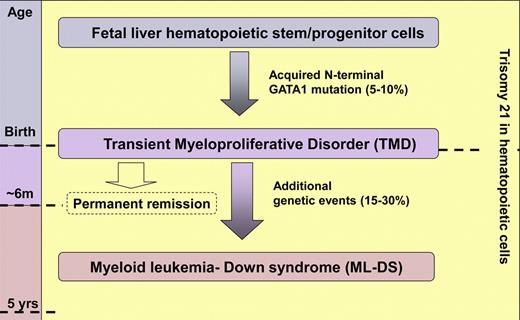
TMD is a clonal myeloproliferation unique to neonates with DS that is characterized by increased circulating blast cells morphologically indistinguishable from those in DS–myeloid leukemia (DS-ML).2-4 Indeed, 20% to 30% of infants with TMD subsequently develop ML-DS; fortunately, in most cases TMD spontaneously resolves without later transformation.4 Although TMD is diagnosed in 5% to 10% of neonates with DS, its exact frequency is expected to be determined by population-based studies currently in progress. Identification of N-terminal mutations in the key megakaryocyte transcription factor GATA1 in both ML-DS and TMD provided important insight into their pathogenesis.5-8 Molecular, biologic, and clinical data indicate that ML-DS is initiated before birth when fetal liver hematopoietic stem and/or progenitor cells trisomic for chromosome 21 (Hsa21) acquire GATA1 mutations.3,4,6,8,9 Cases with paired ML-DS and TMD samples show the same GATA1 mutation, indicating they are clonally linked conditions.8 Thus, TMD and ML-DS provide a tractable model to investigate leukemia intiation and progression (see figure). Nevertheless, many questions remain to be answered before accumulating molecular and clinical data can be assembled into a model that accounts for the extraordinary variability in the natural history of these conditions.
First, because TMD is only loosely defined even in the World Health Organization classification,2 any DS or mosaic DS neonate could have TMD because there are other causes of circulating blasts. Thus, it is essential to accurately define TMD and establish useful diagnostic criteria. Second, because the clinical presentation of TMD varies from life-threatening organ infiltration to asymptomatic leukocytosis,1,4 it is important to identify which factors reliably predict TMD outcome and therefore which patients are likely to benefit from treatment. Allied to this is the need to establish the most effective treatment regimens for TMD. While the study by Gamis and colleagues cannot answer all of these questions, it is one of the largest prospective studies of the natural history of clinically diagnosed TMD and provides valuable clinical information for hematologists and pediatricians.1 Strengths of the study are the uniform, albeit broad, clinical diagnostic criteria and treatment and monitoring guidelines. A limitation, as the authors admit, is that the study was designed before recognition of GATA1 mutations in TMD which, given that TMD may be asymptomatic, prevents conclusions about the natural history of the full spectrum of this enigmatic disorder.
Hematologists usually encounter TMD through consultation on abnormal hematologic findings. Importantly for diagnosis, most neonates with TMD in the study were not anemic and had platelet counts similar to DS neonates without TMD.1 The principal hematologic abnormalities were leukocytosis (median 32.8 × 103/uL) and increased peripheral blood blasts (median 25%). Interestingly 16% had mosaicism for Hsa21, confirming that phenotypically normal neonates with hematologic findings consistent with TMD should be screened for trisomy 21 because Gamis and colleagues' study confirms others indicating they are similarly at risk of ML-DS.1,4,10 More than 75% of infants underwent bone marrow examination. Because it is unclear what additional information was obtained, it could be argued that marrow examination is unnecessary in infants with trisomy 21 where clinical and hematologic features are typical of TMD, particularly where GATA1 mutations confirm a (pre)leukemic clone.7,8,10
An important dilemma in TMD management is identifying which infants will benefit from treatment and what treatment is most effective in the short- and long-term. Gamis and colleagues approached the first question using prospectively defined criteria for the presence of one or more life-threatening symptoms (LTS), including hepatic dysfunction, hydrops fetalis, or blast count (> 100 000/μL), as sole criteria for instituting treatment at the physician's discretion.1 Almost half of those with LTS treated according to the guidelines (13/29) succumbed to TMD or treatment complications. By contrast, TMD resolved completely without treatment in all patients without LTS, as found previously where similar treatment guidelines were used.4 These data support the conclusion that neonates without LTS (at diagnosis or while hematologic evidence of TMD persists) can safely be monitored without treatment because their outcome is favorable.
Previous reports confirmed here show that TMD patients with high-risk features defined as organ infiltration, especially hepatic, and/or high leukocyte count (> 100,000/uL) have a mortality of more than 30%.1,3,4 Klusmann et al's study showed that treatment of patients with high-risk features (which also included ascites, prematurity, and failure of remission of TMD), improved survival in the first months of life. Gamis and colleagues did not find improved survival of TMD patients with treatment (cytarabine 3.33 mg/kg/24 hours by continuous infusion for 5 days), perhaps because their guidelines permitted treatment for less severe TMD (a single LTS) and/or because cytarabine-related toxicity was high (96% grade 3/4 toxicity). Although the main aim of treating high-risk TMD is improvement in short-term survival, eradicating the (pre)leukemic clone(s) and consequent reduction in risk of later ML-DS is a potential long-term benefit. Unfortunately, no studies, including that of Gamis and colleagues, have yet demonstrated a significant impact of treatment on the likelihood of developing ML-DS.1,4 Thus, further studies are needed to refine treatment intervention criteria for TMD and the most effective treatment regimen for short-term and long-term benefit.
The findings of Gamis et al are nevertheless helpful for clinicians caring for neonates and children with DS and go some way to answering important clinical questions. However, many other questions remain: What is the relationship between TMD, as clinically defined, and the presence of GATA1 mutation(s)? Does the presence of GATA1 mutation(s) in the absence of typical clinical and hematologic features constitute TMD and does this carry the same risk of transformation to ML-DS? Can patients without GATA1 mutations at birth develop ML-DS, and does this share the same characteristic time window of presentation (< 5 years of age) and immunophenotypic (typically megakaryoblastic) and genetic features, characteristically seen in patients with GATA1 mutations in the neonatal period? Answers to such questions continue to fascinate all interested in Hsa21 and its many enigmatic links to leukemia.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal